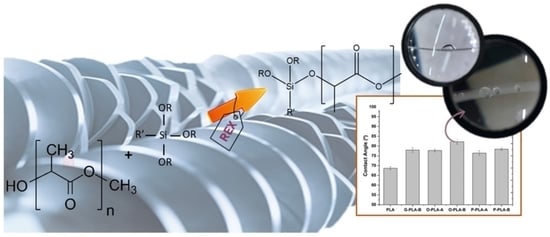
Improved Mechanical, Thermal, and Hydrophobic Properties of PLA Modified with Alkoxysilanes by Reactive Extrusion Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.2.1. Reactive Extrusion
2.2.2. Flat Tape Extrusion
2.2.3. Characterization Methods
3. Results
3.1. PLA/Alkoxysilanes Reaction
3.2. Characterization of the Functionalized Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe and European Association of Plastics Recycling and Recovery Organisations (EPRO). 2019. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 30 April 2021).
- European Environment Agency. The Plastic Waste Trade in the Circular Economy. 2019. Available online: https://www.eea.europa.eu/themes/waste/resource-efficiency/the-plastic-waste-trade-in (accessed on 31 May 2021).
- Sintim, H.; Bandopadhyay, S.; English, M.E.; Bary, A.I.; DeBruyn, J.M.; Schaeffer, S.M.; Miles, C.A.; Reganold, J.P.; Flury, M. Impacts of biodegradable plastic mulches on soil health. Agric. Ecosyst. Environ. 2018, 273, 36–49. [Google Scholar] [CrossRef]
- Haider, T.; Völker, C.; Kramm, J.; Landfester, K. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2018, 58, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formela, K.; Zedler, L.; Hejna, A.; Tercjak, A. Reactive Extrusion of Bio-Based Polymer Blends and Composites. Curr. Trends Future Dev. 2018, 12, 24–57. [Google Scholar]
- The Global Bio-Based Polymer Market 2019—A Revised View on a Turbulent and Growing Market. Bio-Based News. 2020. Available online: http://news.bio-based.eu/the-global-bio-based-polymer-market-2019-a-revised-view-on-a-turbulent-and-growing-market/ (accessed on 28 June 2021).
- European Bioplastics, Nova-Institute. 2019. Available online: https://www.european-bioplastics.org/market/ (accessed on 30 April 2021).
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stabil. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Bio-Sourced Biodegradable PLA Fibres Will Replace Polyester in Textiles. Bioplastics News. 2017. Available online: https://bioplasticsnews.com/2017/08/04/bio-sourced-biodegradable-pla-fibres-polyester-textiles/ (accessed on 28 June 2021).
- Radicci Company. Available online: https://www.radicigroup.com/en/products/fibres-and-nw/poy-starlight/biopolymer-cornleaf (accessed on 31 May 2021).
- Avinc, O.; Khoddami, A. Overview of Poly(Lactic Acid) (PLA) Fibre: Part I: Production, Properties, Performance, Environmental Impact, and End-Use Applications of Poly(Lactic Acid). Fibres 2009, 41, 391–401. [Google Scholar] [CrossRef]
- Zhang, J.F.; Sun, X. Poly(Lactic Acid)-Based Bioplastics; Woodhead Publishing: Cambridge, UK, 2005; pp. 251–288. [Google Scholar]
- Farrington, D.W.; Lunt, J.; Davies, S.; Blackburn, R.S. Polylactic acid fibres. In Biodegradable and Sustainable Fibres; Blackburn, R.S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2005. [Google Scholar]
- Auras, R.A.; Lim, L.T.; Selke, S.E.; Tsuji, H. Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. Part IV: Degradation and Environmental Issues; John Wiley Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Karst, D.; Hain, M.; Yang, Y. Care of PLA Textiles. Res. J. Text. Apparel 2009, 13, 69. [Google Scholar] [CrossRef]
- Avinc, O.; Wilding, M.; Phillips, D. Investigation of the influence of different commercial softeners on the stability of poly(lactic acid) fabrics during storage. Polym. Degrad. Stab. 2010, 95, 214. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, W. Improving the hydrolysis resistance of poly(lactic acid) fiber by hydrophobic finishing. Ind. Eng. Chem. Res. 2015, 54, 2599–2605. [Google Scholar] [CrossRef]
- Patankar, N.A. Mimicking the Lotus Effect: Influence of double roughness structures and slender pillars. Langmuir 2004, 20, 8209–8213. [Google Scholar] [CrossRef]
- eCoatsolutions Company. Available online: https://www.eco-coatsolutions.com/greenhouse--solar-series-self-cleaning-nano-ceramic-coatings.html (accessed on 31 May 2021).
- Kaseema, M.; Fatimah, S.; Nashrah, N.; Gun Ko, Y. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Hamad, K.; Gun Ko, Y.; Kaseem, M.; Deri, F. Effect of acrylonitrile–butadiene–styrene on flow behavior and mechanical properties of polylactic acid/low density polyethylene blend. Asia Pac. J. Chem. Eng. 2014, 9, 349–353. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Costas, A.V.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.; Tambe, C.; Narayan, R. Using Reactive Extrusion to Manufacture Greener Products: From Laboratory Fundamentals to Commercial Scale. In Biomass Extrusion and Reaction Technologies: Principles to Practices and Future Potential; American Chemical Society: Washington, DC, USA, 2018; Volume 1304, pp. 1–23. [Google Scholar]
- Marinho, J.F.; Braga, N.F.; Krohn, A.; Myata, F.S.; Silveira, L.H.; Cabral Neto, A.; Fechine, G.J.M. Melt Processing of Polymer Biocomposites. Polímeros 2015, 25, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Formela, K.; Hejna, A.; Haponiuk, J.; Tercjak, A. In Situ Processing of Biocomposites via Reactive Extrusion; Ray, D., Ed.; Woodhead Publishing: Cambridge, UK, 2017; Volume 8, pp. 195–246. [Google Scholar]
- Liao, J.; Brosse, N.; Hoppe, S.; Du, G.; Zhou, X.; Pizzi, A. One-step compatibilization of poly(lactic acid) and tannin via reactive extrusion. Mater. Des. 2020, 191, 108603. [Google Scholar] [CrossRef]
- Nahum, T.; Dodiuk, H.; Dotan, A.; Kenig, S.; Lellouche, J.P. Superhydrophobic durable coating based on UV-photoreactive silica nanoparticles. J. Appl. Polym. Sci. 2014, 131, 41122. [Google Scholar] [CrossRef]
- Kchaou, M.; Torres, E.; Ylla, N.; Colella, M.; Da Cruz-Boisson, F.; Cassagnau, P.; Espuche, E.; Bounor-Legaré, V. Enhanced Hydrophobicity and Reduced Water Transport Properties in Alkylalkoxysilane Modified Poly(Butylene Terephthalate) Using Reactive Extrusion. Mater. Chem. Phys. 2019, 223, 597–606. [Google Scholar] [CrossRef]
- Tam, J.; Feng, B.; Ikuhara, Y.; Ohta, H.; Erb, U. Crystallographic orientation—Surface energy—Wetting property relationships of rare earth oxides. J. Mater. Chem. A 2018, 38, 1–6. [Google Scholar] [CrossRef]
- Schondelmaier, D.; Cramm, S.; Klingeler, R.; Morenzin, J.; Zilkens, C.; Eberhardt, W. Orientation and self-assembly of hydrophobic fluoroalkylsilanes. Langmuir 2002, 18, 6242–6245. [Google Scholar] [CrossRef] [Green Version]
- Vink, E.H.T.; Rábago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorksTM polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Gogolewski, S.; Pennings, A. Resorbable materials of poly(L-lactide). II. Fibers spun from solutions of poly(L-lactide) in good solvents. J. Appl. Polym. Sci. 1983, 28, 1045–1061. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; García-Sanoguera, D.; Balart, R. Effect of Miscibility on Mechanical and Thermal Properties of Poly(Lactic Acid)/Polycaprolactone Blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane Coupling Agents used for Natural Fiber/Polymer Composites: A Review. Compos. Part A 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [Green Version]
- Chumeka, W.; Pasetto, P.; Pilard, J.-F. Bio-based triblock copolymers from natural rubber and poly(lactic acid): Synthesis and application in polymer blending. Polymer 2014, 55, 4478–4487. [Google Scholar] [CrossRef]
- He, Z.; Jiang, L.; Chuan, Y.; Li, H.; Yuan, M. Ring-Opening Polymerization of L-Lactic Acid O-Carboxyanhydrides Initiated by Alkoxy Rare Earth Compounds. Molecules 2013, 18, 12768–12776. [Google Scholar] [CrossRef] [Green Version]
- Kricheldorf, A.R.; Hachmann-Thiessen, H.; Schwarz, G. Telechelic and Star-Shaped Poly(L-lactide)s by Means of Bismuth(III) Acetate as Initiator. Biomacromolecules 2004, 5, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Popelka, S.; Rypácek, F. Synthesis of polylactide with thiol end groups. Collect. Czech. Chem. Commun. 2003, 68, 1131–1140. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [Green Version]
- Taubner, V.; Shishoo, R. Influence of processing parameters on the degradation of poly(L-lactide) during extrusion. J. Appl. Polym. Sci. 2001, 79, 2128–2135. [Google Scholar] [CrossRef]
- Meng, X.; Nguyen, N.; Tekinalp, H.; Lara-Curzio, E.; Ozcan, S. Supertough PLA-silane nanohybrids by in situ condensation and grafting. ACS Sustain. Chem. Eng. 2018, 6, 1289–1298. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Tsuji, H.; Noda, I.; Ozaki, Y. Structural Changes and Crystallization Dynamics of Poly(l-lactide) during the Cold-Crystallization Process Investigated by Infrared and Two-Dimensional Infrared Correlation Spectroscopy. Macromolecules 2004, 37, 6433–6439. [Google Scholar] [CrossRef]
- Xu, R.; Xie, J.; Lei, C. Influence of Melt-Draw Ratio on the Crystalline Behaviour of a Polylactic Acid Cast Film with a Chi Structure. RSC Adv. 2017, 7, 39914–39921. [Google Scholar] [CrossRef] [Green Version]
- Coltelli, M.-B.; Mallegni, N.; Rizzo, S.; Cinelli, P. Improved Impact Properties in Poly(lactic acid) (PLA) Blends Containing Cellulose Acetate (CA) Prepared by Reactive Extrusion. Materials 2019, 12, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.Q.; Qu, J.P. Mechanical and rheological properties of epoxidized soybean oil plasticized poly (lactic acid). J. Appl. Polym. Sci. 2009, 112, 3185–3191. [Google Scholar] [CrossRef]
- Lee, J.C.; Choi, M.-C.; Choi, D.-H.; Ha, C.-S. Toughness Enhancement of Poly(Lactic Acid) through Hybridisation with Epoxide-Functionalized Silane via Reactive Extrusion. Polym. Degrad. Stab. 2019, 160, 195–202. [Google Scholar] [CrossRef]
- Ku Marsilla, K.I.; Verbeek, C.J.R. Modification of poly(lactic acid) using itaconic anhydride by reactive extrusion. Eur. Polym. J. 2015, 67, 213–223. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.; Kittikorn, T.; Strömberg, E.; Martínez-Felipe, A.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Hydrothermal ageing of polylactide/sisal biocomposites. Studies of water absorption behaviour and Physico-Chemical performance. Polym. Degrad. Stab. 2014, 108, 212–222. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Ogami, A.; Okamoto, M.; Ueda, K. New Polylactide/Layered Silicate Nanocomposite: Nanoscale Control Over Multiple Properties. Macromol. Rapid Commun. 2002, 23, 943–947. [Google Scholar] [CrossRef]
- Balart, J.F.; Montanes, N.; Fombuena, V.; Boronat, T.; Sánchez-Nacher, L. Disintegration in Compost Conditions and Water Uptake of Green Composites from Poly(Lactic Acid) and Hazelnut Shell Flour. J. Polym. Environ. 2017, 26, 701–715. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Ferri, J.M.; Cardona, S.C.; Lora, J.; Fombuena, V. Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (Salvia hispanica L.) to Improve Ductility and Thermal Properties of Poly(Lactic Acid). Polymers 2021, 13, 1283. [Google Scholar] [CrossRef]
- Garlotta, D.A. Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stabil. 2012, 10, 1898–1914. [Google Scholar] [CrossRef]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates. 1. Polylactide: Degradation under isothermal conditions, thermal degradation mechanism and photolysis of the polymer. Polym. Degrad. Stabil. 1985, 11, 309–326. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, M.; Ju, Z.; Tam, P.Y.; Hua, T.; Younas, M.W.; Kamrul, H.; Hu, H. Poly(lactic acid) fibers, yarns and fabrics: Manufacturing, properties and applications. Text. Res. J. 2020, 91, 1–29. [Google Scholar]
- Liv, S.; Tan, H.; Gu, J.; Zhang, Y. Silane Modified Wood Flour Blended with Poly(Lactic Acid) and Its Effects on Composite Performance. Bioresources 2015, 10, 5426–5439. [Google Scholar] [CrossRef] [Green Version]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Fombuena, V.; Balart, J.; Boronat, T.; Sánchez-Nácher, L.; Garcia-Sanoguera, D. Improving mechanical performance of thermoplastic adhesion joints by atmospheric plasma. Mater. Des. 2013, 47, 49–56. [Google Scholar] [CrossRef]
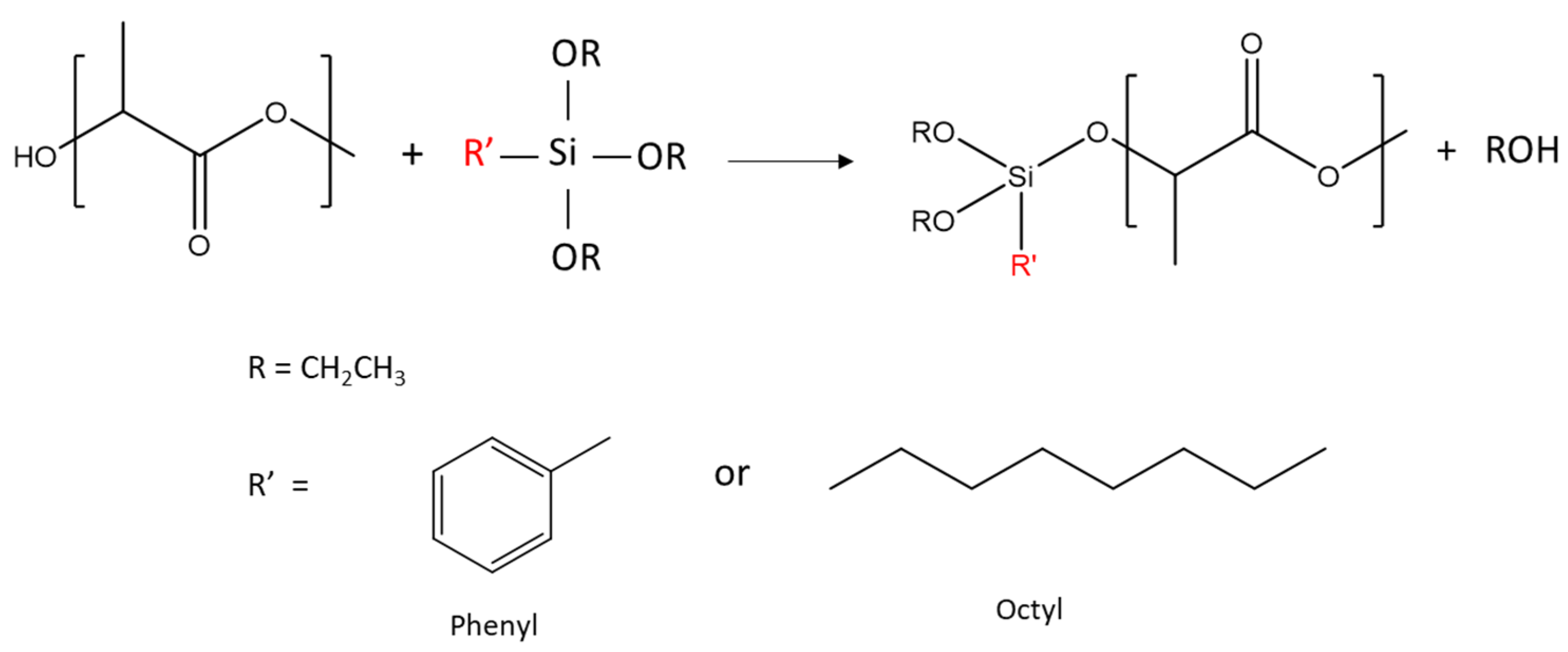
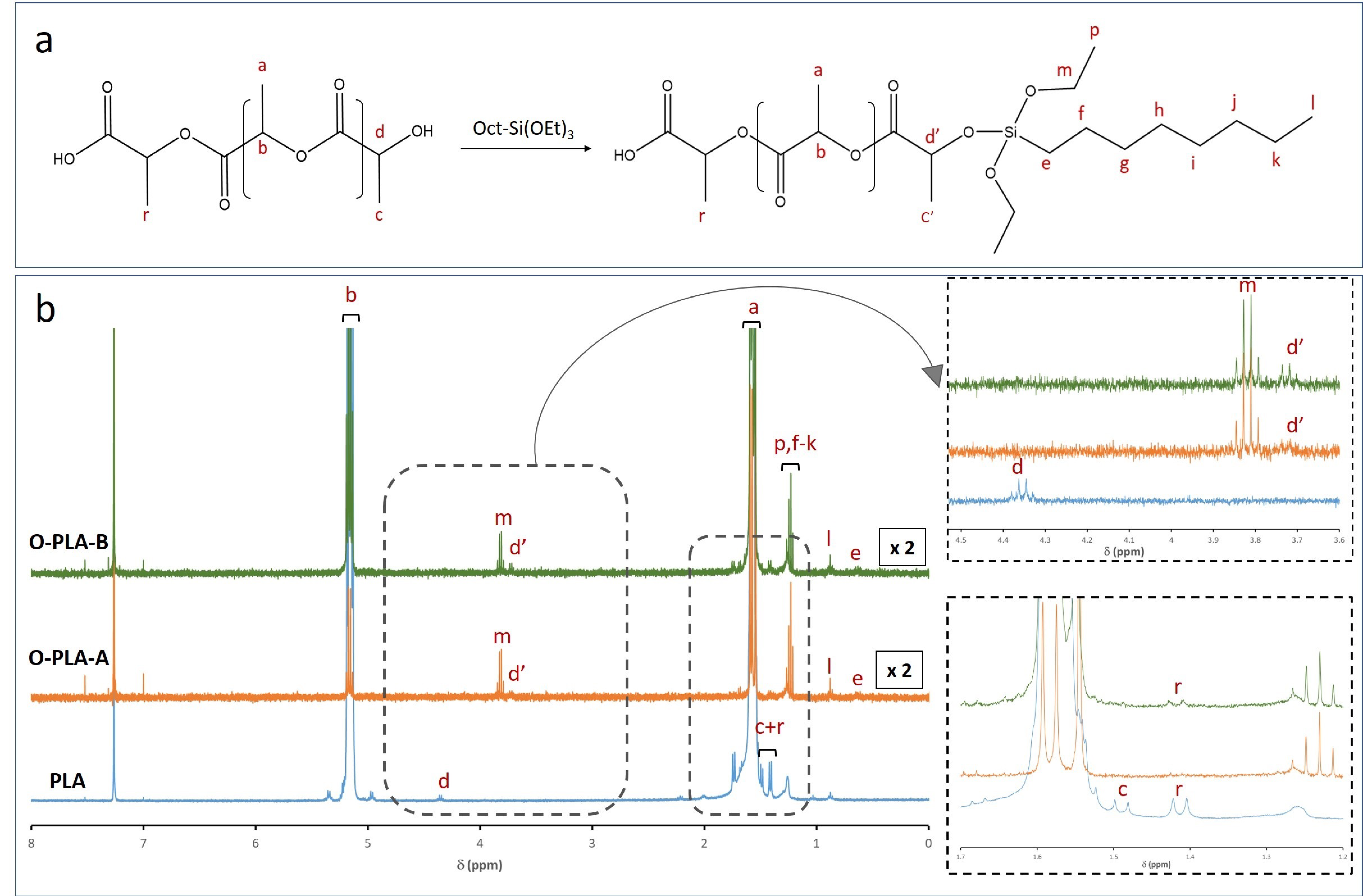
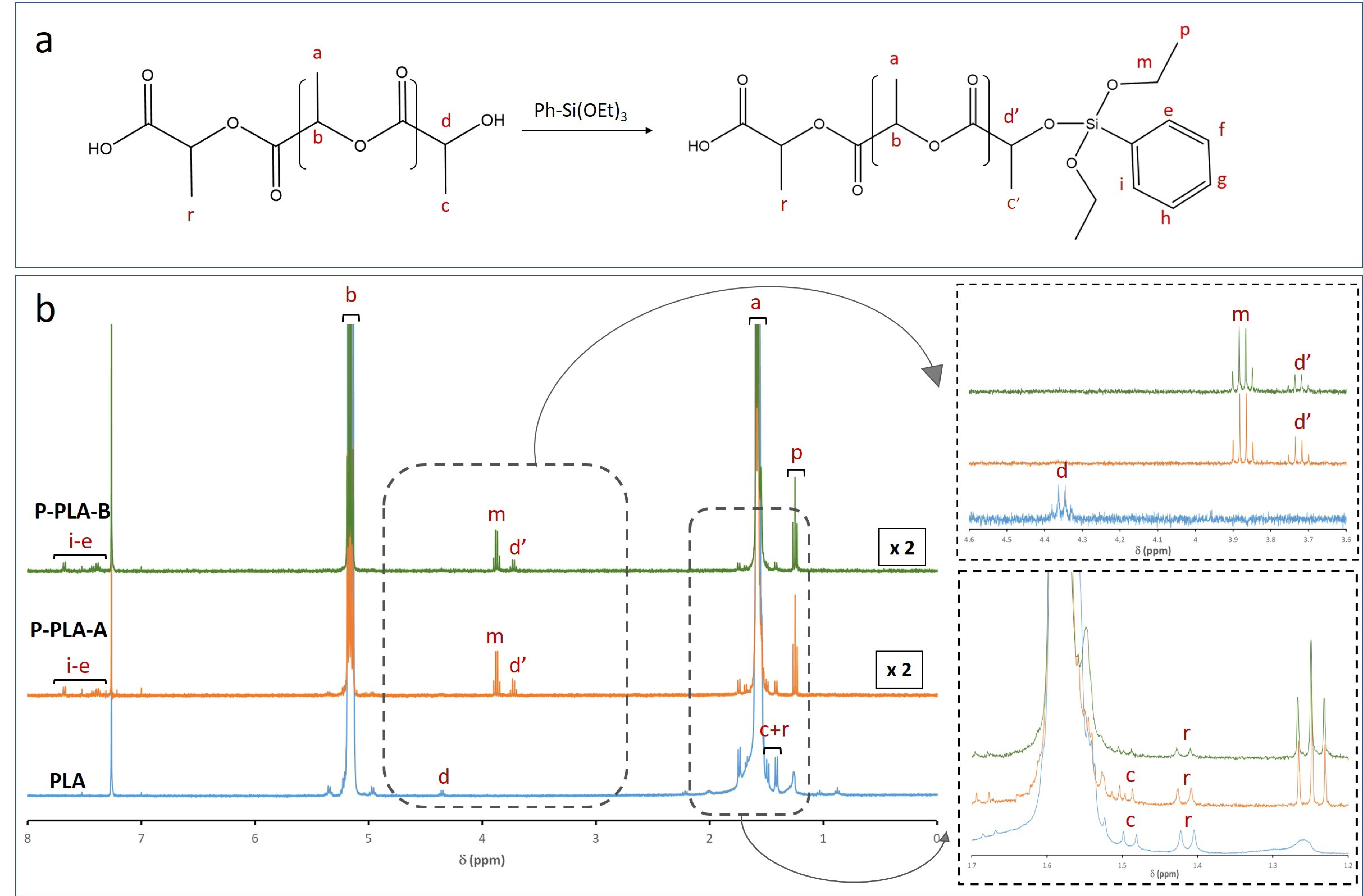
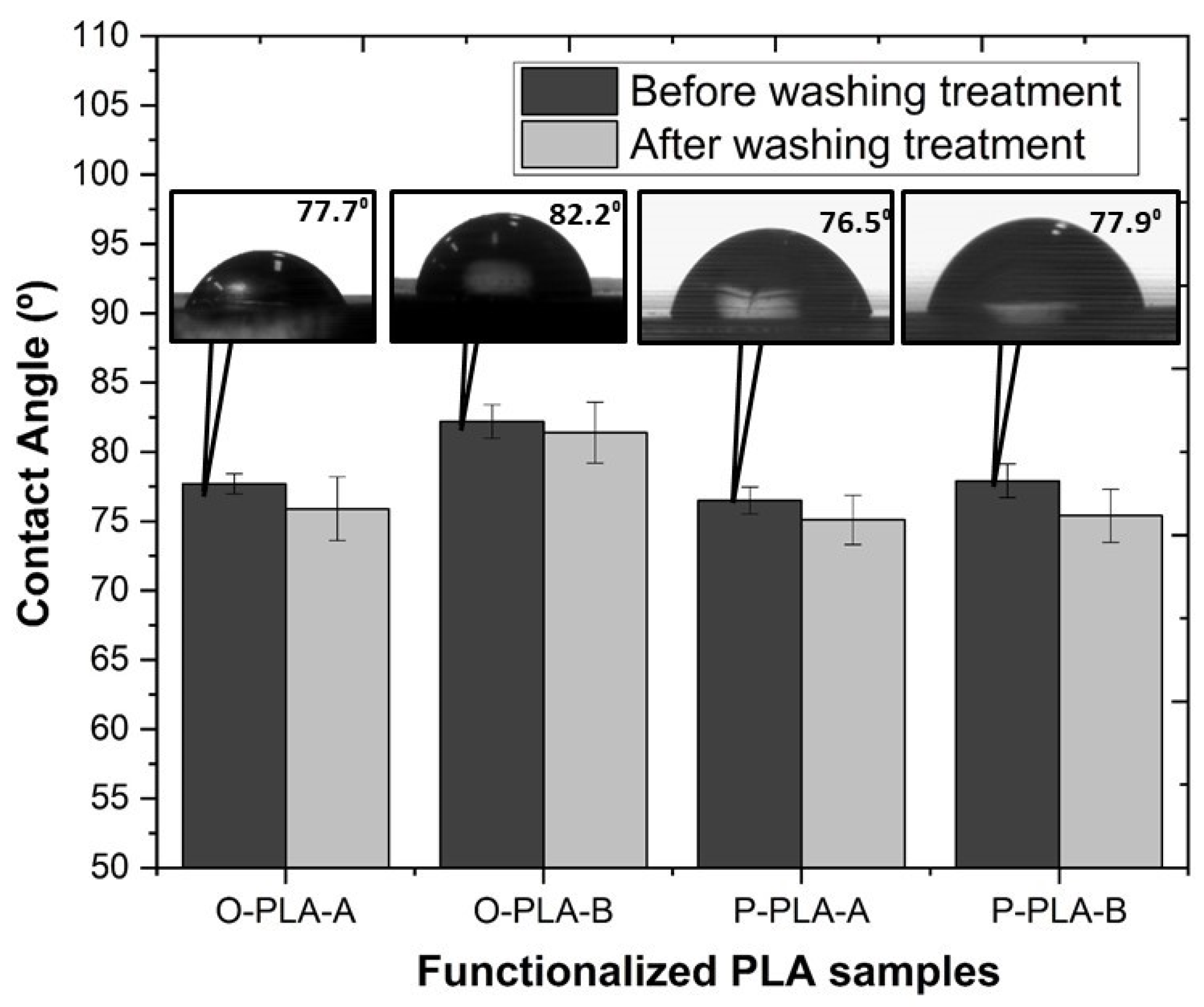
| Sample | Functional Molecule (R’) | Concentration (wt%) | Molar Concentration (mol/h) |
|---|---|---|---|
| PLA (control) | ------- | ------- | 0.031 |
| O-PLA-A | Oct-Si(OEt)3 | 1.5 | 0.167 |
| O-PLA-B | Oct-Si(OEt)3 | 3.0 | 0.337 |
| P-PLA-A | Ph-Si(OEt)3 | 1.3 | 0.167 |
| P-PLA-B | Ph-Si(OEt)3 | 2.7 | 0.337 |
| Sample | Vgodet1 (m/min) | Vgodet4 (m/min) | Draw Ratio (DR) | Dimensions (Width × Thickness, mm) |
|---|---|---|---|---|
| PLA | 10 | 45 | 4.5 | 2.70 × 0.14 |
| O-PLA-A | 10 | 55 | 5.5 | 2.34 × 0.13 |
| O-PLA-B | 10 | 66 | 6.6 | 2.40 × 0.14 |
| P-PLA-A | 10 | 55 | 5.5 | 2.32 × 0.13 |
| P-PLA-B | 10 | 62 | 6.2 | 2.20 × 0.14 |
| Sample | Concentration (ppm) | Si (%) | Incorporation (%) a |
|---|---|---|---|
| O-PLA-A | 259 ± 16 | 0.026 | 16.6 |
| O-PLA-B | 364 ± 22 | 0.036 | 12.2 |
| P-PLA-A | 727 ± 55 | 0.073 | 48.2 |
| P-PLA-B | 1594 ± 410 | 0.159 | 50.4 |
| Sample ID | Assignment | Chemical Signal (ppm) | Integration a |
|---|---|---|---|
| O-PLA-A | d’ | 3.7 | 1 |
| p | 1.21 | 9.58 | |
| O-PLA-B | d’ | 3.7 | 1 |
| p | 1.21 | 12.61 | |
| P-PLA-A | d’ | 3.7 | 1 |
| p | 1.21 | 3.45 | |
| P-PLA-B | d’ | 3.7 | 1 |
| p | 1.21 | 6.59 |
| Sample | Mn (g·mol−1) | Mw (g·mol−1) | D |
|---|---|---|---|
| Neat PLA | 97,000 ± 20,000 | 173,000 ± 20,000 | 1.85 |
| Extruded PLA | 75,000 ± 20,000 | 116,000 ± 20,000 | 1.56 |
| O-PLA-A | 48,000 ± 6000 | 94,000 ± 6000 | 1.96 |
| O-PLA-B | 45,000 ± 9000 | 90,000 ± 15,000 | 1.99 |
| P-PLA-A | 61,000 ± 6000 | 101,000 ± 4000 | 1.66 |
| P-PLA-B | 66,000 ± 4000 | 117,000 ± 2000 | 1.62 |
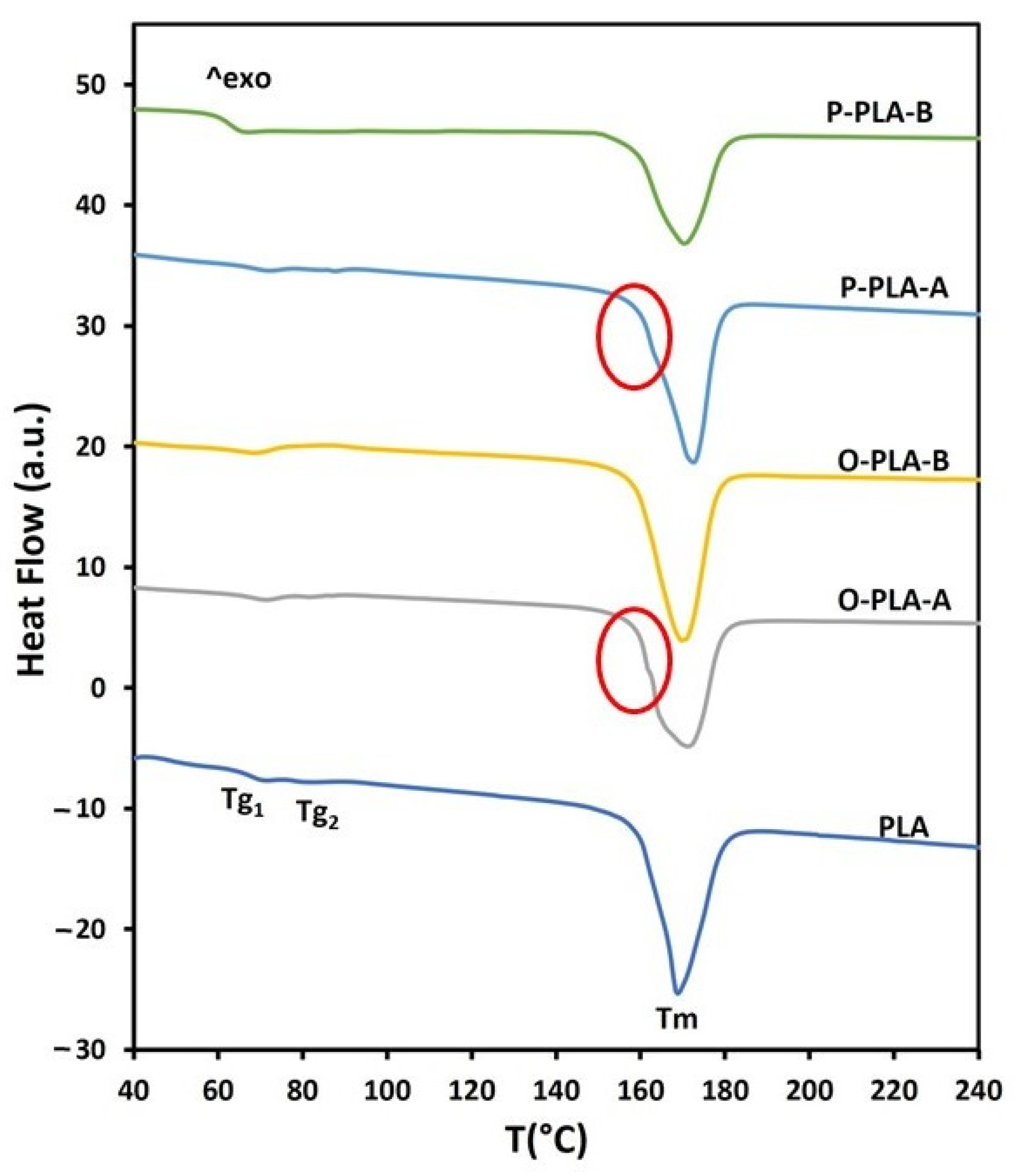
| Sample | Tg1 (°C) | Tg2 (°C) | Tm (°C) | ∆Hm (J·g−1) | Xc (%) a |
|---|---|---|---|---|---|
| PLA | 71 | 82 | 168 | 43.8 | 47 |
| O-PLA-A | 72 | 88 | 170 | 48.4 | 52 |
| O-PLA-B | 67 | ---- | 169 | 52.6 | 56 |
| P-PLA-A | 72 | 82 | 171 | 48.1 | 51 |
| P-PLA-B | 69 | ---- | 170 | 48.4 | 52 |
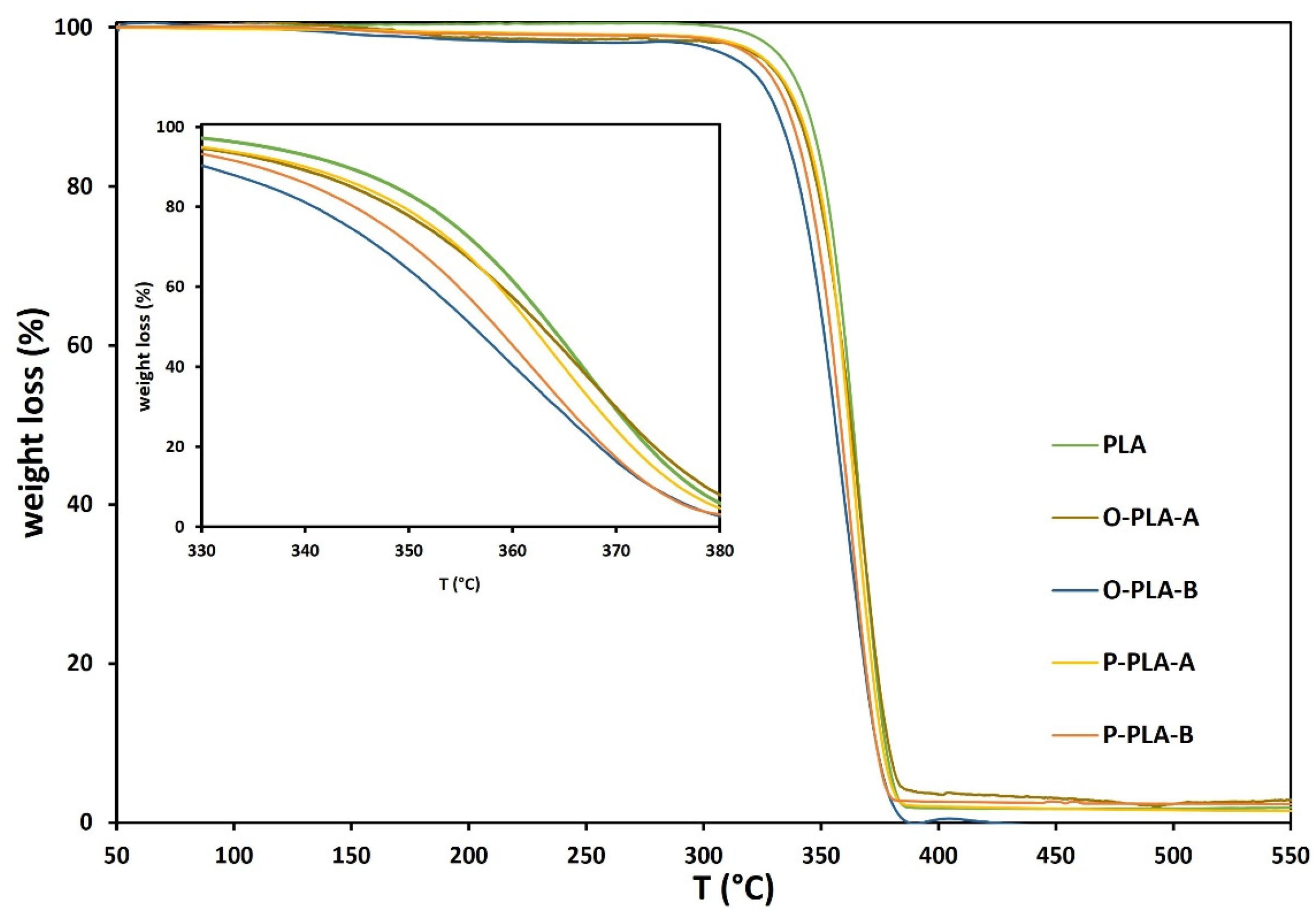
| Sample | T0 (°C) | T50 (°C) | Tf (°C) |
|---|---|---|---|
| PLA | 292 | 364 | 385 |
| O-PLA-A | 294 | 363 | 385 |
| O-PLA-B | 292 | 356 | 387 |
| P-PLA-A | 298 | 362 | 385 |
| P-PLA-B | 298 | 358 | 380 |
| Sample | DR | Dimensions (mm) (Width × Thickness) | Tensile Strength at Break (MPa) | Young’s Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| PLA | 4.5 | 2.70 × 0.14 | 157 ± 10 | 4.6 ± 0.1 | 40.2 ± 2.6 |
| O-PLA-A | 5.5 | 2.34 × 0.13 | 238 ± 5 | 5.1 ± 0.1 | 30.9 ± 1.0 |
| O-PLA-B | 6.6 | 2.40 × 0.14 | 215 ± 10 | 4.8 ± 0.2 | 31.9 ± 2.7 |
| P-PLA-A | 5.5 | 2.32 × 0.13 | 212 ± 15 | 5.1 ± 0.1 | 29.4 ± 3.9 |
| P-PLA-B | 6.2 | 2.37 × 0.14 | 177 ± 8 | 4.9 ± 0.1 | 23.4 ± 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, E.; Gaona, A.; García-Bosch, N.; Muñoz, M.; Fombuena, V.; Moriana, R.; Vallés-Lluch, A. Improved Mechanical, Thermal, and Hydrophobic Properties of PLA Modified with Alkoxysilanes by Reactive Extrusion Process. Polymers 2021, 13, 2475. https://doi.org/10.3390/polym13152475
Torres E, Gaona A, García-Bosch N, Muñoz M, Fombuena V, Moriana R, Vallés-Lluch A. Improved Mechanical, Thermal, and Hydrophobic Properties of PLA Modified with Alkoxysilanes by Reactive Extrusion Process. Polymers. 2021; 13(15):2475. https://doi.org/10.3390/polym13152475
Chicago/Turabian StyleTorres, Elena, Aide Gaona, Nadia García-Bosch, Miguel Muñoz, Vicent Fombuena, Rosana Moriana, and Ana Vallés-Lluch. 2021. "Improved Mechanical, Thermal, and Hydrophobic Properties of PLA Modified with Alkoxysilanes by Reactive Extrusion Process" Polymers 13, no. 15: 2475. https://doi.org/10.3390/polym13152475
APA StyleTorres, E., Gaona, A., García-Bosch, N., Muñoz, M., Fombuena, V., Moriana, R., & Vallés-Lluch, A. (2021). Improved Mechanical, Thermal, and Hydrophobic Properties of PLA Modified with Alkoxysilanes by Reactive Extrusion Process. Polymers, 13(15), 2475. https://doi.org/10.3390/polym13152475