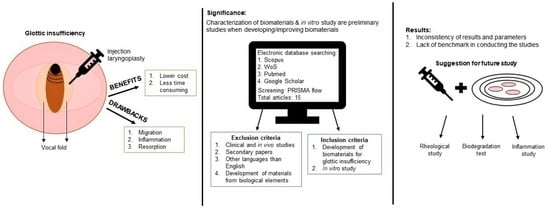
In Vitro Evaluation of Biomaterials for Vocal Fold Injection: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Management
2.5. Quality Assessment
3. Result
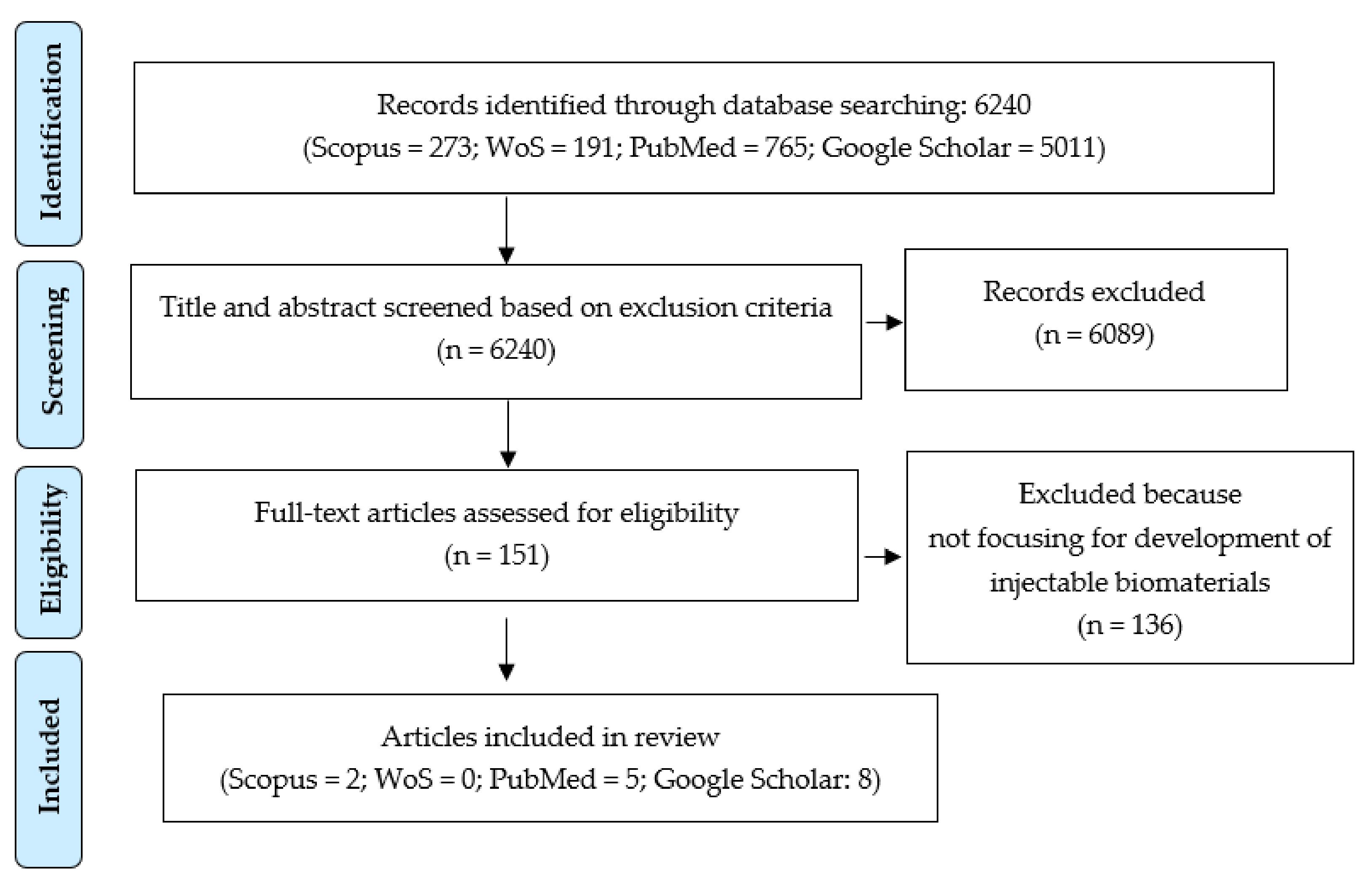
3.1. Searching Result
3.2. Study Characteristics
3.3. Rheological Properties
3.4. Other Characterization Parameters
3.5. Cellular Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CaHA | Calcium Hydroxypatite |
| CMC | Carboxymethylcellulose |
| CNTs | Carbon nanotube |
| COOH | Carboxylic |
| COX2 | Cyclooxygenase |
| ECM | Extracellular matrix |
| HA | Hyaluronic acid |
| HAMA | HA-methacrylate MP |
| HVFF | Human vocal fold fibroblast |
| IL-1β | Interleukin-1 beta |
| IFN-γ | Interferon-gamma |
| LSR | Linear skin rheometry |
| MAP | Microporous annealed particle |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MP | Microparticles |
| NF-ĸB | Necrosis factor kappa beta |
| OH | Hydroxylic |
| PDA | Polydopamine |
| PDMS | Poly(dimethysiloxane) microspheres |
| PEG | Polyethylene glycol-diamine |
| PEGDA | Poly(ethylene glycol) diacrylate |
| PTP | Phonation threshold pressure |
| RLN | Recurrent laryngeal nerve |
| RLP | Resilin-like-polypeptide |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumor necrosis factor-alpha |
| TWE | Torsional wave experiment |
References
- De Araújo Pernambuco, L.; Espelt, A.; Balata, P.M.M.; de Lima, K.C. Prevalence of voice disorders in the elderly: A systematic review of population-based studies. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Onwordi, L.N.; Yaghchi, C.A. Airway Glottic Insufficiency. In StatPearls; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Mattsson, P.; Hydman, J.; Svensson, M. Recovery of laryngeal function after intraoperative injury to the recurrent laryngeal nerve. Gland. Surg. 2015, 4, 27. [Google Scholar] [PubMed]
- Lynch, J.; Parameswaran, R. Management of unilateral recurrent laryngeal nerve injury after thyroid surgery: A review. Head Neck 2017, 39, 1470–1478. [Google Scholar] [CrossRef]
- Williams, M.J.; Utzinger, U.; Barkmeier-Kraemer, J.M.; Vande Geest, J.P. Differences in the microstructure and biomechanical properties of the recurrent laryngeal nerve as a function of age and location. J. Biomech. Eng. 2014, 136, 810081–810089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimberg, A.S.; Wellenstein, D.J.; van den Broek, E.M.; Honings, J.; van den Hoogen, F.J.A.; Marres, H.A.M.; Takes, R.P.; van den Broek, G.B. Office-based vs. operating room-performed laryngopharyngeal surgery: A review of cost differences. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 2963–2973. [Google Scholar] [CrossRef] [Green Version]
- Sulica, L.; Rosen, C.A.; Postma, G.N.; Simpson, B.; Amin, M.; Courey, M.; Merati, A. Current practice in injection augmentation of the vocal folds: Indications, treatment principles, techniques, and complications. Laryngoscope 2010, 120, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Modi, V.K. Vocal fold injection medialization laryngoplasty. Adv. Otorhinolaryngol. 2012, 73, 90–94. [Google Scholar] [CrossRef]
- Mansor, W.N.W.; Azman, M.; Remli, R.; Yunus, M.R.M.; Baki, M.M. Primary Nonselective Laryngeal Reinnervation in Iatrogenic Acute Recurrent Laryngeal Nerve Injury: Case Series and Literature Review. Ear Nose Throat J. 2021, 1–6. [Google Scholar] [CrossRef]
- Mau, T.; Muhlestein, J.; Callahan, S.; Chan, R.W. Modulating phonation through alteration of vocal fold medial surface contour. Laryngoscope 2012, 122, 2005–2014. [Google Scholar] [CrossRef] [Green Version]
- Lungova, V.; Thibeault, S.L. Mechanisms of larynx and vocal fold development and pathogenesis. Cell. Mol. Life Sci. 2020, 77, 3781–3795. [Google Scholar] [CrossRef]
- Koike, Y.; Hirano, M.; Morio, M.; Kasuya, T. Function of the laryngeal muscles on the position and shape of the vocal cord (author’s transl). Nippon. Jibiinkoka Gakkai Kaiho 1975, 78, 1249–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.S.; Mohad, V.; Gowda, M.; Thibeault, S.L. Insights into the Role of Collagen in Vocal Fold Health and Disease. J. Voice 2017, 31, 520–527. [Google Scholar] [CrossRef]
- Jiang, J.; Lin, E.; Hanson, D.G. Vocal fold physiology. Otolaryngol. Clin. N. Am. 2000, 33, 699–718. [Google Scholar] [CrossRef]
- Chhetri, D.K.; Rafizadeh, S. Young’s modulus of canine vocal fold cover layers. J. Voice 2014, 28, 406–410. [Google Scholar] [CrossRef] [Green Version]
- Miri, A.K.; Li, N.Y.K.; Avazmohammadi, R.; Thibeault, S.L.; Mongrain, R.; Mongeau, L. Study of extracellular matrix in vocal fold biomechanics using a two-phase model. Biomech. Model. Mechanobiol. 2015, 14, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.D.; Titze, I.R.; Alipour, F.; Hammond, T.H. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann. Otol. Rhinol. Laryngol. 2000, 109, 77–85. [Google Scholar] [CrossRef]
- Moore, J.; Thibeault, S. Insights into the role of elastin in vocal fold health and disease. J. Voice 2012, 26, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Won, H.-R.; Jung, S.-N.; Yeo, M.-K.; Yi, S.; Liu, L.; Lim, M.A.; Oh, C.; Kang, Y.E.; Chang, J.W.; Rha, K.S.; et al. Effect of Urban Particulate Matter on Vocal Fold Fibrosis through the MAPK/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 6643. [Google Scholar] [CrossRef]
- Enver, N.; Azİzlİ, E.; Akbulut, S.; Çadalli Tatar, E.; Yelken, M.K.; ÖztÜrk, K.; Coşkun, H.; Bİrkent, A.H.; Büyükatalay, Z.Ç.; ÖzgÜrsoy, O.B.; et al. A Multi-Institutional Study of Complication Rates for Vocal Fold Injection with Hyaluronic Acid: Is It Safe to Use in Vocal Folds? Turk. J. Med. Sci. 2020, 51, 819–825. [Google Scholar] [CrossRef]
- Benninger, M.S.; Alessi, D.; Archer, S.; Bastian, R.; Ford, C.; Koufman, J.; Sataloff, R.T.; Spiegel, J.R.; Woo, P. Vocal fold scarring: Current concepts and management. Otolaryngol. Head Neck Surg. 1996, 115, 474–482. [Google Scholar] [CrossRef]
- Xu, H.; Fan, G.-K. The Role of Cytokines in Modulating Vocal Fold Fibrosis: A Contemporary Review. Laryngoscope 2021, 131, 139–145. [Google Scholar] [CrossRef]
- Hortobagyi, D.; Grossmann, T.; Tschernitz, M.; Grill, M.; Kirsch, A.; Gerstenberger, C.; Gugatschka, M. In vitro mechanical vibration down-regulates pro-inflammatory and pro-fibrotic signaling in human vocal fold fibroblasts. PLoS ONE 2020, 15, e0241901. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.; Tateya, I.; Tateya, T.; Muñoz-Del-Río, A.; Bless, D.M. Immediate inflammatory response and scar formation in wounded vocal folds. Ann. Otol. Rhinol. Laryngol. 2006, 115, 921–929. [Google Scholar] [CrossRef]
- Lim, X.; Bless, D.M.; Muñoz-Del-Río, A.; Welham, N.V. Changes in cytokine signaling and extracellular matrix production induced by inflammatory factors in cultured vocal fold fibroblasts. Ann. Otol. Rhinol. Laryngol. 2008, 117, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zeleník, K.; Formánek, M.; Walderová, R.; Formánková, D.; Komínek, P. Five-year results of vocal fold augmentation using autologous fat or calcium hydroxylapatite. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1139–1144. [Google Scholar] [CrossRef]
- DeFatta, R.A.; Chowdhury, F.R.; Sataloff, R.T. Complications of injection laryngoplasty using calcium hydroxylapatite. J. Voice 2012, 26, 614–618. [Google Scholar] [CrossRef]
- Elsaeed, A.; Afsah, O.; Moneir, W.; Elhadidy, T.; Abou-Elsaad, T. Respiratory and voice outcomes of office-based injection laryngoplasty in patients with unilateral vocal fold paralysis. Egypt. J. Otolaryngol. 2021, 37, 1–10. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wu, S.-H.; Tu, Y.-K.; Lin, W.-J.; Liu, S.-A. Hyaluronic Acid Injection Laryngoplasty for Unilateral Vocal Fold Paralysis-A Systematic Review and Meta-Analysis. Cells 2020, 9, 2417. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.T.; Benyamini, L. Voice outcome after vocal fold injection augmentation with carboxymethyl cellulose versus calcium hydroxyapatite. J. Laryngol. Otol. 2020, 134, 263–269. [Google Scholar] [CrossRef]
- Tigges, M.; Hess, M. Glottis injection to improve voice function: Review of more than 500 operations. HNO 2015, 63, 489–496. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Klemuk, S.A.; Lu, X.; Hoffman, H.T.; Titze, I.R. Phonation threshold pressure predictions using viscoelastic properties up to 1400 Hz of injectables intended for Reinke’s space. Laryngoscope 2010, 120, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, H.; Bao, G.; Latifi, N.; Mongeau, L.G. Carbon nanotube composite hydrogels for vocal fold tissue engineering: Biocompatibility, rheology, and porosity. Mater. Sci. Eng. C 2019, 103, 109861. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, J.W.; Park, J.K.; Kim, Y.S.; Kim, H.J.; Shin, Y.S.; Kim, C.H. Efficiency and durability of hyaluronic acid of different particle sizes as an injectable material for VF augmentation. Acta Otolaryngol. 2015, 135, 1311–1318. [Google Scholar] [CrossRef]
- Fu, C.; Ren, F.; Zhang, Q.; Lao, G.; Zhang, L.M. Effects of collagen incorporation on thermogelation and hydrogel characteristics of aqueous Pluronic F127 copolymer system. Colloid Polym. Sci. 2015, 293, 2191–2200. [Google Scholar] [CrossRef]
- Kazemirad, S.; Heris, H.K.; Mongeau, L. Viscoelasticity of hyaluronic acid-gelatin hydrogels for vocal fold tissue engineering. J. Biomed. Mater. Res. 2016, 104, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.E.; Gulka, C.P.; Giordano, J.E.M.; Montero, M.P.; Hoang, A.; Carroll, T.L. Injectable Silk Protein Microparticle-based Fillers: A Novel Material for Potential Use in Glottic Insufficiency. J. Voice 2019, 33, 773–780. [Google Scholar] [CrossRef]
- Li, L.; Stiadle, J.M.; Levendoski, E.E.; Lau, H.K.; Susan, L.; Kiick, K.L.; Surgery, N. Biocompatibility of Injectable Resilin-based Hydrogels. J. Biomed. Mater. Res. Part A 2018, 106, 2229–2242. [Google Scholar] [CrossRef]
- Chung, E.J.; Jun, D.R.; Kim, D.W.; Han, M.J.; Kwon, T.K.; Choi, S.W.; Kwon, S.K. Prevention of polydimethylsiloxane microsphere migration using a mussel-inspired polydopamine coating for potential application in injection therapy. PLoS ONE 2017, 12, e0186877. [Google Scholar] [CrossRef] [Green Version]
- Pruett, L.; Koehn, H.; Martz, T.; Churnin, I.; Ferrante, S.; Salopek, L.; Cottler, P.; Griffin, D.R.; Daniero, J.J. Development of a microporous annealed particle hydrogel for long-term vocal fold augmentation. Laryngoscope 2020, 130, 2432–2441. [Google Scholar] [CrossRef]
- Karajanagi, S.S.; Lopez-Guerra, G.; Park, H.; Kobler, J.B.; Galindo, M.; Aanestad, J.; Mehta, D.D.; Kumai, Y.; Giordano, N.; D’Almeida, A.; et al. Assessment of canine vocal fold function after injection of a new biomaterial designed to treat phonatory mucosal scarring. Ann. Otol. Rhinol. Laryngol. 2011, 120, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, H.; Mohraz, A.; Verma, S.P. Evaluation of heating and shearing on the viscoelastic properties of calcium hydroxyapatite used in injection laryngoplasty. Otolaryngol. Head Neck Surg. 2016, 154, 498–501. [Google Scholar] [CrossRef]
- Kimura, M.; Ted, M.; Roger, W.C. Viscoelastic properties of phonosurgical biomaterials at phonatory frequencies. Laryngoscope 2010, 120, 764–768. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Kim, N.J.; Klemuk, S.; Jang, Y.H.; Park, I.S.; Ahn, K.H.; Lim, J.Y.; Kim, Y.M. Preservation of viscoelastic properties of rabbit vocal folds after implantation of hyaluronic acid-based biomaterials. Otolaryngol. Head Neck Surg. 2012, 147, 515–521. [Google Scholar] [CrossRef]
- Coburn, P.T.; Herbay, A.C.; Berrini, M.; Li-Jessen, N.Y.K. An in vitro assessment of the response of THP-1 macrophages to varying stiffness of a glycol-chitosan hydrogel for vocal fold tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 109, 1–16. [Google Scholar] [CrossRef]
- Chan, K.M.C.; Li, R.H.; Chapman, J.W.; Trac, E.M.; Kobler, J.B.; Zeitels, S.M.; Langer, R.; Karajanagi, S.S. Functionalizable hydrogel microparticles of tunable size and stiffness for soft-tissue filler applications. Acta Biomater. 2014, 10, 2563–2573. [Google Scholar] [CrossRef] [Green Version]
- Fishman, J.M.; Long, J.; Gugatschka, M.; De Coppi, P.; Hirano, S.; Hertegard, S.; Thibeault, S.L.; Birchall, M.A. Stem cell approaches for vocal fold regeneration. Laryngoscope 2016, 126, 1865–1870. [Google Scholar] [CrossRef]
- Levental, I.; Georges, P.C.; Janmey, P.A. Soft biological materials and their impact on cell function. Soft Matter 2007, 3, 299–306. [Google Scholar] [CrossRef]
- Thomas, L.V.; Rahul, V.G.; Prabha, D.N. Effect of stiffness of chitosan-hyaluronic acid dialdehyde hydrogels on the viability and growth of encapsulated chondrocytes. Int. J. Biol. Macromol. 2017, 104, 1925–1935. [Google Scholar] [CrossRef]
- Chan, R.W.; Titze, I.R. Viscoelastic shear properties of human vocal fold mucosa: Measurement methodology and empirical results. J. Acoust. Soc. Am. 1999, 106, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Goodyer, E.; Hemmerich, S.; Müller, F.; Kobler, J.B.; Hess, M. The shear modulus of the human vocal fold, preliminary results from 20 larynxes. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Mills, R.D.; Jiang, J.J. Quantitative Study of the Effects of Dehydration on the Viscoelastic Parameters in the Vocal Fold Mucosa. J. Voice 2017, 31, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Miri, A.K. Mechanical characterization of vocal fold tissue: A review study. J. Voice 2014, 28, 657–667. [Google Scholar] [CrossRef]
- Teller, S.S.; Farran, A.J.E.; Xiao, L.; Jiao, T.; Duncan, R.L.; Clifton, R.J.; Jia, X. High-frequency viscoelastic shear properties of vocal fold tissues: Implications for vocal fold tissue engineering. Tissue Eng. Part A 2012, 18, 2008–2019. [Google Scholar] [CrossRef] [Green Version]
- Kazemirad, S.; Bakhshaee, H.; Mongeau, L.; Kost, K. Non-invasive in vivo measurement of the shear modulus of human vocal fold tissue. J. Biomech. 2014, 47, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Chan, R.W. Nonlinear viscoelastic characterization of human vocal fold tissues under large-amplitude oscillatory shear (LAOS). J. Rheol. 2018, 62, 695–712. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhao, X.; Lu, X.; Kassab, G. Non-linear micromechanics of soft tissues. Int. J. Non-Linear Mech. 2013, 58, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Gaston, J.; Thibeault, S.L. Hyaluronic acid hydrogels for vocal fold wound healing. Biomatter 2013, 3, e23799. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.D.; Sataloff, R.T. Complications of collagen injection of the vocal fold: Report of several unusual cases and review of the literature. J. Voice 2004, 18, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Ford, C.N.; Bless, D.M. Teflon vocal fold augmentation: Failures and management in 28 cases. Otolaryngol. Head Neck Surg. 1993, 109, 493–498. [Google Scholar] [CrossRef]
- Hadden, J.W.; Warfel, A.H.; Smithyman, A.M. Mechanisms of Granuloma Formation BT. In Immunodermatology; Safai, B., Good, R.A., Eds.; Springer: Boston, MA, USA, 1981; ISBN 978-1-4615-7228-2. [Google Scholar]
- Hamdan, A.-L.; Khalifee, E. Adverse Reaction to Restylane: A Review of 63 Cases of Injection Laryngoplasty. Ear Nose Throat J. 2019, 98, 212–216. [Google Scholar] [CrossRef]
- King, S.N.; Chen, F.; Jetté, M.E.; Thibeault, S.L. Vocal fold fibroblasts immunoregulate activated macrophage phenotype. Cytokine 2013, 61, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Kumai, Y. Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability. Int. J. Mol. Sci. 2019, 20, 2551. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, R.S.; Thibeault, S.L.; Prestwich, G.D. Therapeutic potential of gel-based injectables for vocal fold regeneration. Biomed. Mater. 2012, 7, 24103. [Google Scholar] [CrossRef]
- Chen, H.; Erndt-Marino, J.; Diaz-Rodriguez, P.; Kulwatno, J.; Jimenez-Vergara, A.C.; Thibeault, S.L.; Hahn, M.S. In vitro evaluation of anti-fibrotic effects of select cytokines for vocal fold scar treatment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1056–1067. [Google Scholar] [CrossRef]
- Vyas, B.; Ishikawa, K.; Duflo, S.; Chen, X.; Thibeault, S.L. Inhibitory effects of hepatocyte growth factor and interleukin-6 on transforming growth factor-beta1 mediated vocal fold fibroblast-myofibroblast differentiation. Ann. Otol. Rhinol. Laryngol. 2010, 119, 350–357. [Google Scholar] [CrossRef]
- Kwon, T.-K.; Buckmire, R. Injection laryngoplasty for management of unilateral vocal fold paralysis. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 538–542. [Google Scholar] [CrossRef]
- Dominguez, L.M.; Tibbetts, K.M.; Simpson, C.B. Inflammatory reaction to hyaluronic acid: A newly described complication in vocal fold augmentation. Laryngoscope 2017, 127, 445–449. [Google Scholar] [CrossRef]
- Tamariz, E.; Rios-Ramírez, A. Biodegradation of Medical Purpose Polymeric Materials and Their Impact on Biocompatibility. In Biodegradation—Life of Science; Chamy, R., Rosenkranz, F., Eds.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Long, J.L. Tissue engineering for treatment of vocal fold scar. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Mallur, P.S.; Rosen, C.A. Vocal fold injection: Review of indications, techniques, and materials for augmentation. Clin. Exp. Otorhinolaryngol. 2010, 3, 177–182. [Google Scholar] [CrossRef]
- Yung, K.C.; Likhterov, I.; Courey, M.S. Effect of temporary vocal fold injection medialization on the rate of permanent medialization laryngoplasty in unilateral vocal fold paralysis patients. Laryngoscope 2011, 121, 2191–2194. [Google Scholar] [CrossRef]
- Ab Rani, A.; Azman, M.; Ubaidah, M.A.; Mohamad Yunus, M.R.; Sani, A.; Mat Baki, M. Nonselective Laryngeal Reinnervation versus Type 1 Thyroplasty in Patients with Unilateral Vocal Fold Paralysis: A Single Tertiary Centre Experience. J. Voice 2021, 35, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Mat Baki, M.; Clarke, P.; Birchall, M.A. Immediate selective laryngeal reinnervation in vagal paraganglioma patients. J. Laryngol. Otol. 2018, 132, 846–851. [Google Scholar] [CrossRef]
- Simpson, B.; Rosen, C. Principles of Vocal Fold Augmentation BT. In Operative Techniques in Laryngology; Simpson, B., Rosen, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-68107-6. [Google Scholar]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V. Preparation, characterization, and in vitro enzymatic degradation of chitosan-gelatine hydrogel scaffolds as potential biomaterials. J. Biomed. Mater. Res. A 2012, 100, 1655–1667. [Google Scholar] [CrossRef]
- Growney Kalaf, E.A.; Pendyala, M.; Bledsoe, J.G.; Sell, S.A. Characterization and restoration of degenerated IVD function with an injectable, in situ gelling alginate hydrogel: An in vitro and ex vivo study. J. Mech. Behav. Biomed. Mater. 2017, 72, 229–240. [Google Scholar] [CrossRef]
- Wassenaar, J.W.; Braden, R.L.; Osborn, K.G.; Christman, K.L. Modulating In Vivo Degradation Rate of Injectable Extracellular Matrix Hydrogels. J. Mater. Chem. B 2016, 4, 2794–2802. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Latifi, N.; Asgari, M.; Vali, H.; Mongeau, L. A tissue-mimetic nano-fibrillar hybrid injectable hydrogel for potential soft tissue engineering applications. Sci. Rep. 2018, 8, 1047. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Guo, X.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Kasper, F.K.; Mikos, A.G. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells In Vitro. Biomacromolecules 2009, 10, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Woo, P. Injection laryngoplasty with micronized dermis: A 10-year experience with 381 injections in 344 patients. Laryngoscope 2010, 120, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Mallur, P.S.; Morrison, M.P.; Postma, G.N.; Amin, M.R.; Rosen, C.A. Safety and efficacy of carboxymethylcellulose in the treatment of glottic insufficiency. Laryngoscope 2012, 122, 322–326. [Google Scholar] [CrossRef]
- Hertegård, S.; Hallén, L.; Laurent, C.; Lindström, E.; Olofsson, K.; Testad, P.; Dahlqvist, A. Cross-linked hyaluronan versus collagen for injection treatment of glottal insufficiency: 2-year follow-up. Acta Otolaryngol. 2004, 124, 1208–1214. [Google Scholar] [CrossRef]
- Woo, J.H.; Baek, M.K.; Kim, D.Y.; Park, H.-M.; An, S.; Moon, K.H.; Cha, H.E. Injection Laryngoplasty for The Treatment of Vocal Fold Scar, and Sulcus. J. Korean Soc. Laryngol. Phoniatr. Logop. 2016, 27, 25–29. [Google Scholar] [CrossRef]
- Rudolf, R.; Sibylle, B. Laryngoplasty With Hyaluronic Acid in Patients with Unilateral Vocal Fold Paralysis. J. Voice 2012, 26, 785–791. [Google Scholar] [CrossRef]
| No. | Terms |
|---|---|
| 1 | Biomaterial* |
| 2 | Material* |
| 3 | Regenerative therap* |
| 4 | Hyaluronic acid* |
| 5 | Vocal fold injection* |
| 6 | Glottic insufficienc* |
| 7 | Vocal fold medialization* |
| 8 | Vocal fold augmentation* |
| 9 | Or/1–4 |
| 10 | Or/5–8 |
| 11 | And/9 & 10 |
| 12 | Line 11: Restrict to period: January 2010 to March 2021 |
| Author, Year | Type of Biomaterials | Study Measure Outcome | Summary of Results | Conclusion |
|---|---|---|---|---|
| 1. Klemuk et al., 2010 [36] | 1. Carbomer hydrogel 2. Micronized dermal graft tissue 3. Crosslinked HA hydrogel 4. HA and gelatin crosslinked hydrogel | (a) Rheology measurement by rotational (for 0.1 to 100 Hz) and piezoelectric rheometer (up to 2000 Hz): shear elastic (G′) and viscous moduli (G″) (linear) (b) Pressure threshold projection (PTP) vocal fold length and frequency: (i) For males: 15.8 mm and 125 Hz (ii) For females: 10.63 mm, 200 Hz | Shear elastic (G′) Mean values: 100 to 10,000 Pa Viscous moduli (G″) Mean values: 10 to 5000 Pa Loss tangent (G″/G′) in 100 to 1000 Hz Hylan B, Extracel and Cbmr: 0.08 to 0.66 (widest range) PTP value relative to nominal PTP value (0.283 kPa) Sample 2 had highest value (3× to 21×) and sample 4 had lowest value (0.01× to 0.05×) | Crosslinked HA hydrogel, HA and gelatin crosslinked hydrogel and carbomer hydrogel are suitable for voice production. |
| 2. Mahboubi, Mohraz & Verma 2016 [46] | 1. CaHA 2. CMC | (a) Viscosity modulus (ƞ) by rotational rheometer (b) Shear elastic (G′) and loss moduli (G″) with frequency sweeps at 0.01% strain and 0.1 to 200 Hz by oscillatory rheometer (linear) | Viscosity CaHA (43,100 Pa.s) was 10 times more viscous than CMC (4540 Pa.s). Heating and shearing G″ for CaHA reduced by 52% | Heating and shearing potentially reduces viscosity of CaHA. |
| 3. Kimura, Mau & W Chan 2010 [47] | 1. 3% bovine collagen (atelocollagen) 2. micronized Alloderm 3. CaHA 4. 2.4% crosslinked HA gel | (a) Elastic shear modulus (G′) tested with 0.3 to 0.5 mm gap size, 1 to 2% strain and 1 to 250 Hz. (b) Dynamic viscosity (n′) by custom build, controlled strain, linear simple shear rheometer system | Elastic shear modulus (G′) Atelocollagen performed the nearest value (about 1000 Pa) to the vocal fold superficial layer′s value. Dynamic viscosity (ƞ′) Atelocollagen performed the nearest value (about 0.7 Pa.s) to the vocal fold superficial layer′s value at around 135 Hz. | All biomaterials had stiffer properties compared to earlier studies, hence suggested for deep injection into the vocal fold but not into lamina propria. |
| 4. Ravanbakhsh et al., 2019 [37] | 1. Carboxylic (COOH) multi-walled functionalized carbon nanotube (CNTs) 2. Hydroxylic (OH) multi-walled functionalized CNTs | Storage modulus tested at 0.1 to 10 Hz and 1000 µm gap size, <5% shear strain by rotational rheometer (linear) | Storage modulus* - Increased with higher CNT concentration - OH-CNT had higher storage modulus than COOH-CNT | Mechanical strength of hydrogel was not influenced by the concentration of CNT. |
| 5. Kim et al., 2015 [38] | 1. Commercial HA 2. Unequal particle-sized middle viscosity HA (3,000,000 cP) 3. Unequal particle-sized low viscosity HA (30,000 cP) | Elasticity at 0.02 Hz (not specified) | Elasticity Commercial HA: 200 to 400 Pa Mid HA: 300 Pa Low HA: 3 Pa | Unequal particle-size HA showed better outcomes than commercial HA in vivo. |
| 6. Choi et al., 2012 [48] | 1. Rofilan (non animal stabilized biomaterial) 2. Restylane (double crosslinked 3. Dextran beads in HA (dextran microspheres) | (a) Shear viscosity (ƞ) (b) Mean elastic modulus (G′) (c) Mean viscous modulus (G″) at frequency of 0.1 to 10 Hz with strain controlled rheometer (linear) | Steady state viscosity (ƞ) Restylane had the highest (19.138 Pa∙s) value. Mean elastic modulus (G′) Reviderm had the highest (464.1 Pa∙s) value. Mean viscous modulus (G″) Reviderm had the highest (167.8 Pa·s) value. | All HA-based hydrogels had similar shear viscosities and the values were higher than reported human vocal fold but in vivo study showed that HA-based hydrogels were compatible with viscoelasticity of rabbit vocal fold. |
| 7. Chan et al., 2014 [50] | 1. PEG microparticles (MP) 2. Gelatin methacrylate MP 3. HA-methacrylate (HAMA) MP 4. Semi-IPN MP of HAMA & gelatine | Shear storage modulus (G′) and shear loss modulus (G″) at 0.6% strain by rotational rheometer (linear). | Viscoelasticity for PEG-DA:PEG When the ratio of PEG-DA:PEG increased 50 to 100%, G′ increased from 523 Pa to 1599 a; G″ increased from 38 Pa to 111 Pa | Photopolymerization method was able to synthesize soft MP with varying stiffness which was independent of its size. |
| 8. Coburn et al., 2020 [49] | Glycol-chitosan hydrogel with different crosslinker concentrations: 0.005%, 0.01% and 0.02% | Mechanical characterization at 0.1 to 100 rad/s by rotational rheometer (linear) | Storage modulus (G′) and loss modulus (G″) 0.005%: around 50 and 18 Pa 0.01%: around 340 and 17 Pa 0.02%: around 740 and 15 Pa G′ value > G″: showing elastic property | Hydrogel with higher stiffness potentially caused inflammation but delayed expression of IL-10 at 72 h caused higher macrophage apoptosis. |
| 9. Fu et al., 2015 [39] | Pluronic F127 with collagen of 1%, 2% and 3% | (a) Storage modulus (b) Loss modulus Rheology measurement at 1.0 rad/s and 0.5% strain by rotational rheometer (linear) | Elastic modulus (G′) at 1.0 rad/s Pluronic F127 with highest collagen (3%) exhibited lowest G′ (94.0 kPa). Viscous modulus (G″) at 10 to 100 rad/s* Pluronic F127 with collagen did not reduce sharply compared to without collagen. | Pluronic F127 with collagen enhanced the drug release time and favoured cell growth. |
| 10. Kazemirad, Heris & Mongeau 2016 [40] | 1. Sample 1 to 3: 0.50%HA + crosslinker PEGDA of 0.25%, 0.5% & 1.0% 2. Sample 4 to 5: 0.45%HA + 0.05% gelatin (Ge) of 0.1% and 0.2% | (a) Shear storage (G′) (b) Loss moduli (G″) at frequency up to 4000 Hz (linear) | Shear storage (G′) & Loss moduli (G″) Sample 4 (G′: 19.61 Pa; G″: 5.00 Pa) and 5 (G′: 12.24 Pa; G″: 8.50 Pa) were comparable to viscoelasticity with human vocal fold. | With optimized concentration of HA, Ge and crosslinker, the hydrogel showed comparable viscoelasticity. |
| 11. Brown et al., 2019 [41] | Silk protein-based in HA suspension | Mechanical properties at 0.1 to 10.0 Hz and 1% strain by dynamic rotation shear rheometer (linear) | Mechanical properties - Silk suspension increased stiffness less rapid (5× lesser) than CaHA-CMC - Injection of silk suspension (1.5 times) yield lesser stiffness in vocal fold than CaHA-CMC (4.0 times) | Silk-HA had similar viscoelasticity properties with porcine vocal fold. |
| 12. Li et al., 2018 [42] | Chemically crosslinked resilin-like-polypeptide (RLP) hydrogel: 1. Sample 1: 10 wt% 2. Sample 2: 15 wt% | (a) Shear storage modulus at 0.1 to 100 rad/s (b) Storage moduli (G′) and loss moduli (G″) by stress controlled rheometer (c) strain sweep test of 0.01% to 1000% (linear) | Shear storage modulus Sample 1 and 2 increased rapidly until 1000 Pa and 2000 Pa respectively Storage moduli (G′) and loss moduli (G″) G′ was higher than G″ for 100 to 200 fold. Strain sweep Sample 1: 265% Sample 2: 245% High resistance to break. | Rheological properties of the hydrogels were in the range of native vocal fold tissue. |
| 13. Pruett et al., 2020 [44] | Fabrication of microporous annealed particle (MAP) by water-in-oil emulsion | Young’s modulus (linear), compare with vocalis muscle | Young’s modulus 1.9 wt% MAP showed with porcine vocal fold’s muscle comparable (~15,000 Pa). | MAP gel exhibited similar rheological properties with porcine vocal fold tissue. |
| 14. Karajanagi et al., 2011 [45] | PEG30 hydrogel | Elastic shear properties (G′) from 1 to 10 Hz with 0.6% strain by rotational rheometer (linear) | Viscoelasticity* PEG30 showed softer hydrogel compared to value reported in literature review. | PEG30 demonstrated optimal physical properties for vocal fold injection. |
| Author, Year | Type of Biomaterials | Study Measure Outcome | Summary of Results | Conclusion |
|---|---|---|---|---|
| 1. Ravanbakhsh et al., 2019 [37] | 1. Carboxylic (COOH) multi-walled functionalized CNTs 2. Hydroxylic (OH) multi-walled functionalized CNTs | (a) Morphology (b) Pore size (c) Swelling ratio (d) Gelation time | Pore size Diameter of CNTs: 45 ± 5 nm Increased by 33% with increased concentration of COOT-CNT but not significant in OH-CNT. Swelling ratio Increased by 5% with increased COOT-CNT concentration. OH-CNT had no effect on this property. Gelation time* Increased starting 750 μg/mL of CNT | COOH-CNT hydrogel showed larger pore size which might enhance cell migration. |
| 2. Kim et al., 2015 [38] | 1. Commercial HA (Restylane) 2. Unequal particle-sized middle viscosity HA 3. Unequal particle-sized low viscosity HA | (a) Particle size (b) Swelling ratio | Particle size Restylane: 200 µm Mid HA: 300 to 500 µm Low HA: No size Swelling ratio Restylane: 100 to 200% Mid HA: 130% Low HA: 200% | Unequal particle-size HA showed better outcomes than Restylane® in vivo. |
| 3. Chan et al., 2014 [50] | 1. PEG MP 2. Gelatin methacrylate MP 3. HA-methacrylate (HAMA) MP 4. Semi-IPN MP of HAMA & gelatin | (a) Particle size (b) Drug release test | Ability to inject Can be injected through 22 gauge needle Particle size (D90) Range from 136 µm to 162 µm MP produced had uniform particle distribution with ~1.5 polydispersity (PDI). Increased stirring speed up to 600 rpm or surfactant reduced the size from 515 to 140 µm. Drug release test* Drug encapsulation and release in PEG NS/MP was lower than from NS alone. | Higher stirring speed and surfactant concentration reduced size of MP and drug release time. |
| 4. Fu et al., 2015 [39] | 1. Pluronic F127 with collagen of 1%, 2% and 3% | (a) Morphology (b) Drug release test | Pore size With increased concentration of collagen incorporated in Pluronic F127, pore size was increased (from 5–20 µm to 20–40 µm). Drug release Collagen incorporated in Pluronic F127 reduced drug (ofloxacin) release (43.6% to 48.1%). | Pluronic F127 with collagen enhanced the drug release time and favoured cell growth. |
| 5. Brown et al., 2019 [41] | Silk protein-based in HA suspension | (a) Pore size (b) Injection force through 24 G long needle and 50 cm catheter with 1.05 mm inner diameter at 13 mm/min speed | Pore size Diameter ranging 10 to 100 um and had ability to return into original shape after compression. Injection force Silk protein (34.9 N) needed less force than CaHA-CMC (51.4 N) as control. | Particle size of silk-HA allowed macrophage passage, tissue adherence and was biocompatible. |
| 6. Chung et al., 2017 [43] | PDMScoated with PDA | (a) Particle size (b) Morphology | Particle size 79.23 µm ± 2.23 with 2.81% coefficient of variation. (less than 5% showed highly uniform size distribution) Morphology PDMS microsphere with PDA had a rougher surface while without PDA had a smoother surface. | PDMS was injectable, non-absorbable and showed better cell adherence. |
| Author, Year | Type of Biomaterials | Study Measure Outcome | Summary of Results | Conclusion |
|---|---|---|---|---|
| 1. Ravanbakhsh et al., 2019 [37] | 1. Carboxylic (COOH) multi-walled functionalized CNTs 2. Hydroxylic (OH) multi-walled functionalized CNTs | Cell viability of HVFF | Cell viability 1. COOH-CNT up to 750 ug/mL; 2. OH-CNT up to 1250 ug/mL as the cytotoxicity level increased after this threshold. | COOH-CNT had higher cytotoxicity than OH-CNT |
| 2. Chan et al., 2014 [50] | 1. PEG MP 2. Gelatin methacrylate MP 3. HA-methacrylate (HAMA) MP 4. Semi-IPN MP of HAMA & gelatin | Cytocompatibility test on NIH/3T3 cells | Cytocompatibility test 0.1 to 50 mg/mL PEG50 culture resulted in cell viability of 80%. | Hydrogel produced by photopolymerization was cytocompatible. |
| 3. Coburn et al., 2020 [49] | Glycol-chitosan hydrogel with concentration: 1. 0.005% 2. 0.01% 3. 0.02% | (a) Production of cytokines by macrophage (b) Macrophage viability (c) Macrophage phenotyping: -CD11b (+) CD33/CD80:proinflammatory CD33/CD206:anti- inflammatory | Production of cytokines TNF-α and IL-10 increased in hydrogel culture and with increased stiffness of hydrogel. Cell viability Macrophage viability was reduced in hydrogel culture. Cell phenotyping More CD33/CD206 (anti-inflammatory) expressing macrophage in macrophage+ VFF+hydrogel than macrophage+hydrogel. | Hydrogel with higher stiffness potentially caused inflammation but delayed expression of IL-10 at 72 h caused higher macrophage apoptosis. |
| 4. Fu et al., 2015 [39] | Pluronic F127 with collagen of 1%, 2% and 3% | Cell viability (Hacat cells) | Cell viability Collagen incorporated in Pluronic F127 improved cell adhesion and viability. | Pluronic F127 loaded with collagen improved cell adhesion and viability. |
| 5. Chung et al., 2017 [43] | PDMS PDA | Cell adhesion test (mouse NIH-3T3 embryonic fibroblast) | Cell adhesion test PDMS microsphere with PDA had more cells attached on its surface. | PDMS with PDA demonstrated better cell adherence. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan-Chiew, N.; Baki, M.M.; Fauzi, M.B.; Lokanathan, Y.; Azman, M. In Vitro Evaluation of Biomaterials for Vocal Fold Injection: A Systematic Review. Polymers 2021, 13, 2619. https://doi.org/10.3390/polym13162619
Wan-Chiew N, Baki MM, Fauzi MB, Lokanathan Y, Azman M. In Vitro Evaluation of Biomaterials for Vocal Fold Injection: A Systematic Review. Polymers. 2021; 13(16):2619. https://doi.org/10.3390/polym13162619
Chicago/Turabian StyleWan-Chiew, Ng, Marina Mat Baki, Mh Busra Fauzi, Yogeswaran Lokanathan, and Mawaddah Azman. 2021. "In Vitro Evaluation of Biomaterials for Vocal Fold Injection: A Systematic Review" Polymers 13, no. 16: 2619. https://doi.org/10.3390/polym13162619