Starch–Mucilage Composite Films: An Inclusive on Physicochemical and Biological Perspective
Abstract
:1. Introduction
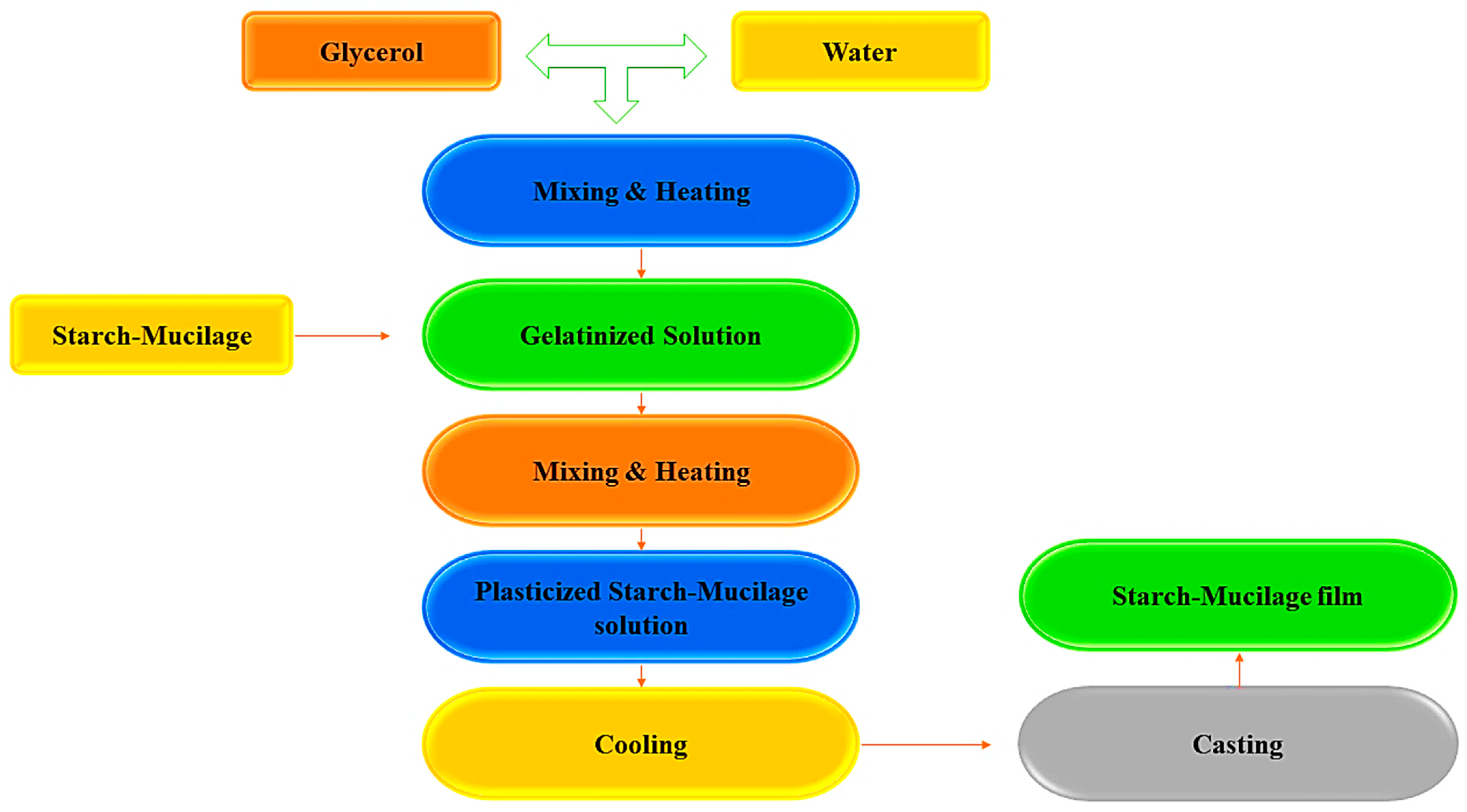
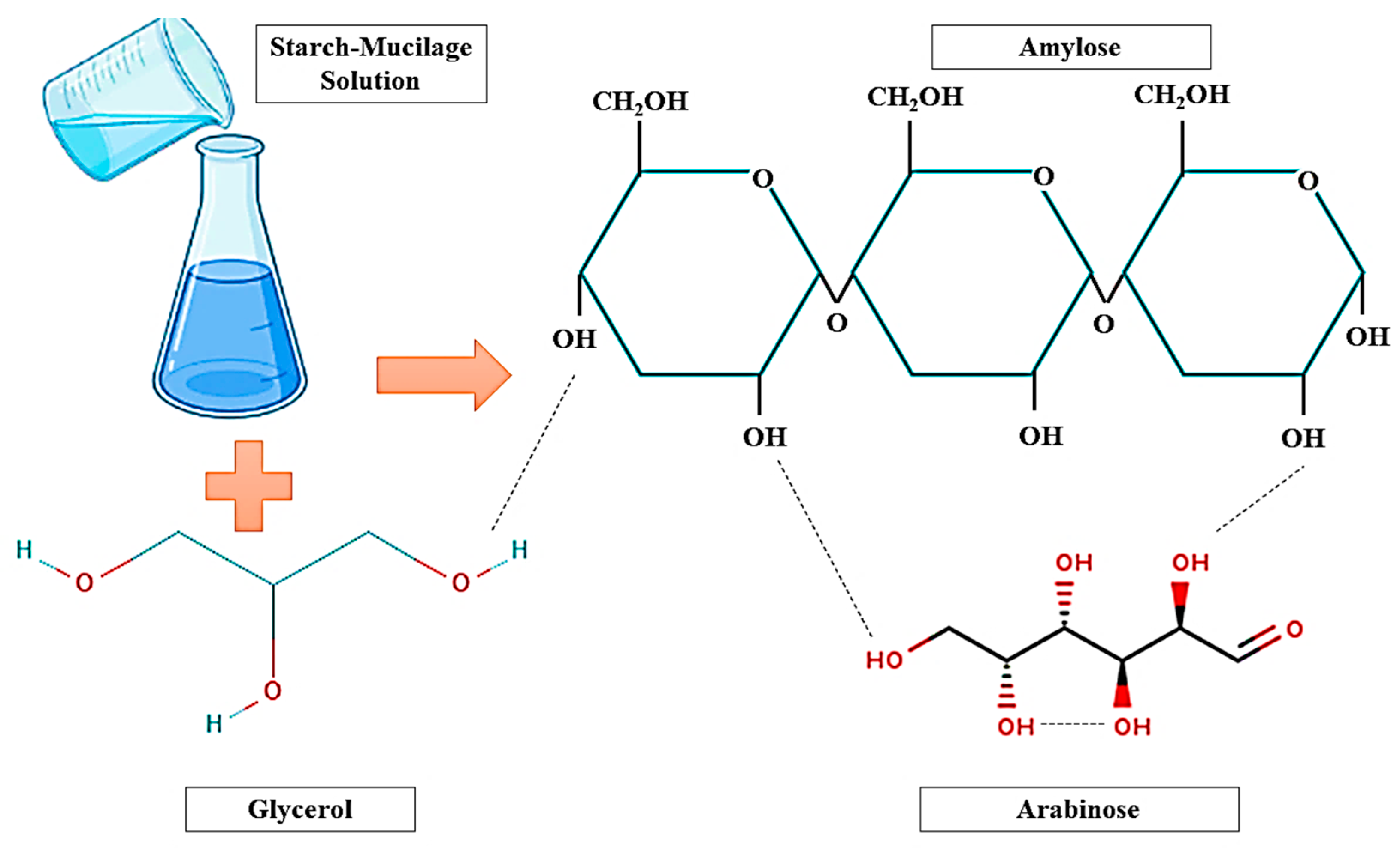
2. Synthesis of Starch–Mucilage Composite Films
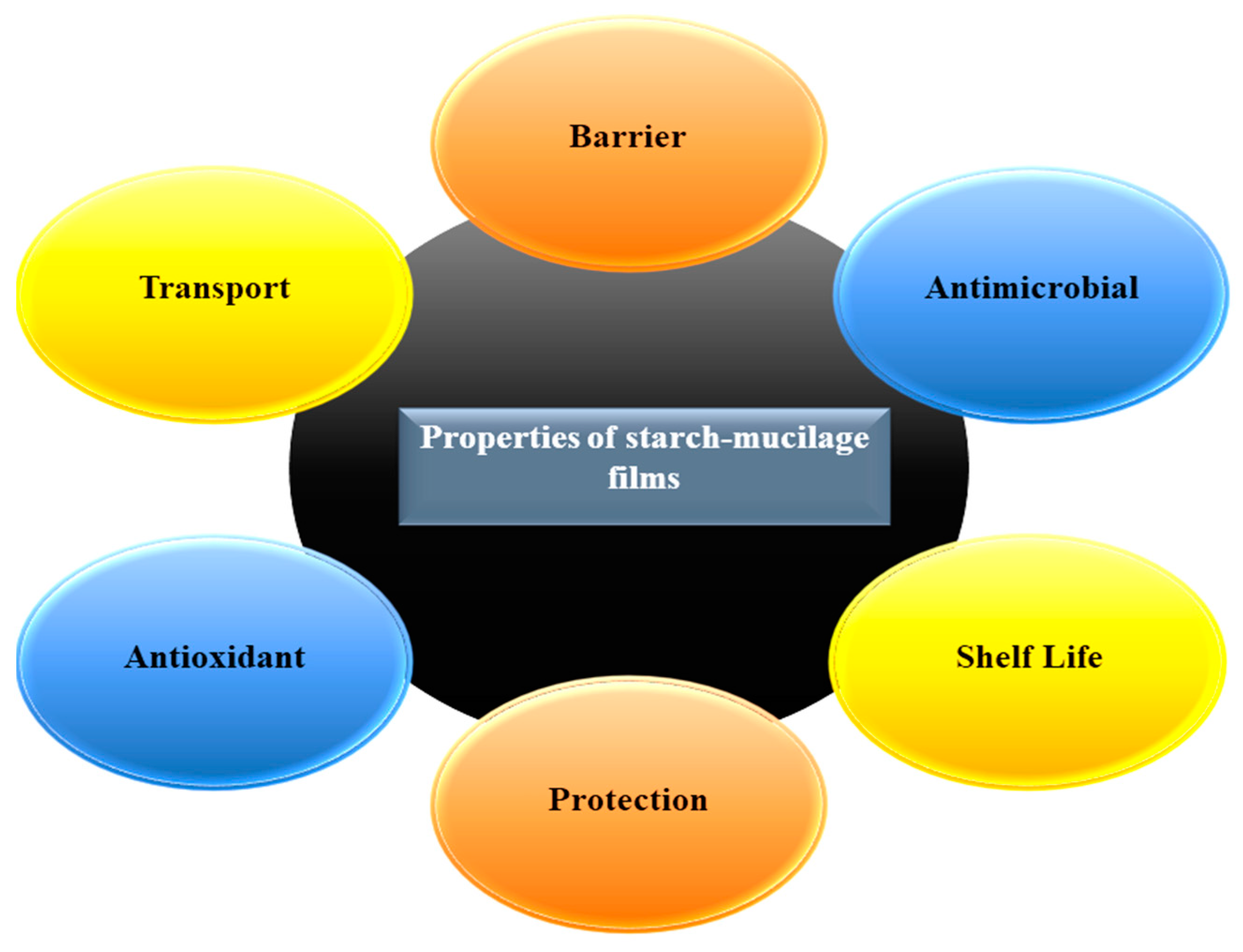
3. Physicochemical Properties and Characterization of the Starch–Mucilage Film
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. Scanning Electron Microscopy (SEM)
3.3. Thermal Stability of Films
4. Mechanical and Physical Properties of Starch–Mucilage Films
4.1. Tensile Strength
4.2. Water Solubility and Water Vapor Transmission Rate (WVTR)
4.3. Transparency and Thickness of the Film
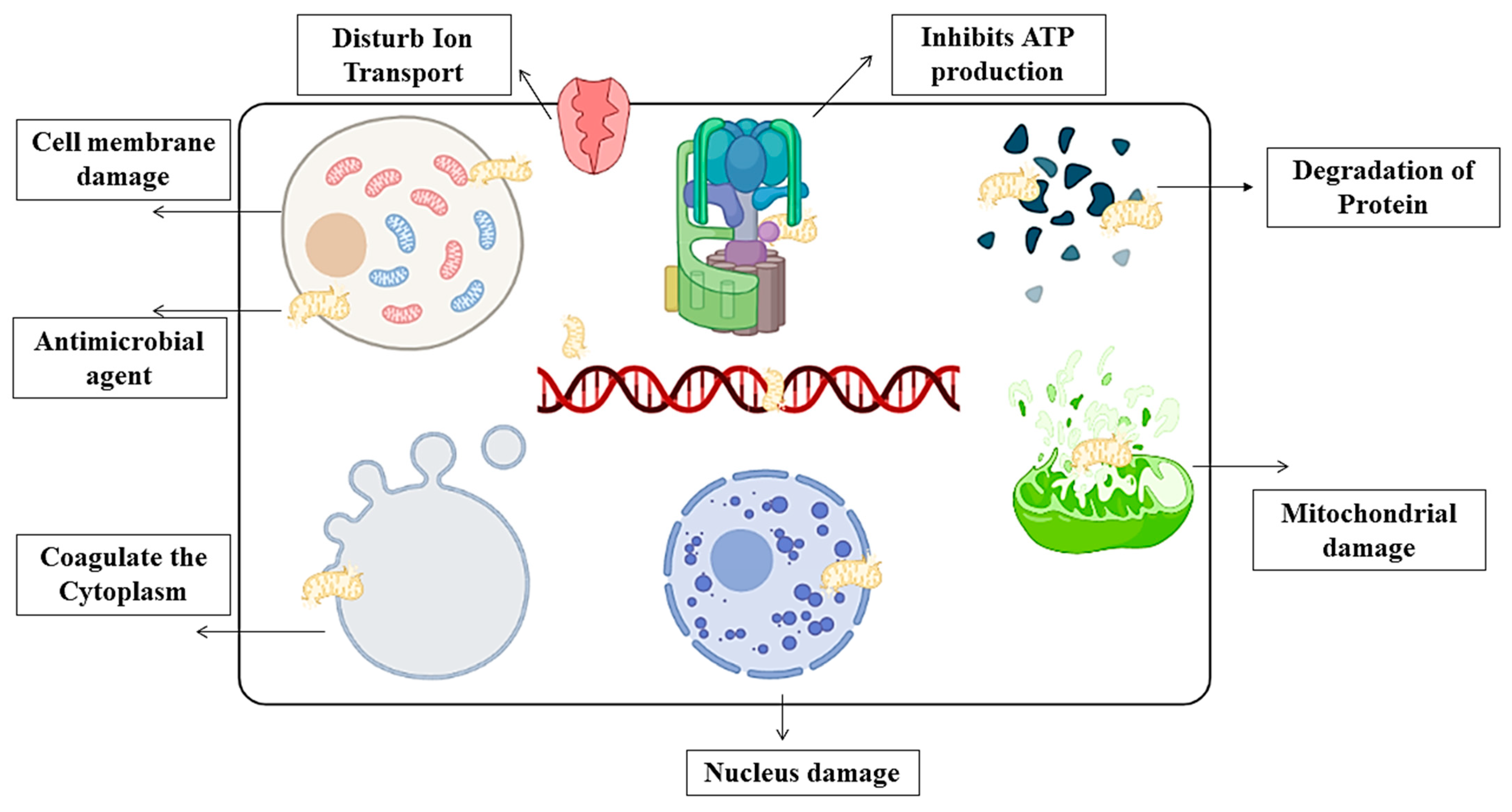
4.4. Antimicrobial Activity and Bio-Degradation of Film
5. Techno-Economic Challenges of Starch–Mucilage Films
6. Future Research Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Mujtaba, M.; Koç, B.; Salaberria, A.M.; Ilk, S.; Duman, D.C.; Akyüz, L.; Cakmak, Y.S.; Kaya, M.; Khawar, K.M.; Labidi, J.; et al. Production of novel chia-mucilage nanocomposite films with starch nanocrystals; An inclusive biological and physicochemical perspective. Int. J. Biol. Macromol. 2019, 133, 663–673. [Google Scholar] [CrossRef]
- Chillo, S.; Flores, S.; Mastromatteo, M.; Conte, A.; Gerschenson, L.; Del Nobile, M. Influence of glycerol and chitosan on tapioca starch-based edible film properties. J. Food Eng. 2008, 88, 159–168. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, C.; Wang, X.; Xiong, L.; Sun, Q. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT Food Sci. Technol. 2016, 69, 251–257. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Grzyb, J.; Fiedorowicz, M. Formation and properties of hyaluronan/nano Ag and hyaluronan-lecithin/nano Ag films. Carbohydr. Polym. 2016, 151, 452–457. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Rukmanikrishnan, B.; Ramalingam, S.; Rajasekharan, S.K.; Lee, J.; Lee, J. Binary and ternary sustainable composites of gellan gum, hydroxyethyl cellulose and lignin for food packaging applications: Biocompatibility, antioxidant activity, UV and water barrier properties. Int. J. Biol. Macromol. 2020, 153, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Arce, V.B.; Garcia, M.A. Nanocomposite starch-based films containing silver nanoparticles synthesized with lemon juice as reducing and stabilizing agent. Carbohydr. Polym. 2021, 252, 117208. [Google Scholar] [CrossRef]
- Valencia, G.A.; Zare, E.N.; Makvandi, P.; Gutiérrez, T.J. Self-Assembled Carbohydrate Polymers for Food Applications: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2009–2024. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Effect of glycerol on the morphology of nanocomposites made from thermoplastic starch and starch nanocrystals. Carbohydr. Polym. 2011, 84, 203–210. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Zhang, Q.-X.; Yu, Z.-Z.; Xie, X.-L.; Naito, K.; Kagawa, Y. Preparation and crystalline morphology of biodegradable starch/clay nanocomposites. Polymer 2007, 48, 7193–7200. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.; Chawla, P.; Walasek-Janusz, M. A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication. Polymer 2021, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.; Galvao, A.; Filho, C.J.A.D.S.; Mendes, F.; Oliveira, M.; Barbosa, F.; Filho, M.S.; Bastos, M. Okra mucilage and corn starch bio-based film to be applied in food. Polym. Test. 2018, 71, 352–361. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Galicia-García, T.; Martínez-Bustos, F.; Grosso, R.F.; González-Núñez, R. Effects of Nopal Mucilage (Opuntia ficus-indica) as Plasticizer in the Fabrication of Laminated and Tubular Films of Extruded Acetylated Starches. Int. J. Polym. Sci. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Tantiwatcharothai, S.; Prachayawarakorn, J. Property improvement of antibacterial wound dressing from basil seed (O. basilicum L.) mucilage-ZnO nanocomposite by borax crosslinking. Carbohydr. Polym. 2020, 227, 115360. [Google Scholar] [CrossRef]
- Jensen, A.; Lim, L.-T.; Barbut, S.; Marcone, M. Development and characterization of soy protein films incorporated with cellulose fibers using a hot surface casting technique. LWT Food Sci. Technol. 2015, 60, 162–170. [Google Scholar] [CrossRef]
- Kumari, M.; Mahajan, H.; Joshi, R.; Gupta, M. Development and structural characterization of edible films for improving fruit quality. Food Packag. Shelf Life 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Khanzadi, M.; Jafari, S.M.; Mirzaei, H.; Chegini, F.K.; Maghsoudlou, Y.; Dehnad, D. Physical and mechanical properties in biodegradable films of whey protein concentrate–pullulan by application of beeswax. Carbohydr. Polym. 2015, 118, 24–29. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymer 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; do AmaralSobral, P.J.; Menegalli, F.C. Effect of drying conditions and plasticizer type on some physical and mechanical properties of amaranth flour films. LWT Food Sci. Technol. 2013, 50, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Krystyjan, M.; Khachatryan, G.; Ciesielski, W.; Buksa, K.; Sikora, M. Preparation and characteristics of mechanical and functional properties of starch/Plantago psyllium seeds mucilage films. Starch-Stärke 2017, 69, 1700014. [Google Scholar] [CrossRef]
- Ayquipa-Cuellar, E.; Salcedo-Sucasaca, L.; Azamar-Barrios, J.A.; Chaquilla-Quilca, G. Assessment of Prickly Pear Peel Mucilage and Potato Husk Starch for Edible Films Production for Food Packaging Industries. Waste Biomass Valorization 2021, 12, 321–331. [Google Scholar] [CrossRef]
- Fitch-Vargas, P.R.; Aguilar-Palazuelos, E.; de Jesús Zazueta-Morales, J.; Vega-García, M.O.; Valdez-Morales, J.E.; Martínez-Bustos, F.; Jacobo-Valenzuela, N. Physicochemical and Microstructural Characterization of Corn Starch Edible Films Obtained by a Combination of Extrusion Technology and Casting Technique. J. Food Sci. 2016, 81, E2224–E2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yu, Z.; Alsammarraie, F.K.; Kong, F.; Lin, M.; Mustapha, A. Properties and antimicrobial activity of polyvinyl alcohol-modified bacterial nanocellulose packaging films incorporated with silver nanoparticles. Food Hydrocoll. 2020, 100, 105411. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutiérrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Clerici, M.T.P.S.; Kil Chang, Y.K.; Steel, C.J. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Wang, H.; Xia, L.; Chen, M.; Li, L.; Cheng, J.; Li, X.; Jiang, S. Colorimetric film based on polyvinyl alcohol/okra mucilage polysaccharide incorporated with rose anthocyanins for shrimp freshness monitoring. Carbohydr. Polym. 2020, 229, 115402. [Google Scholar] [CrossRef]
- Seetharaman, S.; Balya, H.; Kuppusamy, G. Preparation and Evaluation of Cefixime Nanoparticles Prepared Using Fenugreek Seed Mucilage and Chitosan as Natural Polymers. Int. J. Pharm. Clin. Res. 2016, 8. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. Functional properties and applications of basil seed gum: An overview. Food Hydrocoll. 2017, 73, 313–325. [Google Scholar] [CrossRef]
- Cao, L.; Si, J.Y.; Liu, Y.; Sun, H.; Jin, W.; Li, Z.; Zhao, X.H.; Le Pan, R. Essential oil composition, antimicrobial and antioxidant properties of Mosla chinensis Maxim. Food Chem. 2009, 115, 801–805. [Google Scholar] [CrossRef]
- Rivera-Corona, J.L.; Rodríguez-González, F.; Rendón-Villalobos, R.; García-Hernández, E.; Solorza-Feria, J. Thermal, structural and rheological properties of sorghum starch with cactus mucilage addition. LWT Food Sci. Technol. 2014, 59, 806–812. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem. 2016, 212, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Huang, Q.; Ghaffar, A.; Abid, M.A.; Zafar, M.S.; Khurshid, Z.; Latif, M. A Smart Drug Delivery System Based on Biodegradable Chitosan/Poly(allylamine hydrochloride) Blend Films. Pharmaceutics 2020, 12, 131. [Google Scholar] [CrossRef] [Green Version]
- Ruggero, F.; Carretti, E.; Gori, R.; Lotti, T.; Lubello, C. Monitoring of degradation of starch-based biopolymer film under different composting conditions, using TGA, FTIR and SEM analysis. Chemosphere 2020, 246, 125770. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, X.; Liu, L.; Chen, W.; Bai, J.; Ma, F.; Liu, X.; Kang, W. Preparation and characterization of edible films composed of Dioscorea opposita Thunb. mucilage and starch. Polym. Test. 2020, 90, 106708. [Google Scholar] [CrossRef]
- Askari, F.; Sadeghi, E.; Mohammadi, R.; Rouhi, M.; Taghizadeh, M.; Shirgardoun, M.H.; Kariminejad, M. The physicochemical and structural properties of psyllium gum/modified starch composite edible film. J. Food Process. Preserv. 2018, 42, e13715. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Gattia, D.M.; Roselli, G.; Persia, F.; De Angelis, U.; Santulli, C. Thermoplastic Starch (TPS) Films Added with Mucilage from Opuntia Ficus Indica: Mechanical, Microstructural and Thermal Characterization. Materials 2020, 13, 1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, F.R.S.; Bastos, M.S.R.; Mendes, L.G.; Silva, A.R.A.; Sousa, F.D.; Monteiro-Moreira, A.C.O.; Cheng, H.N.; Biswas, A.; Moreira, R.A. Preparation and evaluation of hemicellulose films and their blends. Food Hydrocoll. 2017, 70, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, T.J.; Tapia, M.S.; Pérez, E.; Famá, L. Edible films based on native and phosphated 80:20 waxy:normal corn starch. Starch-Stärke 2015, 67, 90–97. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, B.; Chen, J.; Zhao, B.; Wang, X.; Wang, L.; Zha, S.; Wang, Y.; Zhang, J.; Wang, Y. Effect of drying methods on physicochemical properties and antioxidant activities of wolfberry (Lycium barbarum) polysaccharide. Carbohydr. Polym. 2015, 127, 176–181. [Google Scholar] [CrossRef]
- Loo, C.P.; Sarbon, N.M. Chicken skin gelatin films with tapioca starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Chen, G. A comparative study on the starch-based biocomposite films reinforced by nanocellulose prepared from different non-wood fibers. Cellulose 2019, 26, 2425–2435. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Golding, J.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q. Amylose-lipid complex as a measure of variations in physical, mechanical and barrier attributes of rice starch- ι -carrageenan biodegradable edible film. Food Packag. Shelf Life 2017, 14, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Šešlija, S.; Nesic, A.; Ružić, J.; Krusic, M.K.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible blend films of pectin and poly(ethylene glycol): Preparation and physico-chemical evaluation. Food Hydrocoll. 2018, 77, 494–501. [Google Scholar] [CrossRef]
- Behrouzian, F.; Razavi, S.M.; Phillips, G.O. Cress seed (Lepidium sativum) mucilage, an overview. Bioact. Carbohydr. Diet. Fibre 2014, 3, 17–28. [Google Scholar] [CrossRef]
- Thessrimuang, N.; Prachayawarakorn, J. Development, modification and characterization of new biodegradable film from basil seed (Ocimum basilicum L.) mucilage. J. Sci. Food Agric. 2019, 99, 5508–5515. [Google Scholar] [CrossRef]
- dos Santos Caetano, K.; Almeida Lopes, N.; Haas Costa, T.M.; Brandelli, A.; Rodrigues, E.; Hickmann Flôres, S.; Cladera-Olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Lutfi, Z.; Hasnain, A. Mango kernel starch-gum composite films: Physical, mechanical and barrier properties. Int. J. Biol. Macromol. 2017, 98, 869–876. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Oliveira, L.M.; Cereda, M.P.; Alves, A.J.; Scamparini, A.R.P. Mechanical properties, hydrophilicity and water activity of starch-gum films: Effect of additives and deacetylated xanthan gum. Food Hydrocoll. 2005, 19, 341–349. [Google Scholar] [CrossRef]
- Kim, S.R.B.; Choi, Y.-G.; Kim, J.-Y.; Lim, S.-T. Improvement of water solubility and humidity stability of tapioca starch film by incorporating various gums. LWT Food Sci. Technol. 2015, 64, 475–482. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films—A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Nurazzi, N.M.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of sugar palm nanofibrillated cellulose concentrations on morphological, mechanical and physical properties of biodegradable films based on agro-waste sugar palm (Arenga pinnata (Wurmb.) Merr) starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Liu, W.; Qiu, X. High performance PVA/lignin nanocomposite films with excellent water vapor barrier and UV-shielding properties. Int. J. Biol. Macromol. 2020, 142, 551–558. [Google Scholar] [CrossRef]
- PirouziFard, M.; Yorghanlu, R.A.; Pirsa, S. Production of active film based on potato starch containing Zedo gum and essential oil of Salvia officinalis and study of physical, mechanical, and antioxidant properties. J. Thermoplast. Compos. Mater. 2020, 33, 915–937. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.D.A.; Menegalli, F.C. Nanocomposites based on banana starch reinforced with cellulose nanofibers isolated from banana peels. J. Colloid Interface Sci. 2017, 505, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Priya, B.; Gupta, V.K.; Pathania, D.; Singha, A.S. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef]
- Cerqueira, J.C.; da Silva Penha, J.; Oliveira, R.S.; Guarieiro, L.L.N.; da Silva Melo, P.; Viana, J.D.; Machado, B.A.S. Production of biodegradable starch nanocomposites using cellulose nanocrystals extracted from coconut fibers. Polímeros 2017, 27, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Francisco, C.B.; Pellá, M.G.; Silva, O.A.; Raimundo, K.F.; Caetano, J.; Linde, G.A.; Colauto, G.A.L.; Dragunski, D.C. Shelf-life of guavas coated with biodegradable starch and cellulose-based films. Int. J. Biol. Macromol. 2020, 152, 272–279. [Google Scholar] [CrossRef]
- Andrade, L.A.; de Oliveira Silva, D.A.; Nunes, C.A.; Pereira, J. Experimental techniques for the extraction of taro mucilage with enhanced emulsifier properties using chemical characterization. Food Chem. 2020, 327, 127095. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Rao, M.S.; Kanatt, S.R.; Chawla, S.P.; Sharma, A. Chitosan and guar gum composite films: Preparation, physical, mechanical and antimicrobial properties. Carbohydr. Polym. 2010, 82, 1243–1247. [Google Scholar] [CrossRef]
- Soleimani-Rambod, A.; Zomorodi, S.; Raeisi, S.N.; Asl, A.K.; Shahidi, S.-A. The Effect of Xanthan Gum and Flaxseed Mucilage as Edible Coatings in Cheddar Cheese during Ripening. Coatings 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite films based on CMC, okra mucilage and ZnO nanoparticles: Physico mechanical and antibacterial properties. Carbohydr. Polym. 2018, 181, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind. Crop. Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Fahami, A.; Fathi, M. Development of cress seed mucilage/PVA nanofibers as a novel carrier for vitamin A delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Yazdi, F.T.; Shahidi, F.; Hesarinejad, M.A.; Mortazavi, S.A.; Mohebbi, M. Plantago major seed mucilage: Optimization of extraction and some physicochemical and rheological aspects. Carbohydr. Polym. 2017, 155, 68–77. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, R.; Punia, S. Characterization of mucilages extracted from different flaxseed (Linum usitatissiumum L.) cultivars: A heteropolysaccharide with desirable functional and rheological properties. Int. J. Biol. Macromol. 2018, 117, 919–927. [Google Scholar] [CrossRef]
- Hasan, M.; Gopakumar, D.A.; Olaiya, N.G.; Zarlaida, F.; Alfian, A.; Aprinasari, C.; Alfatah, T.; Rizal, S.; Khalil, H.A. Evaluation of the thermomechanical properties and biodegradation of brown rice starch-based chitosan biodegradable composite films. Int. J. Biol. Macromol. 2020, 156, 896–905. [Google Scholar] [CrossRef]
- Appiah-Nkansah, N.B.; Li, J.; Rooney, W.; Wang, D. A review of sweet sorghum as a viable renewable bioenergy crop and its techno-economic analysis. Renew. Energy 2019, 143, 1121–1132. [Google Scholar] [CrossRef]
- Capitani, M.I.; Ixtaina, V.Y.; Nolasco, S.M.; Tomás, M.C. Microstructure, chemical composition and mucilage exudation of chia (Salvia hispanica L.) nutlets from Argentina. J. Sci. Food Agric. 2013, 93, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Ji, Y.; Walck, J.L.; Tao, J. Seed Biology of Lepidium apetalum (Brassicaceae), with Particular Reference to Dormancy and Mucilage Development. Plants 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, L.A.; Nunes, C.A.; Pereira, J. Relationship between the chemical components of taro rhizome mucilage and its emulsifying property. Food Chem. 2015, 178, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and Physicochemical Characterization of Mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Baskin, J.M.; Baskin, C.C.; Huang, Z. More than just a coating: Ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 434–442. [Google Scholar] [CrossRef]
| Source of Binary Blended Films | Thickness (mm) | Tensile Strength (MPa) | WVP | Elongation (%) | Water Solubility (%) | References |
|---|---|---|---|---|---|---|
| Dioscorea opposita Thunb. mucilage and starch | 0.21–0.34 | 0.79–4.26 | 51.85–63.08 (g mm/m2 d kPa) | 47.52–153.26 | 41.11–62.74 | [40] |
| Plantago psyllium seed mucilage and starch | 0.130–0.209 | 0.56–1.38 | - | 7.5–15.7 | 16.76–22.85 | [24] |
| Okra Mucilage and Corn Starch | 0.04–0.08 | 66.98–140.30 | 1.32–2.42 (g/m s Pa) | 5.91–5.99 | 11.53–89.82 | [15] |
| Chia-seed mucilage and starch nanocrystals | 0.042–0.045 | 6.7–7.5 | - | 14–19 | 80–86.1 | [3] |
| Nopal mucilage and Rice starch | 0.47–0.50 | 2.80–3.96 | 0.21–3.10 (g mm m−2 h−1 kPa) | 12.07–2.63 | 18.42–22.59 | [16] |
| Prickly pear peel mucilage and Potato husk starch | 0.09–0.22 | - | - | 39.67–54.43 | [25] | |
| Thermoplastic starch and Opuntia ficus indica mucilage | 0.125–0.150 | 0.64–3.75 | - | - | - | [41] |
| Mango kernel starch and guar and xanthan gums | - | 3.57–10.24 | 1.28–4.29 × 10−10 (g m−1 s−1 Pa−1) | 6.28–17.78 | 36.26–44.63 | [52] |
| Potato starch and zedo gums | 0.22–0.207 | - | 5.58–9.53 × 10−11 (g/m s Pa) | - | 25.02–43.67 | [58] |
| Banana starch and peel fibers | - | 8.9–11.1 | 8.9–25.2 × 10−11 (g/m s Pa) | 20.7–25.9 | - | [59] |
| Corn starch and cellulose nanofibers | - | 21.90–28–87 | 3.00–4.73 × 10−7 (g Pa−1 h−1 m−1) | 73.07–103.80 | - | [60] |
| Potato starch and coconut fiber nanocrystals | - | 4.09–8.20 | 23.71–30.40 | - | [61] | |
| Cassava starch and hydroxyethyl cellulose | 0.04–0.08 | - | 12.84–18.94 (g h−1 m−2) | - | 28.73–93.26 | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosif, M.M.; Najda, A.; Bains, A.; Zawiślak, G.; Maj, G.; Chawla, P. Starch–Mucilage Composite Films: An Inclusive on Physicochemical and Biological Perspective. Polymers 2021, 13, 2588. https://doi.org/10.3390/polym13162588
Tosif MM, Najda A, Bains A, Zawiślak G, Maj G, Chawla P. Starch–Mucilage Composite Films: An Inclusive on Physicochemical and Biological Perspective. Polymers. 2021; 13(16):2588. https://doi.org/10.3390/polym13162588
Chicago/Turabian StyleTosif, Mansuri M., Agnieszka Najda, Aarti Bains, Grażyna Zawiślak, Grzegorz Maj, and Prince Chawla. 2021. "Starch–Mucilage Composite Films: An Inclusive on Physicochemical and Biological Perspective" Polymers 13, no. 16: 2588. https://doi.org/10.3390/polym13162588








