Binary Green Blends of Poly(lactic acid) with Poly(butylene adipate-co-butylene terephthalate) and Poly(butylene succinate-co-butylene adipate) and Their Nanocomposites
Abstract
:1. Introduction
2. Synthesis, Structure and Properties of PLA, PBAT and PBSA
2.1. Poly(lactic acid)
2.2. Poly(butylene adipate-co-terephthalate)
2.3. Poly(butylene succinate-co-adipate)
3. PLA/PBAT and PLA/PBSA Blends: Preparation and Characterization
3.1. PLA/PBAT and PLA/PBSA Mixtures by Blending
3.2. PLA/PBAT and PLA/PBSA Mixtures by Reactive Blending
4. PLA/PBAT and PLA/PBSA Blend Nanocomposites: Preparation and Characterization
5. Biodegradation of PLA, PBAT, PBSA and Their Blends
6. Applications of PLA/PBSA and PLA/PBAT Blends and Nanocomposites
7. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Song, M.; Xu, Y.; Wang, W.; Wang, Z.; Zhang, L. Bio-based polyesters: Recent progress and future prospects. Prog. Polym. Sci. 2021, 120, 101430. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. (Eds.) Synthesis, Structure and Properties of Poly(lactic acid), Adv Polymerms Science; Springer: Cham, Switzerland, 2018; p. 279. [Google Scholar]
- Di Lorenzo, M.L.; Androsch, R. (Eds.) Industrial Applications of Poly(lactic acid), Adv Polymerms Science; Springer: Cham, Switzerland, 2018; p. 282. [Google Scholar]
- Dorgan, J.R.; Lehermeier, H.; Mang, M. Thermal and Rheological Properties of Commercial-Grade Poly(Lactic Acid)s. J. Polym. Environ. 2000, 8, 1–9. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA and their functions in widespread applications-A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.L. Poly(L-lactic acid)/Poly(butylene succinate) Biobased Biodegradable Blends. Polym. Rev. 2020, 1–37. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Gan, Z.; Kuwabara, K.; Yamamoto, M.; Abe, H.; Doi, Y. Solid-state structures and thermal properties of aliphatic–aromatic poly(butylene adipate-co-butylene terephthalate) copolyesters. Polym. Degrad. Stab. 2004, 83, 289–300. [Google Scholar] [CrossRef]
- Herrera, R.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. Characterization and degradation behavior of poly(butylene adipate-co-terephthalate)s. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4141–4157. [Google Scholar] [CrossRef]
- Pérez-Camargo, R.A.; Fernández-d’Arlas, B.; Cavallo, D.; Debuissy, T.; Pollet, E.; Avérous, L.; Müller, A.J. Tailoring the structure, morphology, and crystallization of isodimorphic poly(butylene succinate-ran-butylene adipate) random co-polymers by changing composition and thermal history. Macromolecules 2017, 50, 597–608. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable aliphatic polyesters. Part, I. Properties and biodegradation of poly(butylene succinate-co-butylene adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Li, K.-A.; Du, A.-K. Compatibilization strategies in poly(lactic acid)-based blends. RSC Adv. 2015, 5, 32546–32565. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Toughening of Polylactic Acid: An Overview of Research Progress. Polym. Technol. Eng. 2015, 55, 1623–1652. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, Y.-D.; Zeng, J.-B. Progress in Toughening Poly(lactic acid) with Renewable Polymers. Polym. Rev. 2017, 57, 557–593. [Google Scholar] [CrossRef]
- Sangeetha, V.; Deka, H.; Varghese, T.; Nayak, S.K. State of the art and future prospectives of poly(lactic acid) based blends and composites. Polym. Compos. 2018, 39, 81–101. [Google Scholar] [CrossRef]
- Koh, J.J.; Zhang, X.; He, C. Fully biodegradable Poly(lactic acid)/Starch blends: A review of toughening strategies. Int. J. Biol. Macromol. 2018, 109, 99–113. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Anuar, F.H.; Abdullah, I. The contribution of eco-friendly bio-based blends on enhancing the thermal stability and biodegradability of Poly(lactic acid). J. Clean. Prod. 2018, 198, 987–995. [Google Scholar] [CrossRef]
- Jin, F.-L.; Hu, R.-R.; Park, S.-J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. Part B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. A review on tensile and morphological properties of poly (lactic acid) (PLA)/ thermoplastic starch (TPS) blends. Polym. Technol. Mater. 2019, 58, 1945–1964. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ray, S.S. Rheology of poly(lactic acid)-based systems. Polym. Rev. 2019, 59, 465–509. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.F.; Ismail, H. An overview of toughening polylactic acid by an elastomer. Polym. Technol. Mater. 2019, 58, 1399–1422. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(butylene succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(lactic acid): A Review. J. Macromol. Sci. Part C 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Tan, J.; Abdel-Rahman, M.A.; Sonomoto, K. Biorefinery-Based Lactic Acid Fermentation: Microbial Production of Pure Monomer Product. In Synthesis, Structure and Properties of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 27–66. [Google Scholar]
- Byers, J.A.; Biernesser, A.B.; Chiaie, K.D.; Kaur, A.; Kehl, J.A. Catalytic Systems for the Production of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 67–118. [Google Scholar]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Cocca, M.; Malinconico, M. Crystal polymorphism of poly(L-lactic acid) and its influence on thermal properties. Thermochim. Acta 2011, 522, 110–117. [Google Scholar] [CrossRef]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly(L-lactic acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C. Crystal nucleation in random L/D-lactide copolymers. Eur. Polym. J. 2016, 75, 474–485. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Kühnert, I.; Spörer, Y.; Brünig, H.; Tran, N.H.A.; Rudolph, N. Processing of Poly(lactic acid). In Industrial Applications of Poly(lactic Acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–33. [Google Scholar]
- Domenek, S.; Fernandes-Nassar, S.; Ducruet, V. Rheology, Mechanical Properties, and Barrier Properties of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 303–341. [Google Scholar]
- Jacquel, N.; Saint-Loup, R.; Pascault, J.-P.; Rousseau, A.; Fenouillot, F. Bio-based alternatives in the synthesis of aliphatic–aromatic polyesters dedicated to biodegradable film applications. Polymer 2015, 59, 234–242. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Luo, S.; Li, F.; Yu, J.; Cao, A. Synthesis of poly(butylene succinate-co-butylene terephthalate) (PBST) copolyesters with high molecular weights via direct esterification and polycondensation. J. Appl. Polym. Sci. 2010, 115, 2203–2211. [Google Scholar] [CrossRef]
- Lee, P.C.; Lee, W.G.; Lee, S.Y.; Chang, H.N. Succinic Acid Production with Reduced by-Product Formation in the Fermentation of Anaerobiospirillum succiniciproducens Using Glycerol as a Carbon Source. Biotechnol. Bioeng. 2001, 72, 41–48. [Google Scholar] [CrossRef]
- Cheng, K.-K.; Zhao, X.; Zeng, J.; Zhang, J.-A. Biotechnological production of succinic acid: Current state and perspectives. Biofuels Bioprod. Biorefin. 2012, 6, 302–318. [Google Scholar] [CrossRef]
- Bretz, K.; Kabasci, S. Feed-Control Development for Succinic Acid Production with Anaerobiospirillum succinicipro-ducens. Biotechnol. Bioeng. 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Borges, E.R.; Pereira, N. Succinic acid production from sugarcane bagasse hemicellulose hydrolysate by Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 2011, 38, 1001–1011. [Google Scholar] [CrossRef]
- Peters, M.W.; Taylor, J.D.; Jenni, M.; Manzer, L.E.; Henton, D.E. Integrated Process to Selectively Convert Renewable Isobutanol to p-Xylene. U.S. Patent 2011/0087000, 14 April 2011. [Google Scholar]
- Berti, C.; Binassi, E.; Colonna, M.; Fiorini, M.; Kannan, G.; Karanam, S.; Mazzacurati, M.; Odeh, I.; Vannini, M. Bio-based Terephthalate Polyesters. WO Patent 2010/078328, 8 July 2009. [Google Scholar]
- Polen, T.; Spelberg, M.; Bott, M. Toward biotechnological production of adipic acid and precursors from biorenewables. J. Biotechnol. 2013, 167, 75–84. [Google Scholar] [CrossRef]
- Vardon, D.; Franden, M.A.; Johnson, C.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015, 8, 617–628. [Google Scholar] [CrossRef]
- Picataggio, S.; Rohrer, T.; DeAnda, K.; Lanning, D.; Reynolds, R.; Mielenz, J.; Eirich, L.D. Metabolic Engineering of Candida Tropicalis for the Production of Long–Chain Dicarboxylic Acids. Nat. Biotechnol. 1992, 10, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.; Lebeau, J.; Yang, T.; Li, S.; Lynch, M.D. A critical review on the progress and challenges to a more sustainable, cost competitive synthesis of adipic acid. Green Chem. 2021, 23, 3172–3190. [Google Scholar] [CrossRef]
- Mondal, D.; Bhowmick, B.; Mollick, M.R.; Maity, D.; Saha, N.R.; Rangarajan, V.; Rana, D.; Sen, R.; Chattopadhyay, D. Antimicrobial activity and biodegradation behavior of poly(butylene adipate-co-terephthalate)/clay nanocomposites. J. Appl. Polym. Sci. 2013, 131, 40079–40088. [Google Scholar] [CrossRef]
- Shi, X.; Ito, H.; Kikutani, T. Characterization on mixed-crystal structure and properties of poly(butylene adipate-co-terephthalate) biodegradable fibers. Polymer 2005, 46, 11442–11450. [Google Scholar] [CrossRef]
- Cranston, E.; Kawada, J.; Raymond, S.; Morin, F.G.; Marchessault, R.H. Cocrystallization model for synthetic biode-gradable poly(butylene adipate-cobutylene terephthalate). Biomacromolecules 2003, 4, 995–999. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A.; Billon, N.; Combeaud, C. Effect of the simultaneous biaxial stretching on the structural and mechanical properties of PLA, PBAT and their blends at rubbery state. Eur. Polym. J. 2015, 68, 288–301. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Zhu, G.; Huang, F.; Zhang, J. Synthesis,1H-NMR characterization, and biodegradation behavior of aliphatic–aromatic random copolyester. J. Appl. Polym. Sci. 2007, 104, 2643–2649. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Witt, U.; Müller, R.-J.; Deckwer, W.-D. New biodegradable polyester-copolymers from commodity chemicals with favorable use properties. J. Environ. Polym. Degrad. 1995, 3, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.-J.; Kleeberg, I.; Deckwer, W.-D. Biodegradation of polyesters containing aromatic constituents. J. Biotechnol. 2001, 86, 87–95. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bhattacharya, M.; Mano, J.F. Thermal analysis of the multiple melting behavior of poly(butylene succinate-co-adipate). J. Polym. Sci. B Polym. Phys. 2005, 43, 3077–3082. [Google Scholar] [CrossRef]
- Ray, S.S.; Bandyopadhyay, J.; Bousmina, M. Effect of Organoclay on the Morphology and Properties of Poly(propylene)/Poly[(butylene succinate)-co-adipate] Blends. Macromol. Mater. Eng. 2007, 292, 729–747. [Google Scholar] [CrossRef]
- Fujimaki, T. Processability and properties of aliphatic polyesters, ‘BIONOLLE’, synthesized by polycondensation reaction. Polym. Degrad. Stab. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Yang, J.; Pan, P.; Hua, L.; Xie, Y.; Dong, T.; Zhu, B.; Inoue, Y.; Feng, X. Fractionated crystallization, polymorphic crystalline structure, and spherulite morphology of poly(butylene adipate) in its miscible blend with poly(butylene succinate). Polymer 2011, 52, 3460–3468. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, P. Crystallization of biodegradable and biobased polyesters: Polymorphism, cocrystallization, and structure-property relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Avérous, L. Synthesis and characterization of bio-based poly (butylene furandicarboxylate)-b-poly (tetramethylene glycol) copolymers. Eur. Polym. J. 2017, 87, 84–98. [Google Scholar] [CrossRef]
- Nikolic, M.; Djonlagic, J. Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene adipate)s. Polym. Degrad. Stab. 2001, 74, 263–270. [Google Scholar] [CrossRef]
- Su, S.; Duhme, M.; Kopitzky, R. Uncompatibilized PBAT/PLA Blends: Manufacturability, Miscibility and Properties. Materials 2020, 13, 4897. [Google Scholar] [CrossRef]
- Nofar, M.; Oguz, H.; Ovalı, D. Effects of the matrix crystallinity, dispersed phase, and processing type on the morphological, thermal, and mechanical properties of polylactide based binary blends with poly[(butylene adipate)-co-terephthalate] and poly[(butylene succinate)-co-adipate]. J. Appl. Polym. Sci. 2019, 136, 47636. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Sojoudiasli, H.; Park, C.B.; Carreau, P.; Heuzey, M.-C.; Kamal, M. Mechanical and bead foaming behavior of PLA-PBAT and PLA-PBSA blends with different morphologies. Eur. Polym. J. 2017, 90, 231–244. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, C.; Wongwiwattana, P.; Thomas, N.L. Optimising ductility of poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends through co-continuous phase morphology. J. Polym. Environ. 2018, 26, 3802–3816. [Google Scholar] [CrossRef] [Green Version]
- Gigante, V.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rubber Toughening of Polylactic Acid (PLA) with Poly(butylene adipate-co-terephthalate) (PBAT): Mechanical Properties, Fracture Mechanics and Analysis of Ductile-to-Brittle Behavior while Varying Temperature and Test Speed. Eur. Polym. J. 2019, 115, 125–137. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, J.; Yang, X.; Xiao, P. Morphology and properties of biodegradable poly (lactic acid)/poly (butylene adipate-co-terephthalate) blends with different viscosity ratio. Polym. Test. 2017, 60, 58–67. [Google Scholar] [CrossRef]
- Oguz, H.; Dogan, C.; Kara, D.; Ozen, Z.T.; Ovali, D.; Nofar, M. Development of PLA-PBAT and PLA-PBSA bio-blends: Effects of processing type and PLA crystallinity on morphology and mechanical properties. AIP Conf. Proc. 2017, 2055, 030003. [Google Scholar] [CrossRef]
- Nofar, M.; Maani, A.; Sojoudi, H.; Heuzey, M.C.; Carreau, P.J. Interfacial and rheological properties of PLA/PBAT and PLA/PBSA blends and their morphological stability under shear flow. J. Rheol. 2015, 59, 317–333. [Google Scholar] [CrossRef]
- Gua, S.-Y.; Zhang, K.; Ren, J.; Zhan, H. Melt rheology of polylactide/poly(butylene adipate-co-terephthalate) blends. Carbohyrate Polym. 2008, 74, 79–85. [Google Scholar] [CrossRef]
- Dil, E.J.; Carreau, P.J.; Favis, B.D. Morphology, miscibility and continuity development in poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. Polymer 2015, 68, 202–212. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Pivsa-Art, S.; Fujii, K.; Nomura, K.; Ishimoto, K.; Aso, Y.; Yamane, H.; Ohara, H. Compression molding and melt-spinning of the blends of poly(lactic acid) and poly(butylene succinate-co-adipate). J. Appl. Polym. Sci. 2014, 132, 41856. [Google Scholar] [CrossRef]
- Ojijo, V.; Ray, S.S.; Sadiku, R. Concurrent Enhancement of Multiple Properties in Reactively Processed Nanocomposites of Polylactide/Poly[(butylene succinate)-co-adipate] Blend and Organoclay. Macromol. Mater. Eng. 2014, 299, 596–608. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Huang, S.-Y.; Chen, Y.-F.; Kuo, M.-T.; Chiang, T.-Y.; Chang, C.-Y.; Wang, Y.-H. Heat Treatment Effects on the Mechanical Properties and Morphologies of Poly (Lactic Acid)/Poly (Butylene Adipate-co-terephthalate) Blends. Int. J. Polym. Sci. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Quero, E.; Müller, A.J.; Signori, F.; Coltelli, M.-B.; Bronco, S. Isothermal Cold-Crystallization of PLA/PBAT Blends With and Without the Addition of Acetyl Tributyl Citrate. Macromol. Chem. Phys. 2012, 213, 36–48. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Role of Specific Interfacial Area in Controlling Properties of Immiscible Blends of Bio-degradable Polylactide and Poly[(butylene succinate)-co-adipate]. ACS Appl. Mater. Interfaces 2012, 4, 6690–6701. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Pavasupree, S.; O-Charoen, N.; Insuan, U.; Jailak, P.; Pivsa-Art, S. Preparation of Polymer Blends between Poly (L-lactic acid), Poly (butylene succinate-co-adipate) and Poly (butylene adipate-co-terephthalate) for Blow Film Industrial Application. Energy Procedia 2011, 9, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Peng, J.; Turng, L.-S.; Huang, H.-X. Dynamic rheological behavior and morphology of polylactide/poly(butylenes adipate-co-terephthalate) blends with various composition ratios. Adv. Polym. Technol. 2011, 30, 150–157. [Google Scholar] [CrossRef]
- Farsetti, S.; Cioni, B.; Lazzeri, A. Physico-Mechanical Properties of Biodegradable Rubber Toughened Polymers. Macromol. Symp. 2011, 301, 82–89. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Wu, Q.; Ren, J. Preparation, Mechanical, and Thermal Properties of Biodegradable Polyesters/Poly(lactic acid) Blends. J. Nanomater. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-T.; Tsou, C.-H.; Huang, C.-Y.; Chen, K.-N.; Wu, C.-S.; Chai, W.-L. Compatible and crystallization properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. J. Appl. Polym. Sci. 2009, 116, 680–687. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, W.; Yeh, J.-T. Crystallization Behavior of Fully Biodegradable Poly(Lacric acid)/Poly(Butylene Adipate-co-Terephthalate) Blends. J. Appl. Polym. Sci. 2009, 112, 3754–3763. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, W.; Lu, B.; Wang, P.; Wang, G.; Ji, J. PLA-PEG-PLA tri-block copolymers: Effective compatibilizers for promotion of the interfacial structure and mechanical properties of PLA/PBAT blends. Polymer 2018, 146, 179–187. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, W.; Huang, D.; Lu, B.; Wang, P.; Wang, G.; Ji, J. Compatibilization of immiscible PLA-based biodegradable polymer blends using amphiphilic di-block copolymers. Eur. Polym. J. 2019, 118, 45–52. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Bronco, S.; Chinea, C. The effect of free radical reactions on structure and properties of poly(lactic acid) (PLA) based blends. Polym. Degrad. Stab. 2010, 95, 332–341. [Google Scholar] [CrossRef]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P. In-situ compatibilization of poly(lactic acid) and poly(butylene adipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- Signori, F.; Boggioni, A.; Righetti, M.C.; Rondán, C.E.; Bronco, S.; Ciardelli, F. Evidences of Transesterification, Chain Branching and Cross-Linking in a Biopolyester Commercial Blend upon Reaction with Dicumyl Peroxide in the Melt. Macromol. Mater. Eng. 2014, 300, 153–160. [Google Scholar] [CrossRef]
- Ai, X.; Li, X.; Yu, Y.; Pan, H.; Yang, J.; Wang, N.; Yang, H.; Zhang, H.; Dong, L. The Mechanical, Thermal, Rheological and Morphological Properties of PLA/PBAT Blown Films by Using Bis(tert-butyl dioxy isopropyl) Benzene as Crosslinking Agent. Polym. Eng. Sci. 2019, 59, E227–E236. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Toncelli, C.; Ciardelli, F.; Bronco, S. Compatible blends of biorelated polyesters through catalytic trans-esterification in the melt. Polym. Degrad. Stab. 2011, 96, 982–990. [Google Scholar] [CrossRef]
- Lin, S.; Guo, W.; Chen, C.; Ma, J.; Wang, B. Mechanical properties and morphology of biodegradable poly(lactic ac-id)/poly(butylene adipate-co-terephthalate) blends compatibilized by transesterification. Mater. Design 2012, 36, 604–608. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Toughening of Biodegradable Polylactide/Poly(butylene succinate-co-adipate) Blends via in Situ Reactive Compatibilization. ACS Appl. Mater. Interfaces 2013, 5, 4266–4276. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Q.; Ren, J.; Wang, L. Preparation and properties of biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blend with glycidyl methacrylate as reactive processing agent. J. Mater. Sci. 2009, 44, 250–256. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, H. Mechanical properties and phase morphology of super-tough PLA/PBAT/EMA-GMA multicomponent blends. Mater. Lett. 2017, 192, 17–20. [Google Scholar] [CrossRef]
- Eslami, H.; Kamal, M.R. Effect of a chain extender on the rheological and mechanical properties of biodegradable poly(lactic acid)/poly[(butylene succinate)-co-adipate] blends. J. Appl. Polym. Sci. 2013, 129, 2418–2428. [Google Scholar] [CrossRef]
- Mallegni, N.; Phuong, T.V.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A. Poly(lactic acid) (PLA) Based Tear Resistant and Bio-degradable Flexible Films by Blown Film Extrusion. Materials 2018, 11, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhao, B.; Wei, B.; Wei, Y.; Yao, J.; Zhang, H.; Chen, X.; Shao, Z. Enhanced compatibility between poly(lactic acid) and poly (butylene adipate-co-terephthalate) by incorporation of N-halamine epoxy precursor. Int. J. Biol. Macromol. 2020, 165, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Biopolymer Blends Based on Poly (lactic acid): Shear and Elongation Rheology/Structure/Blowing Process Relationships. Polymers 2015, 7, 939–962. [Google Scholar] [CrossRef]
- Arruda, L.C.; Magaton, M.; Bretas, R.E.S.; Ueki, M.M. Influence of chain extender on mechanical, thermal and morphological properties of blown films of PLA/PBAT blends. Polym. Test. 2015, 43, 27–37. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S. Super toughened biodegradable polylactide blends with non-linear copolymer interfacial archi-tecture obtained via facile in-situ reactive compatibilization. Polymer 2015, 80, 1–17. [Google Scholar] [CrossRef]
- Palai, B.; Mohanty, S.; Nayak, S.K. Synergistic effect of polylactic acid(PLA) and Poly(butylene succinate-co-adipate) (PBSA) based sustainable, reactive, super toughened eco-composite blown films for flexible packaging applications. Polym. Test. 2020, 83, 106130. [Google Scholar] [CrossRef]
- Dong, W.; Zou, B.; Yan, Y.; Ma, P.; Chen, M. Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly(lactide)/Poly(butylene adipate-co-terephthalate) Blends. Int. J. Mol. Sci. 2013, 14, 20189–20203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Peng, S.; Chen, H.; Yu, X.; Zhao, X. Mechanical properties, rheological behaviors, and phase morphologies of high-toughness PLA/PBAT blends by in-situ reactive compatibilization. Compos. Part. B Eng. 2019, 173, 107028. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Yang, J.; Pan, H.; Gao, G.; Zhang, H.; Dong, L. Improvement of compatibility and mechanical properties of the poly(lactic acid)/poly(butylene adipate-co -terephthalate) blends and films by reactive extrusion with chain extender. Polym. Eng. Sci. 2018, 58, 1868–1878. [Google Scholar] [CrossRef]
- Li, X.; Ai, X.; Pan, H.; Yang, J.; Gao, G.; Zhang, H.; Yang, H.; Dong, L. The morphological, mechanical, rheological, and thermal properties of PLA/PBAT blown films with chain extender. Polym. Adv. Technol. 2018, 29, 1706–1717. [Google Scholar] [CrossRef]
- Wu, D.D.; Guo, Y.; Huang, A.P.; Xu, R.W.; Liu, P. Effect of the multi-functional epoxides on the thermal, mechanical and rheological properties of poly(butylene adipate-co-terephthalate)/polylactide blends. Polym. Bull. 2020, 1–25. [Google Scholar] [CrossRef]
- Farias da Silva, J.M.; Soares, B.G. Epoxidized cardanol-based prepolymer as promising biobased compatibilizing agent for PLA/PBAT blends. Polym. Test. 2021, 93, 106889. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Reactive extrusion of PLA, PBAT with a multi-functional epoxide: Physico-chemical and rheological properties. Eur. Polym. J. 2014, 58, 90–102. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Ferri, J.M.; Dominici, F.; Boronat, T.; Sánchez-Nacher, L.; Balart, R.; Torre, L. Manufacturing and compatibilization of PLA/PBAT binary blends by cottonseed oil-based derivatives. Express Polym. Lett. 2018, 12, 808–823. [Google Scholar] [CrossRef]
- Teamsinsungvon, A.; Ruksakulpiwat, Y.; Jarukumjorn, K. Preparation and Characterization of Poly(lactic acid)/Poly(butylene adipate-co-terepthalate) Blends and Their Composite. Polymer-Plastics Technol. Engineering 2013, 52, 1362–1367. [Google Scholar] [CrossRef]
- He, H.; Wang, G.; Chen, M.; Xiong, C.; Li, Y.; Tong, Y. Effect of Different Compatibilizers on the Properties of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate) Blends Prepared under Intense Shear Flow Field. Materials 2020, 13, 2094. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmus, A. Ductility improvements of PLA-based binary and ternary blends with controlled morphology using PBAT, PBSA, and nanoclay. Compos. Part. B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Malwela, T.; Sinha Ray, S. Role of Organoclay in Controlling the Morphology and Crystal-Growth Behavior of Biodegradable Polymer-Blend Thin Films Studied Using Atomic Force Microscopy. Macromol. Mater. Eng. 2014, 299, 1106–1115. [Google Scholar] [CrossRef]
- Eslami, H.; Kamal, M.R. Elongational rheology of biodegradable poly (lactic acid)/poly [(butylene succinate)-co-adipate] binary blends and poly (lactic acid)/poly [(butylene succinate)-co-adipate]/clay ternary nanocomposites. J. Appl. Polym. Sci. 2012, 127, 2290–2306. [Google Scholar] [CrossRef]
- Ojijo, V.; Ray, S.S.; Sadiku, R. Effect of Nanoclay Loading on the Thermal and Mechanical Properties of Biodegradable Polylactide/Poly[(butylene succinate)-co-adipate] Blend Composites. ACS Appl. Mater. Interfaces 2012, 4, 2395–2405. [Google Scholar] [CrossRef]
- Mhlabeni, T.; Pillai, S.K.; Ray, S.S. Effect of organically modified layered double hydroxides on the properties of poly(lactic acid)/poly[(butylene succinate)-co-adipate] immiscible blends. J. Appl. Polym. Sci. 2019, 137, 48654. [Google Scholar] [CrossRef]
- Shahlari, M.; Lee, S. Mechanical and morphological properties of poly(butylene adipate-co-terephthalate) and poly(lactic acid) blended with organically modified silicate layers. Polym. Eng. Sci. 2012, 52, 1420–1428. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.; Song, H.Y.; Hyun, K. Characterization of the Effect of Clay on Morphological Evaluations of PLA/Biodegradable Polymer Blends by FT-Rheology. Macromolecules 2019, 52, 7904–7919. [Google Scholar] [CrossRef]
- Nofar, M.; Heuzey, M.-C.; Carreau, P.J.; Kamal, M.R. Effects of nanoclay and its localization on the morphology stabilization of PLA/PBAT blends under shear flow. Polymer 2016, 98, 353–364. [Google Scholar] [CrossRef]
- Guo, Y.; He, S.; Yang, K.; Xue, Y.; Zuo, X.; Yu, Y.; Liu, Y.; Chang, C.-C.; Rafailovich, M.H. Enhancing the Mechanical Properties of Biodegradable Polymer Blends Using Tubular Nanoparticle Stitching of the Interfaces. ACS Appl. Mater. Interfaces 2016, 8, 17565–17573. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT Bionanocomposites with Antimicrobial Natural Rosin for Green Packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, B.; Zhang, J. Properties of Poly(lactic acid)/Poly(butylene adipate-co-terephthalate)/Nanoparticle Ternary Composites. Ind. Eng. Chem. Res. 2009, 48, 7594–7602. [Google Scholar] [CrossRef]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Rahail Parvaiz, M. Effect of glycidyl methacrylate (GMA) on the thermal, me-chanical and morphological property of biodegradable PLA/PBAT blend and its nanocomposites. Bioresour. Technol. 2010, 101, 8406–8415. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S.K. Study of Thermo-Mechanical and Morphological Behaviour of Biodegradable PLA/PBAT/Layered Silicate Blend Nanocomposites. J. Polym. Environ. 2014, 22, 398–408. [Google Scholar] [CrossRef]
- Mirzadeh, A.; Ghasemi, H.; Mahrous, F.; Kamal, M.R. Reactive extrusion effects on rheological and mechanical properties of poly(lactic acid)/poly[(butylene succinate)-co-adipate]/epoxy chain extender blends and clay nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42664. [Google Scholar] [CrossRef]
- Perrone de, L.; Freitas, A.L.; Tonini Filho, L.R.; Schmid Calvao, P.; Catelli de Souza, A.M. Effect of montmorillonite and chain extender on rheological, morphological and biodegradation behavior of PLA/PBAT blends. Polym. Test. 2017, 62, 189–195. [Google Scholar] [CrossRef]
- Ko, S.W.; Hong, M.K.; Park, B.J.; Gupta, R.K.; Choi, H.J.; Bhattacharya, S.N. Morphological and rheological characteri-zation of multi-walled carbon nanotube/PLA/PBAT blend nanocomposites. Polym. Bull. 2009, 63, 125–134. [Google Scholar] [CrossRef]
- Urquijo, J.; Aranburu, N.; Dagréou, S.; Guerrica-Echevarría, G.; Eguiazábal, J. CNT-induced morphology and its effect on properties in PLA/PBAT-based nanocomposites. Eur. Polym. J. 2017, 93, 545–555. [Google Scholar] [CrossRef]
- Zhou, S.; Hrymak, A.N.; Kamal, M.R. Properties of microinjection-molded multi-walled carbon nanotubes-filled poly(lactic acid)/poly[(butylene succinate)-co-adipate] blend nanocomposites. J. Mater. Sci. 2018, 53, 9013–9025. [Google Scholar] [CrossRef]
- Dil, E.J.; Favis, B.D. Localization of micro- and nano-silica particles in heterophase poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. Polymer 2015, 76, 295–306. [Google Scholar] [CrossRef]
- Dil, E.J.; Virgilio, N.; Favis, B.D. The effect of the interfacial assembly of nano-silica in poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends on morphology, rheology and mechanical properties. Eur. Polym. J. 2016, 85, 635–646. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ray, S.S. Influence of nanoparticles and their selective localization on the structure and properties of polylactide-based blend nanocomposites. Compos. Part B 2021, 215, 108845. [Google Scholar] [CrossRef]
- Prudnikova, S.V.; Vinogradova, O.N.; Trusova, M.Y. Specific character of bacterial biodegradation of polyhydroxyalkanoates with different chemical structure in soil. Dokl. Biochem. Biophys. 2017, 473, 94–97. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Sevim, K.; Pan, J. A model for hydrolytic degradation and erosion of biodegradable polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shockley, M.F.; Muliana, A.H. Modeling temporal and spatial changes during hydrolytic degradation and erosion in bi-odegradable polymers. Polym. Degrad. Stab. 2020, 180, 109298. [Google Scholar] [CrossRef]
- Johnson, A.N.; Barlow, D.E.; Kelly, A.L.; Varaljay, V.A.; Crookes-Goodson, W.J.; Biffinger, J.C. Current progress towards understanding the biodegradation of synthetic condensation polymers with active hydrolases. Polym. Int. 2021, 70, 977–983. [Google Scholar] [CrossRef]
- Lu, Z.; Reif, R.; Gan, J. Isomer-specific biodegradation of nonylphenol in an activated sludge bioreactor and structure-biodegradability relationship. Water Res. 2015, 68, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Genovese, L. New eco-friendly random copolyesters based on poly(propylene cyclohexanedicarboxylate): Structure-properties relationships. Express Polym. Lett. 2015, 9, 972–983. [Google Scholar] [CrossRef]
- Beran, E.; Hull, S.; Steininger, M. The Relationship Between the Chemical Structure of Poly(alkylene glycol)s and Their Aerobic Biodegradability in an Aqueous Environment. J. Polym. Environ. 2013, 21, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Corti, A.; Cinelli, P.; D’Antone, S.; Kenawy, E.-R.-; Solaro, R. Biodegradation of poly(vinyl alcohol) in soil environment: Influence of natural organic fillers and structural parameters. Macromol. Chem. Phys. 2002, 203, 1526–1531. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Chiellini, F.; Imam, S.H. Environmentally Degradable Bio-Based Polymeric Blends and Composites. Macromol. Biosci. 2004, 4, 218–231. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Husárová, L.; Pekarova, S.; Stloukal, P.; Kucharzcyk, P.; Verney, V.; Commereuc, S.; Ramone, A.; Koutny, M. Identification of important abiotic & biotic factors in the biodegradation of PLA. Int. J. Biol. Macromol. 2014, 71, 155–162. [Google Scholar] [CrossRef]
- Mueller, R.-J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process. Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Jarerat, A. Biodegradation of poly(l-lactide). Biotechnol. Lett. 2004, 26, 771–777. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. New polylactide-layered silicate nanocomposites. 2. Concurrent improvements of material properties, biodegradability and melt rheology. Polymer 2003, 44, 857–866. [Google Scholar] [CrossRef]
- Stloukal, P.; Pekařová, S.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Chitu, L.; Bodner, S.C.; Maier, G.; Slouf, M.; et al. Kinetics and mechanism of the biodegradation of PLA/clay nanocomposites during thermophilic phase of composting process. Waste Manag. 2015, 42, 31–40. [Google Scholar] [CrossRef]
- Fukushima, K.; Gimenez, E.; Cabedo, L.; Lagaron, J.M.; Feijoo, J.L. Biotic degradation of poly(DL-lactide) based na-naocomposits. Polym. Degrad. Stab. 2012, 97, 1278–1284. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Cividanes, L.; Gouveia, R.; Lona, L. An overview on properties and applications of poly(butylene adipate-co-terephthalate)-PBAT based composites. Polym. Eng. Sci. 2019, 59, E7–E15. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Palsikowski, P.A.; Kuchnier, C.N.; Pinheiro, I.F.; Morales, A.R. Biodegradation in Soil of PLA/PBAT Blends Compatibilized with Chain Extender. J. Polym. Environ. 2017, 26, 330–341. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(lactic acid) (PLA), and Their Blend in Freshwater with Sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef] [PubMed]
- Girdthep, S.; Worajittiphon, P.; Leejarkpai, T.; Molloy, R.; Punyodom, W. Effect of silver-loaded kaolinite on real ageing, hydrolytic degradation, and biodegradation of composite blown films based on poly(lactic acid) and poly(butylene adipate-co-terephthalate). Eur. Polym. J. 2016, 82, 244–259. [Google Scholar] [CrossRef]
- Ratto, J.A.; Stenhouse, P.J.; Auerbach, M.; Mitchell, J.; Farrell, R. Processing, performance and biodegradability of a thermoplastic aliphatic polyester/starch system. Polymer 1999, 40, 6777–6788. [Google Scholar] [CrossRef]
- Malwela, T.; Sinha Ray, S. Enzymatic degradation behavior of nanoclay reinforcedbiodegradable PLA/PBSA blend composites. Int. J. Biol. Macromol. 2015, 77, 131–142. [Google Scholar] [CrossRef]
- Pivsa-Art, S.; Kord-Sa-Ard, J.; Sijong, W.; Pivsa-Art, W.; Ohara, H.; Yamane, H. Biodegradation in Landfilled of Biode-gradable Micro-braided Yarn. Energy Procedia 2016, 89, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Manias, E.; Polizos, G.; Huh, J.-Y.; Ophir, A.; Songtipya, P.; Jimenez-Gasco, M.D.M. Tailored Polyethylene Nanocomposite Sealants: Broad-Range Peelable Heat-Seals Through Designed Filler/Polymer Interfaces. J. Adhes. Sci. Technol. 2009, 23, 709–737. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, M.K.; Bardet, S.; Nilsen, J.; Fredriksen, S.B. Evaluation and suitability of biomaterials for modified atmosphere packaging of fresh salmon fillets. Packag. Technol. Sci. 2011, 24, 237–248. [Google Scholar] [CrossRef]
- Liewchirakorn, P.; Aht-Ong, D.; Chinsirikul, W. Practical Approach in Developing Desirable Peel-Seal and Clear Lidding Films Based on Poly(Lactic Acid) and Poly(Butylene Adipate-Co-Terephthalate) Blends. Packag. Technol. Sci. 2017, 31, 296–309. [Google Scholar] [CrossRef]
- Busch, D.; Klein, D.; Schmitz, B. Peelable Sealable PLA Film. WO Patent 2007/113077, 11 October 2007. [Google Scholar]
- Narita, J.; Sawai, T.; Takeishi, I.; Itoh, S. Aliphatic Polyester Compositions, Film Made Thereof and Laminates Thereof. U.S. Patent No. 6,521,336 B2, 18 February 2003. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/ β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A review of active packaging in bakery products: Applications and future trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Srisa, A.; Harnkarnsujarit, N. Antifungal films from trans-cinnamaldehyde incorporated poly (lactic acid) and poly (butylene adipate-co-terephthalate) for bread packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef] [PubMed]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly (lactic acid)/poly (butylene-succinate-co-adipate)(PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Ribes, S.; Ruiz-Rico, M.; Pérez-Esteve, É.; Fuentes, A.; Talens, P.; Martínez-Máñez, R.; Barat, J.M. Eugenol and thymol immobilised on mesoporous silica-based material as an innovative antifungal system: Application in strawberry jam. Food Control. 2017, 81, 181–188. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of PLA-PBSA based biodegradable active film and its application to salmon slices. Food Packag. Shelf Life 2019, 22, 100393. [Google Scholar] [CrossRef]
- Lyu, Y.; Chen, Y.; Lin, Z.; Zhang, J.; Shi, X. Manipulating phase structure of biodegradable PLA/PBAT system: Effects on dynamic rheological responses and 3D printing. Compos. Sci. Technol. 2020, 200, 108399. [Google Scholar] [CrossRef]
- Cardoso, P.H.M.; Coutinho, R.R.T.P.; Drummond, F.R.; Da Conceição, M.D.N.; Thiré, R.M.D.S.M. Evaluation of Printing Parameters on Porosity and Mechanical Properties of 3D Printed PLA/PBAT Blend Parts. Macromol. Symp. 2020, 394, 2000157. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Cheng, J.; Anstey, A.; Mohanty, A.K.; Misra, M. Development of toughened blends of poly (lactic acid) and poly (butylene adipate-co-terephthalate) for 3d printing applications: Compatibilization methods and material performance evaluation. ACS Sustain. Chem. Eng. 2020, 8, 6576–6589. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhao, H.; Wen, X.; Lin, L.; Schlarb, A.K.; Shi, X. Optimization of 3D printing parameters for high-performance biodegradable materials. J. Appl. Polym. Sci. 2021, 138, 50782. [Google Scholar] [CrossRef]
- Singamneni, S.; Smith, D.; LeGuen, M.-J.; Truong, D. Extrusion 3D Printing of Polybutyrate-Adipate-Terephthalate-Polymer Composites in the Pellet Form. Polymers 2018, 10, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasong, W.; Muanchan, P.; Ishigami, A.; Thumsorn, S.; Kurose, T.; Ito, H. Properties of 3D Printable Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends and Nano Talc Composites. J. Nanomater. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Prasong, W.; Ishigami, A.; Thumsorn, S.; Kurose, T.; Ito, H. Improvement of Interlayer Adhesion and Heat Resistance of Biodegradable Ternary Blend Composite 3D Printing. Polymers 2021, 13, 740. [Google Scholar] [CrossRef]
- Wan, M.; Liu, S.; Huang, D.; Qu, Y.; Hu, Y.; Su, Q.; Zheng, W.; Dong, X.; Zhang, H.; Wei, Y.; et al. Biocompatible heterogeneous bone incorporated with polymeric biocomposites for human bone repair by 3D printing technology. J. Appl. Polym. Sci. 2021, 138, 50114. [Google Scholar] [CrossRef]
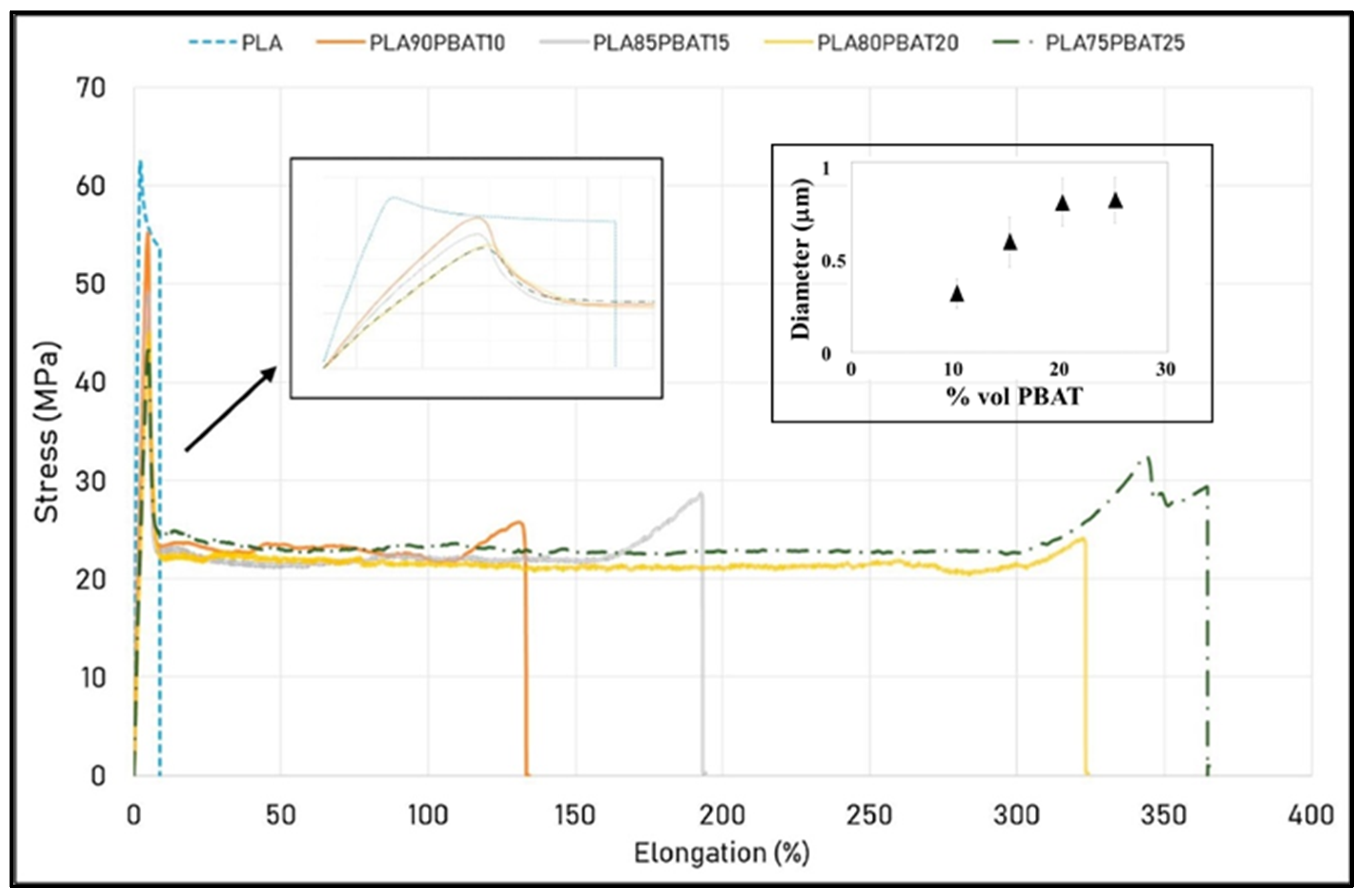
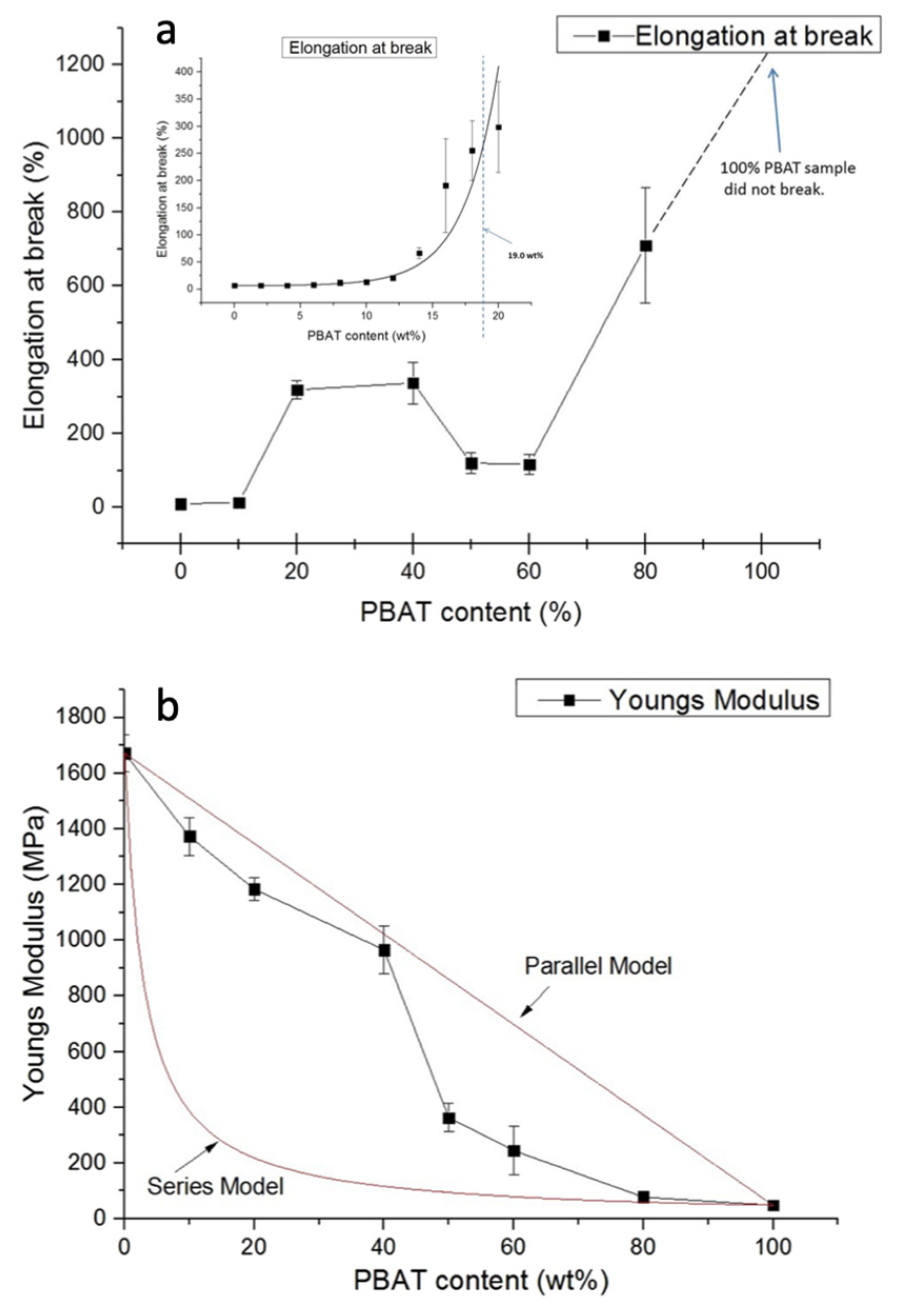
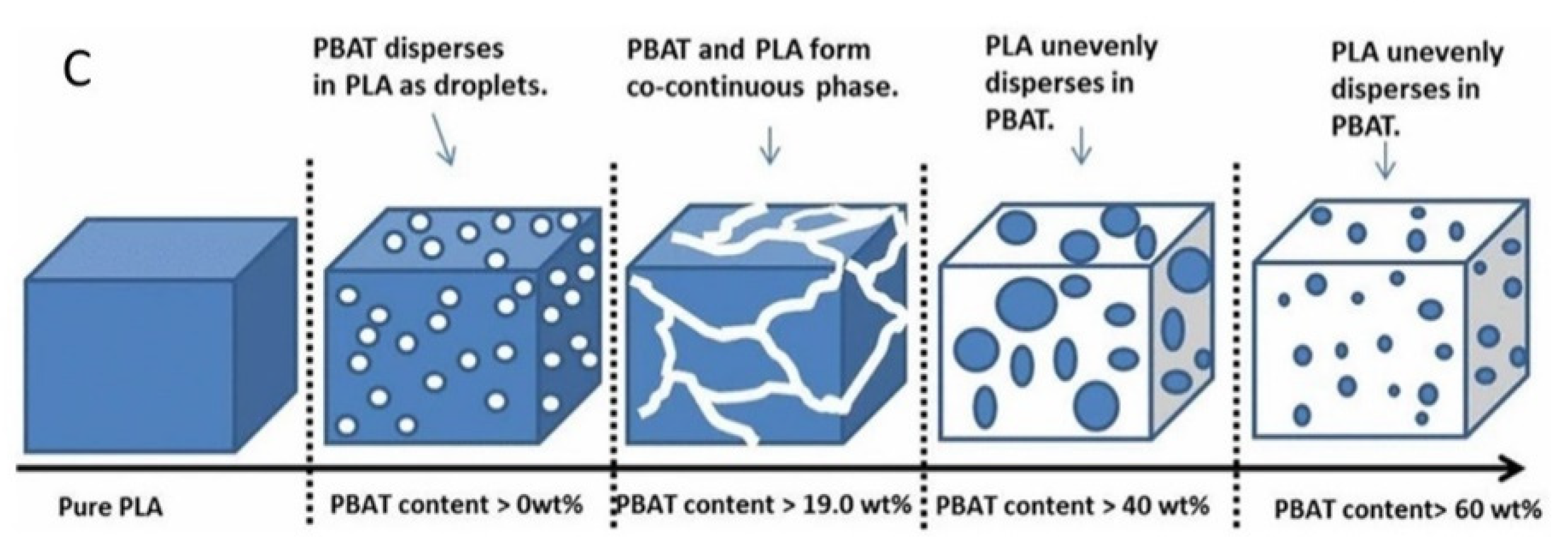
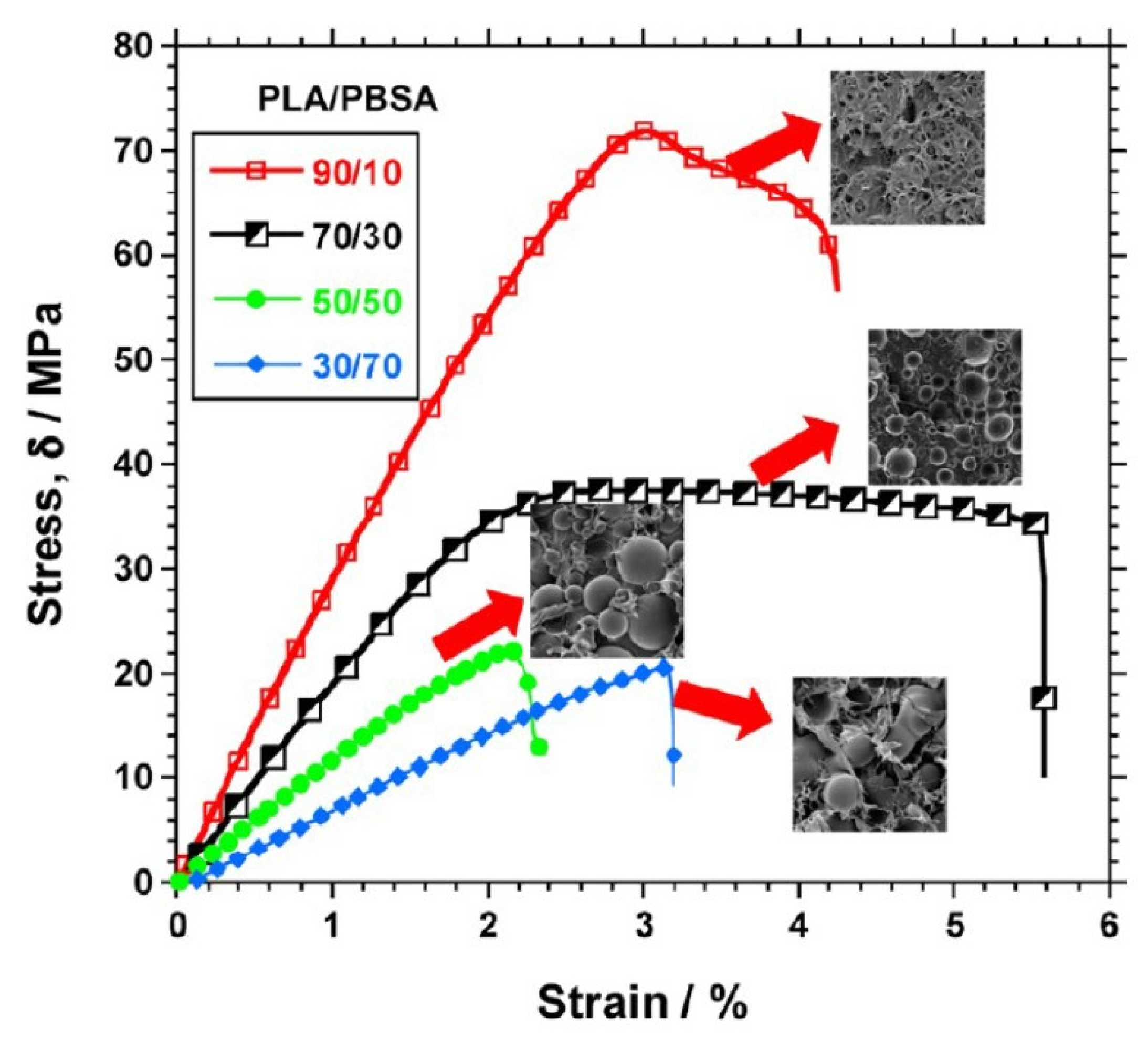
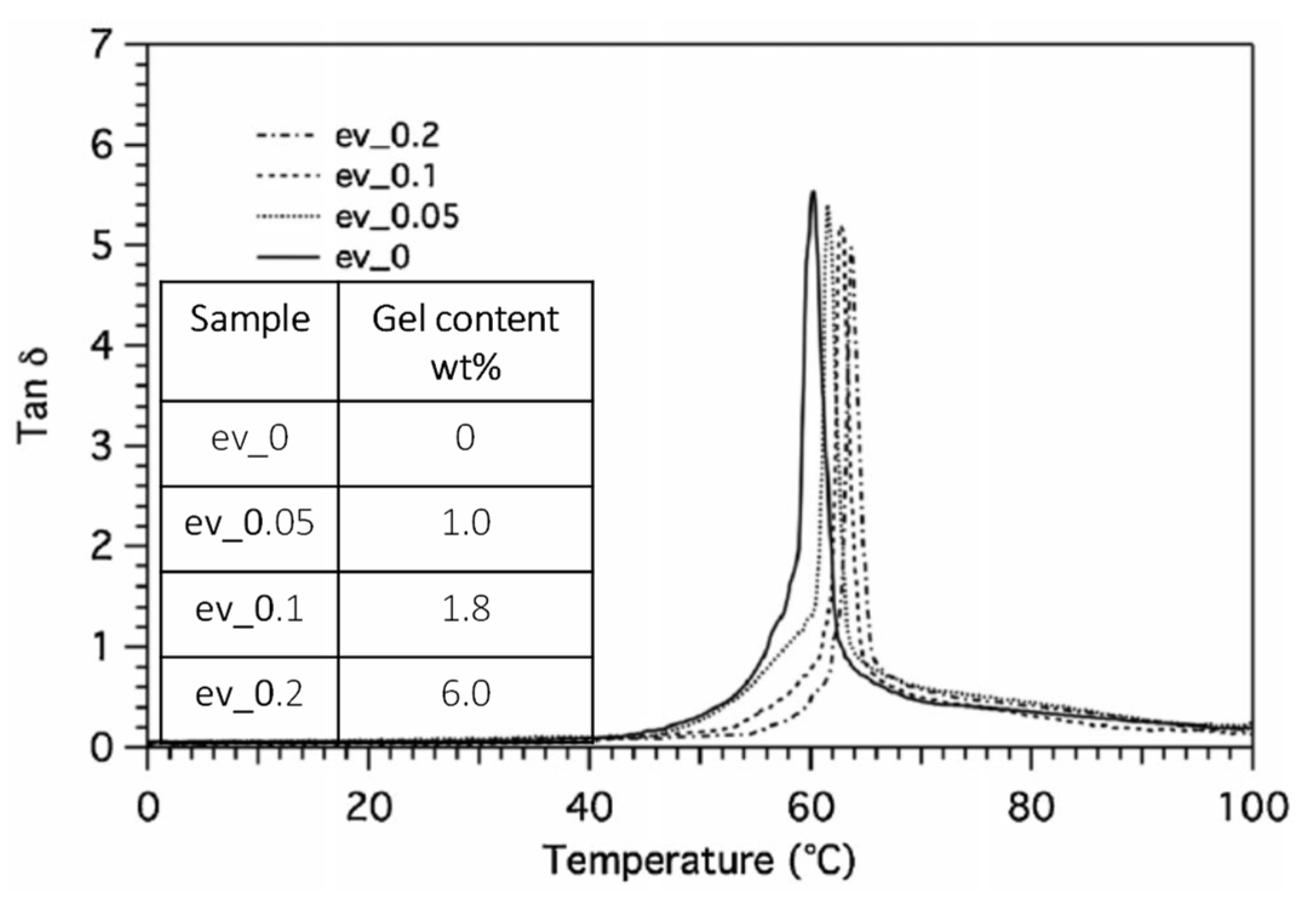

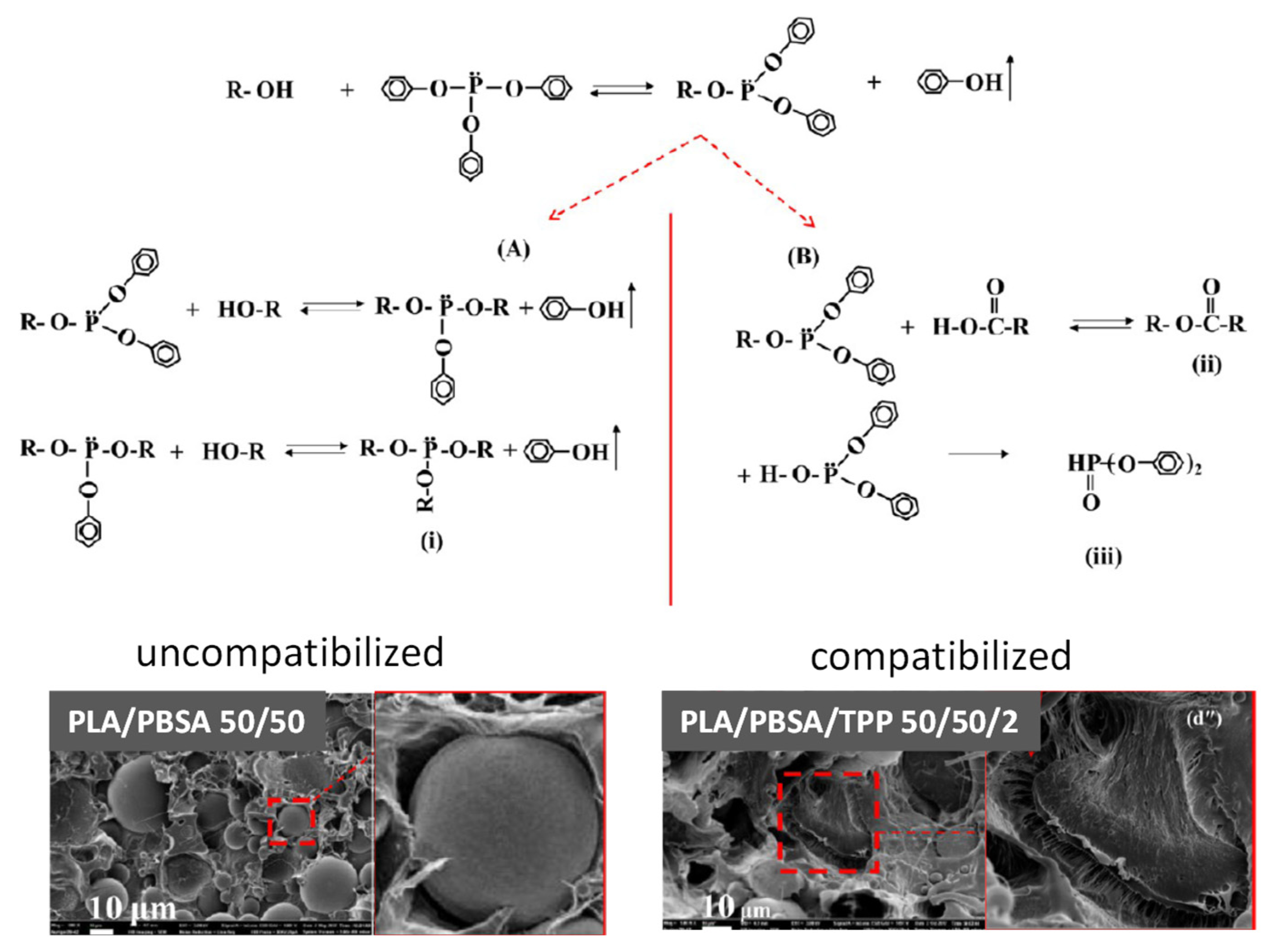


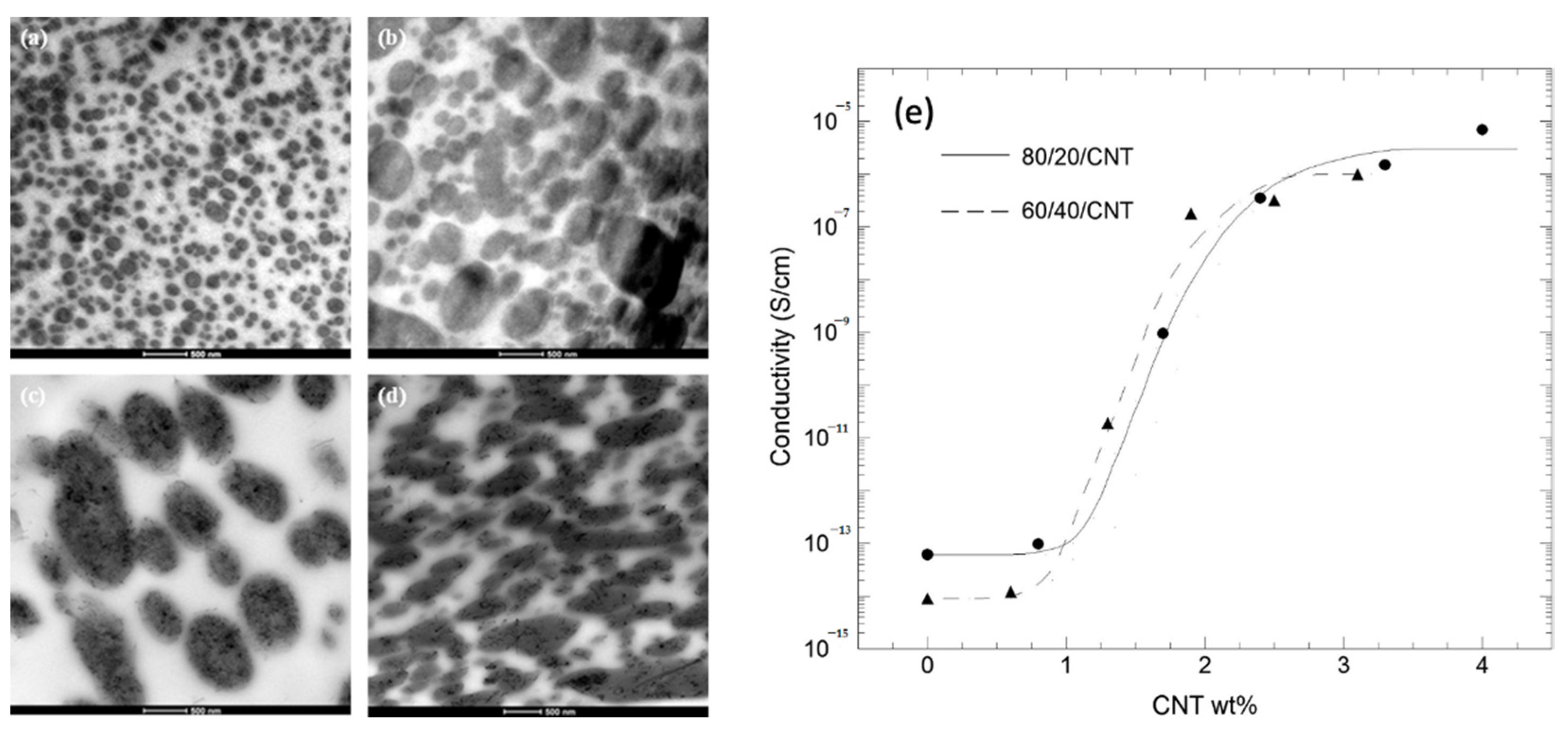

| Polymer | Tg (°C) | Tm (°C) | TS (MPa) | ε (%) | E (MPa) |
|---|---|---|---|---|---|
| PLA | 55–60 [10] | 150–180 [10] | 15–60 [6] | 3–10 [6] | 3500–4000 [6] |
| PBAT | −60–−10 [11] | 50–30 1 70–190 2 [11] | 10–20 [12] | 600–100 1 10–100 2 [12] | 40–100 1 400–100 2 [12] |
| PBSA | −60–−35 [13] | 20–50 3 50–110 4 [13] | 12–10 3 20–10 4 [14] | 380–330 3 430–330 4 [14] | 300 1,5 |
| Blends | Compatibilizer | Trade Name | Process 1 | Compositions | Ref. |
|---|---|---|---|---|---|
| PLA/PBAT | T-GMA | Lotader ARKEMA | TS | 90/10, 80/20, 70/30, 60/40 T-GMA: 1–10 wt% | [100] |
| PLA/PBAT | EMA-GMA | Nd ARKEMA | MM | 90/10, 82/10/0, 75/10/8, 75/10/15, 70/10/20 EMA-GMA: 8–20 wt% | [101] |
| PLA/PBSA | CESA | CESA Extend OMAN (Clariant) | MM | 70/30 CESA: 2 wt% | [102] |
| PLA/PBAT | EJ-400 | EJ-400 (Jsi Co) | TS IM | 67/33 EJ-400: 10 wt% | [103] |
| PLA/PBAT | BETT | synthesized | MM | 50,750 BETT: 2 et5 | [104] |
| PLA/PBAT PLA/PBSA | Joncryl | Joncryl ADR-4368 (BASF) | TS MM | 80/20 ADR-4368: 0.25–1 wt% 60/40, 40/60 ADR-4368: 0.3–0.6 wt% 90/10, 80/20, 70/30, 60/40 ADR-4368: 0.3–1.0 wt% 95/5, 90/10, 80/20 ADR-4368: 3 wt% | [105] [106] [107] [108] [109] |
| PLA/PBAT | Joncryl | Joncryl ADR-4370S (BASF) | MM | 80/20 ADR-4370S: 1 wt% 90/10, 80/20, 70/30, 60/40 ADR-4370S: 0.75 wt% | [110] [111] |
| PLA/PBAT | Joncryl | Joncryl ADR-4370F (BASF) | TS MM | 50/50 ADR-4370F: 0.05–0.2 wt% 80/20, 60740, 40/60, 30/80 ADR-4370F: 0.1 wt% | [112] [113] |
| PLA/PBAT | Joncryl | Joncryl ADR-4368, 4380, 4370 (BASF) | MM | 80/20 ADR: 0.1–0.15, 0.2, 0.3, 0.5 wt% | [114] |
| PLA/PBAT | ECP | ECP Cardolite® NC-514 (Cardolite USA) | MM | 80/20 NC-514: 3 wt% | [115] |
| Additive | Content (wt%) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| none | - | 1700–2100 | 29–33 | 52–60 |
| ECP | 1, 3, 5 | 2100–1600 | 31–29 | 150–190 |
| ECSO | 1, 7.5 | 2400–1900 | 60–40 | 65 |
| MCSO | 1, 7.5 | 3000–2400 | 60–40 | 75–125 |
| Joncryl | 1 | 2200 | 26–37 | 75 |
| Blends | Nanoclay 1 | Modifier | Chain Extender | Ref. |
|---|---|---|---|---|
| PLA/PBSA 75/25, 50/50, 70/30, 25/75 | MMT Cloisite 30B (C30B) | Methyl tallow bis(2-hydroxiethyl) quaternary ammonium salt | - | [120,121,122] |
| PLA/PBSA 90/10, 80/20, 70/30 | MMT Cloisite 30A (C30A) | Dimethyl dehydrogenated tallow quaternary ammonium salt | - | [121,123,126] |
| PLA/PBSA 80/20 | LDH SaLDH | Stearic acid (surface coated) | - | [124] |
| PLA/PBSA 80/20 | MMT Unmodified | Na+ | - | [126] |
| PLA/PBAT 80/20, 70/30, 60/40, 50/50 | MMT Cloisite 30B (C30B) | Methyl tallow bis(2-hydroxiethyl)quaternary ammonium salt | - | [125,126,127] |
| PLA/PBAT 80/20 | MMT Unmodified | Na+ | - | [126] |
| PLA/PBAT 70/30 | MMT (MMT-RDP) | resorcinol diphenyl phosphate (surface coated) | - | [128] |
| PLA/PBAT 75/25, 50/50, 25/75 | MMT (organomodified) | Gum rosin and stearic acid (adsorbed starting from organomodified clay) | - | [129] |
| PLA/PBAT 90/10 | MMT (organomodified) | Methyl tallow bis(2-hydroxiethyl)quaternary ammonium salt | PLA g-MAH | [130] |
| PLA/PBAT 75/25 | MMT Cloisite 30B (C30B) | Methyl tallow bis(2-hydroxiethyl)quaternary ammonium salt | GMA | [131] |
| PLA/PBAT | MMT Cloisite 30B (C30B) | Methyl tallow bis(2-hydroxiethyl)quaternary ammonium salt | MAH + peroxide | [132] |
| PLA/PBSA 90/10, 80/20, | MMT (MEE) | Dipolyoxy ethylene alkyl methyl ammonium salt | TPP | [81] |
| PLA/PBSA 90/10, 80/20, 70/30 | MMT Cloisite 30B (C30B) | Methyl tallow bis(2-hydroxiethyl)quaternary ammonium salt | CESA | [133] |
| PLA/PBAT 45/55 | MMT Cloisite 30A (C30a) | Dimethyl dehydrogenated tallow quaternary ammonium salt | Joncryl ADR-4368 | [134] |
| Sample and Mixing Strategy | Dv (m) |
|---|---|
| PLAT/PBAT 75/25 | 1.3 |
| PLA/PBAT/C30B 75/25/1 method S1 | 0.75 |
| PLA/PBAT/C30B 75/25/1 method S2 | 0.85 |
| PLA/PBAT/C30B 75/25/1 method S3 | 0.70 |
| PLA/PBAT/C30B 75/25/5 method S1 | 0.65 |
| PLA/PBAT/C30B 75/25/5 method S2 | 0.75 |
| PLA/PBAT/C30B 75/25/5 method S3 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coiai, S.; Di Lorenzo, M.L.; Cinelli, P.; Righetti, M.C.; Passaglia, E. Binary Green Blends of Poly(lactic acid) with Poly(butylene adipate-co-butylene terephthalate) and Poly(butylene succinate-co-butylene adipate) and Their Nanocomposites. Polymers 2021, 13, 2489. https://doi.org/10.3390/polym13152489
Coiai S, Di Lorenzo ML, Cinelli P, Righetti MC, Passaglia E. Binary Green Blends of Poly(lactic acid) with Poly(butylene adipate-co-butylene terephthalate) and Poly(butylene succinate-co-butylene adipate) and Their Nanocomposites. Polymers. 2021; 13(15):2489. https://doi.org/10.3390/polym13152489
Chicago/Turabian StyleCoiai, Serena, Maria Laura Di Lorenzo, Patrizia Cinelli, Maria Cristina Righetti, and Elisa Passaglia. 2021. "Binary Green Blends of Poly(lactic acid) with Poly(butylene adipate-co-butylene terephthalate) and Poly(butylene succinate-co-butylene adipate) and Their Nanocomposites" Polymers 13, no. 15: 2489. https://doi.org/10.3390/polym13152489








