Multifunctional and Self-Healable Intelligent Hydrogels for Cancer Drug Delivery and Promoting Tissue Regeneration In Vivo
Abstract
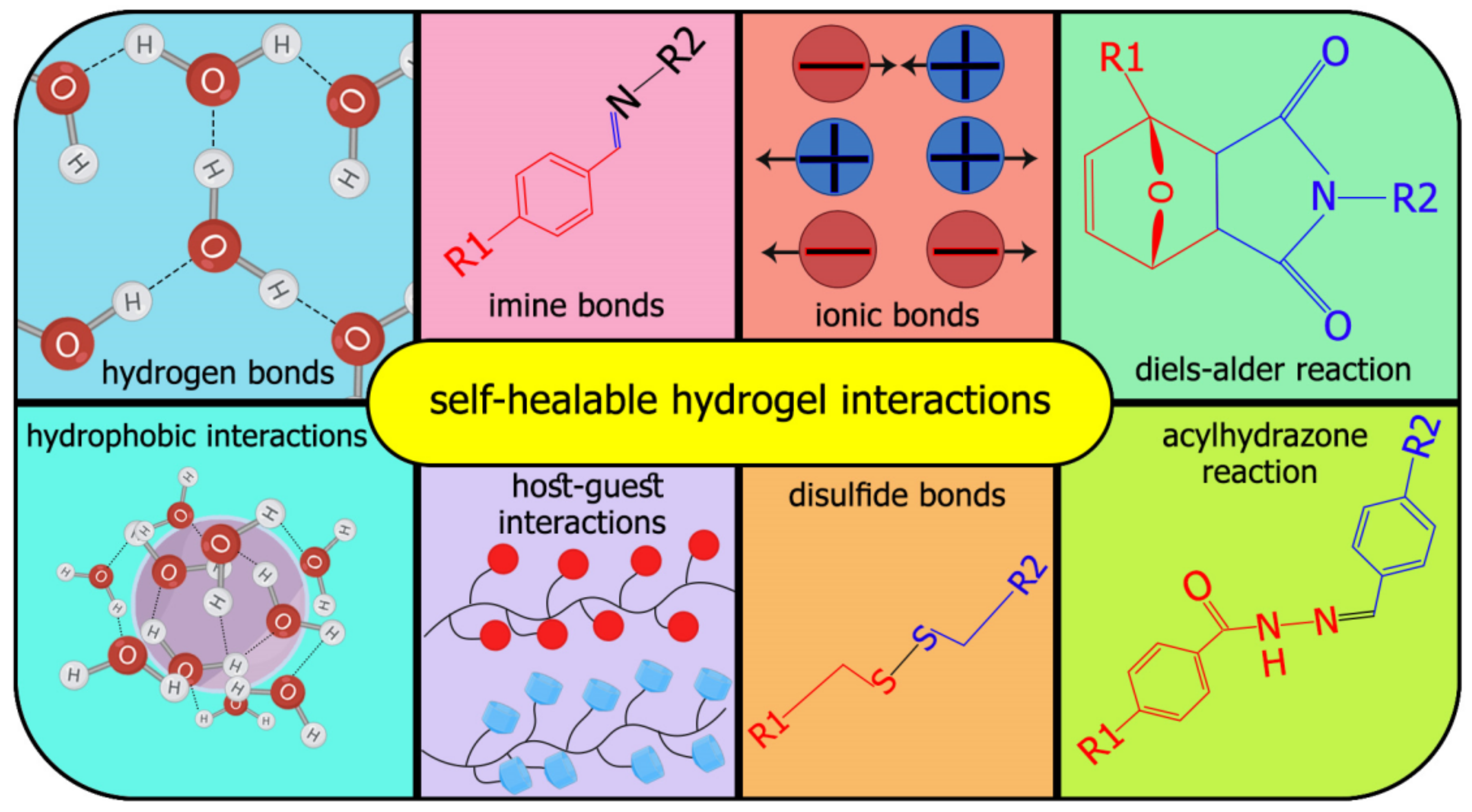
:1. Introduction
2. Tissue Engineering Applications and Cancer Drug Delivery
2.1. Cancer Drug Delivery
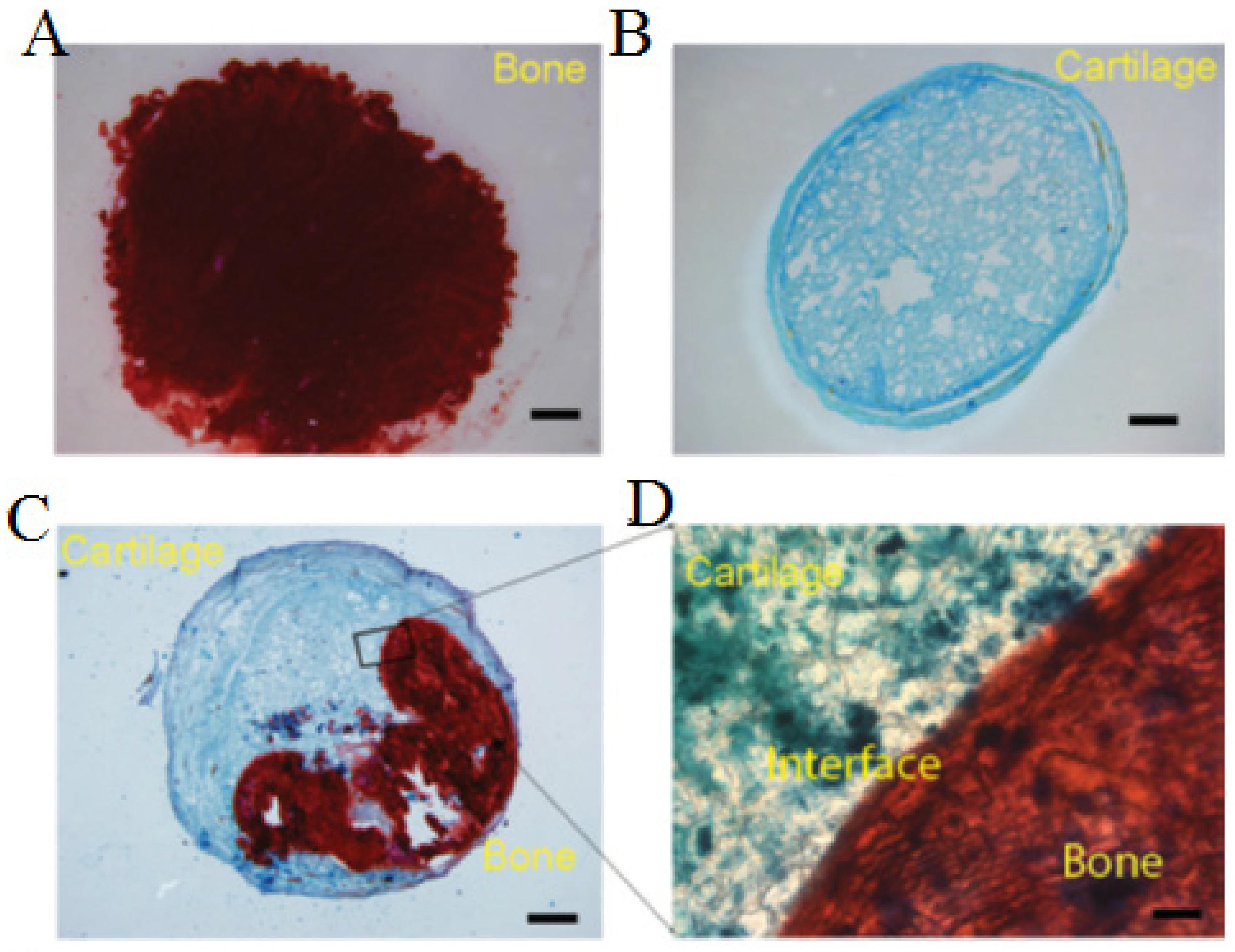
2.2. Bone
Injectable Hydrogels for Bone Tissue Engineering
2.3. Cardiac Tissue Engineering
Injectable Hydrogels for Cardiac Tissue Engineering
2.4. Injectable Hydrogels Used for Neural System Applications
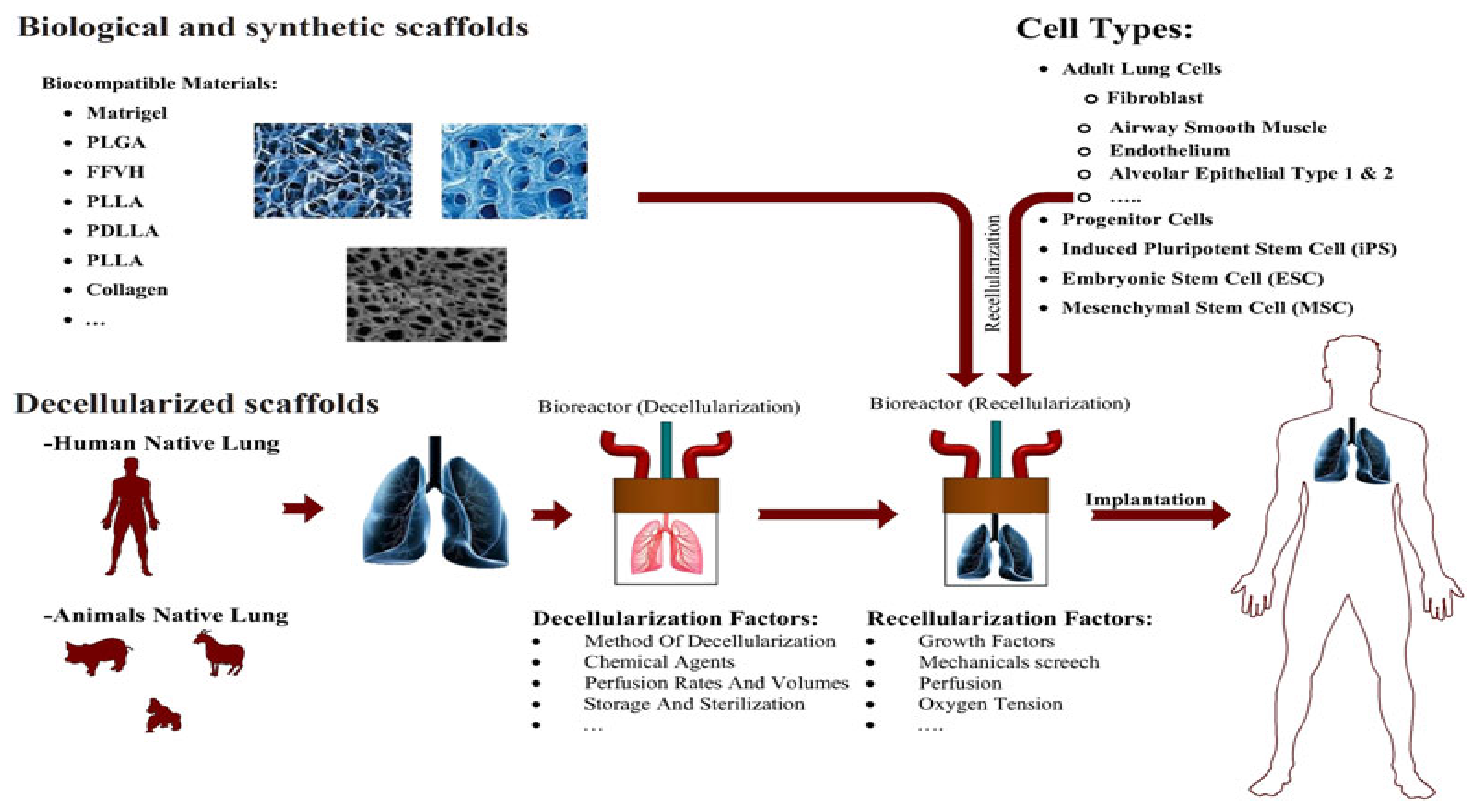
2.5. Injectable Hydrogel Used for Lung-Related Applications
2.6. Injectable Hydrogel Used for Wound Healing Applications
3. Conclusions
4. Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenera-tive medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Bigham, A.; Foroughi, F.; Ghomi, E.R.; Rafienia, M.; Neisiany, R.E.; Ramakrishna, S. The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio-Des. Manuf. 2020, 3, 281–306. [Google Scholar] [CrossRef]
- Eshkalak, S.K.; Ghomi, E.R.; Dai, Y.; Choudhury, D.; Ramakrishna, S. The role of three-dimensional printing in healthcare and medicine. Mater. Des. 2020, 194, 108940. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Eshkalak, S.K.; Singh, S.; Chinnappan, A.; Ramakrishna, S.; Narayan, R. Fused filament printing of specialized biomed-ical devices: A state-of-the art review of technological feasibilities with PEEK. Rapid Prototyp. J. 2021, 194, 108940. [Google Scholar]
- Chang, G.; Chen, Y.; Li, Y.; Li, S.; Huang, F.; Shen, Y.; Xie, A. Self-healable hydrogel on tumor cell as drug delivery system for local-ized and effective therapy. Carbohydr. Polym. 2015, 122, 336–342. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Pishavar, E.; Luo, H.; Naserifar, M.; Hashemi, M.; Toosi, S.; Atala, A.; Ramakrishna, S.; Behravan, J. Advanced Hydrogels as Exosome Delivery Systems for Osteogenic Differentiation of MSCs: Application in Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 6203. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Sun, F. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 2010, 6, 486–493. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, R.M.; Willie, J.A.; Epps, T.H. 100th Anniversary of Macromolecular Science Viewpoint: Polymers from Lignocellulosic Biomass. Current Challenges and Future Opportunities. ACS Macro Lett. 2020, 9, 476–493. [Google Scholar] [CrossRef]
- Guan, Q.; Lin, G.; Gong, Y.; Wang, J.; Tan, W.; Bao, D.; Liu, Y.; You, Z.; Sun, X.; Wen, Z.; et al. Highly efficient self-healable and dual responsive hydrogel-based deformable triboelectric nanogenerators for wearable electronics. J. Mater. Chem. A 2019, 7, 13948–13955. [Google Scholar] [CrossRef]
- Shao, C.; Meng, L.; Cui, C.; Yang, J. An integrated self-healable and robust conductive hydrogel for dynamically self-adhesive and highly conformable electronic skin. J. Mater. Chem. C 2019, 7, 15208–15218. [Google Scholar] [CrossRef]
- Shur, M.; Fallegger, F.; Pirondini, E.; Roux, A.; Bichat, A.; Barraud, Q.; Courtine, G.; Lacour, S.P. Soft Printable Electrode Coating for Neural Interfaces. ACS Appl. Bio Mater. 2020, 3, 4388–4397. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef] [Green Version]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [Green Version]
- Ghomi, E.R.; Khosravi, F.; Neisiany, R.E.; Singh, S.; Ramakrishna, S. Future of additive manufacturing in healthcare. Curr. Opin. Biomed. Eng. 2021, 17, 100255. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Guo, L.; Wang, Y.; Feng, L. Intelligent Hybrid Hydrogels for Rapid in Situ Detection and Photothermal Ther-apy of Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 39685–39694. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, E.R.; Khorasani, S.N.; Kichi, M.K.; Dinari, M.; Ataei, S.; Enayati, M.H.; Koochaki, M.S.; Neisiany, R.E. Synthesis and characterization of TiO2/acrylic acid-co-2-acrylamido-2-methyl propane sulfonic acid nanogel composite and investigation its self-healing performance in the epoxy coatings. Colloid Polym. Sci. 2020, 298, 213–223. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Neisiany, R.E.; Khorasani, S.N.; Dinari, M.; Ataei, S.; Koochaki, M.S.; Ramakrishna, S. Development of an epoxy self-healing coating through the incorporation of acrylic acid-co-acrylamide copolymeric gel. Prog. Org. Coat. 2020, 149, 105948. [Google Scholar] [CrossRef]
- Azevedo, S.; Costa, M.; Andersen, A.; Choi, I.S.; Birkedal, H.; Mano, J.F. Bioinspired Ultratough Hydrogel with Fast Recovery, Self-Healing, Injectability and Cytocompatibility. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, H.; Ma, P.X.; Guo, B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12, 1224–1246. [Google Scholar] [CrossRef]
- Lim, J.; Lin, Q.; Xue, K.; Loh, X. Recent advances in supramolecular hydrogels for biomedical applications. Mater. Today Adv. 2019, 3, 100021. [Google Scholar] [CrossRef]
- Gačanin, J.; Hedrich, J.; Sieste, S.; Glaßer, G.; Lieberwirth, I.; Schilling, C.; Fischer, S.; Barth, H.; Knöll, B.; Synatschke, C.V.; et al. Autonomous Ultrafast Self-Healing Hydrogels by pH-Responsive Functional Nanofiber Gelators as Cell Matrices. Adv. Mater. 2018, 31, e1805044. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Defini-tions, concepts, advances, and outlook. Bone Res. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Hunt, J.A.; Chen, R.; Van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, e1800313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Hamann, A.; Nguyen, A.; Pannier, A.K. Nucleic acid delivery to mesenchymal stem cells: A review of nonviral methods and applications. J. Biol. Eng. 2019, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pishavar, E.; Copus, J.S.; Atala, A.; Lee, S.J. Comparison Study of Stem Cell-Derived Extracellular Vesicles for Enhanced Osteo-genic Differentiation. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef]
- Pishavar, E.; Oroojalian, F.; Salmasi, Z.; Hashemi, E.; Hashemi, M. Recent advances of dendrimer in targeted delivery of drugs and genes to stem cells as cellular vehicles. Biotechnol. Prog. 2021, e3174. [Google Scholar] [CrossRef]
- Pishavar, E.; Attaranzadeh, A.; Alibolandi, M.; Ramezani, M.; Hashemi, M. Modified PAMAM vehicles for effective TRAIL gene delivery to colon adenocarcinoma: In vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, S503–S513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte Campos, D.F.; Bonnin Marquez, A.; O’Seanain, C.; Fischer, H.; Blaeser, A.; Vogt, M.; Corallo, D.; Aveic, S. Exploring cancer cell behavior in vitro in three-dimensional multicellular bioprintable collagen-based hydrogels. Cancers 2019, 11, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, R.; Schmidt, S.K.; Hazur, J.; Detsch, R.; Maurer, E.; Boccaccini, A.R.; Hauptstein, J.; Teßmar, J.; Blunk, T.; Schrüfer, S.; et al. Comparison of Hydrogels for the Development of Well-Defined 3D Cancer Models of Breast Cancer and Melanoma. Cancers 2020, 12, 2320. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepato-cellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S. Stimuli-Responsive Hydrogels for Cancer Treat-ment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, X.; Park, S.; Jin, X.; Ma, P.X. Rapid Self-Integrating, Injectable Hydrogel for Tissue Complex Regeneration. Adv. Health Mater. 2015, 4, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N.; et al. Injectable and In Situ-Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of In Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 17936–17948. [Google Scholar] [CrossRef] [PubMed]
- Jonker, A.M.; Löwik, D.; Van Hest, J.C.M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2011, 24, 759–773. [Google Scholar] [CrossRef]
- Upadhyay, A.; Kandi, R.; Rao, C.P. Injectable, Self-Healing, and Stress Sustainable Hydrogel of BSA as a Functional Biocompatible Material for Controlled Drug Delivery in Cancer Cells. ACS Sustain. Chem. Eng. 2018, 6, 3321–3330. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Gou, Y.; Wang, X.; Zhao, X.; Tao, L. Improving tumor chemotherapy effect using an injectable self-healing hydrogel as drug carrier. Polym. Chem. 2017, 8, 5071–5076. [Google Scholar] [CrossRef]
- Li, T.; Li, C.; Ruan, Z.; Xu, P.; Yang, X.; Yuan, P.; Wang, Q.; Yan, L. Polypeptide-conjugated second near-infrared organic fluorophore for image-guided photothermal therapy. ACS Nano 2019, 13, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Shen, T.; Kirillov, A.; Zhang, Y.; Shan, C.; Li, X.; Liu, W.; Tang, Y. Self-Assembled Upconversion Nanoparticle Clusters for NIR-controlled Drug Release and Synergistic Therapy after Conjugation with Gold Nanoparticles. Inorg. Chem. 2017, 56, 5295–5304. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Tong, L.; Xu, Y.; Zhang, J.; Zhai, X.; Su, P.; Weng, L.; Wang, L. Mussel-inspired functionalization of semiconducting polymer nanoparticles for amplified photoacoustic imaging and photothermal therapy. Nanoscale 2019, 11, 14727–14733. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, N.; Yuan, W. NIR/Thermoresponsive Injectable Self-Healing Hydrogels Containing Polydopamine Nanoparticles for Efficient Synergistic Cancer Thermochemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 9118–9131. [Google Scholar] [CrossRef]
- Li, Q.; Wen, J.; Liu, C.; Jia, Y.; Wu, Y.; Shan, Y.; Qian, Z.; Liao, J. Graphene-Nanoparticle-Based Self-Healing Hydrogel in Preventing Postopera-tive Recurrence of Breast Cancer. ACS Biomater. Sci. Eng. 2019, 5, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Pishavar, E.; Ramezani, M.; Hashemi, M. Co-delivery of doxorubicin and TRAIL plasmid by modified PAMAM dendrimer in colon cancer cells, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2019, 45, 1931–1939. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Pal, S.; Kumar, S.; Kar, A.; Awasthi, A.K.; Naaz, A.; Srivastava, A.; Bajaj, A. Injectable, Self-Healing Chimeric Catechol-Fe(III) Hydrogel for Localized Combination Cancer Therapy. ACS Biomater. Sci. Eng. 2017, 3, 3404–3413. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Mou, Q.; Yan, D.; Zhu, X.; Zhang, C. Floxuridine-containing nucleic acid nanogels for anticancer drug delivery. Nanoscale 2018, 10, 8367–8371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, Y.; Yuan, S.; Xu, X.; Wu, Z.; Wu, Z.; Qi, X. ATP responsive DNA nanogels grown on biocompatible branches for anticancer drug delivery. Soft Matter 2019, 15, 3655–3658. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-Silva, T.L.; Lei, Y.L.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled release of a cyclic di-nucleotide for enhanced cancer immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; Ye, H.; Yu, S.; He, C.; Chen, X. Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. J. Control. Release 2017, 255, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zhang, X.; Yu, S.; Wen, D.; Hu, Q.; Ye, Y.; Bomba, H.; Hu, X.; Liu, Z.; et al. In situ formed reactive oxygen species–responsive scaffold with gem-citabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M. Rational Design of Hydrogels to Enhance Osteogenic Potential. Chem. Mater. 2020, 32, 9508–9530. [Google Scholar] [CrossRef] [PubMed]
- Mauffrey, C.; Barlow, B.T.; Smith, W. Management of segmental bone defects. JAAOS J. Am. Acad. Orthop. Surg. 2015, 23, 143–153. [Google Scholar]
- Khosravi, F.; Khorasani, S.N.; Khalili, S.; Neisiany, R.E.; Ghomi, E.R.; Ejeian, F.; Das, O.; Nasr-Esfahani, M.H. Development of a Highly Proliferated Bilayer Coating on 316L Stainless Steel Implants. Polymers 2020, 12, 1022. [Google Scholar] [CrossRef]
- Khosravi, F.; Khorasani, S.N.; Ghomi, E.R.; Kichi, M.K.; Zilouei, H.; Farhadian, M.; Neisiany, R.E. A bilayer GO/nanofibrous biocomposite coating to enhance 316L stainless steel corrosion performance. Mater. Res. Express 2019, 6, 086470. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Adokoh, C.K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Expert Opin. Drug Deliv. 2017, 15, 77–91. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, Z.; Yang, B.; Feng, Q.; Xu, X.; Yuan, W.; Li, X.; Chen, X.; Duan, L.; Wang, D.; et al. Adaptable Hydrogels Mediate Cofactor-Assisted Activation of Bi-omarker-Responsive Drug Delivery via Positive Feedback for Enhanced Tissue Regeneration. Adv. Sci. 2018, 5, 1800875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, Q.; Xu, J.; Xu, X.; Tian, F.; Yeung, K.W.K.; Bian, L. Self-assembled injectable nanocomposite hydrogels stabilized by bisphosphonate-magnesium (Mg2+) coordination regulates the differentiation of encapsulated stem cells via dual crosslinking. Adv. Funct. Mater. 2017, 27, 1701642. [Google Scholar] [CrossRef]
- Tanaka, S.; Wakabayashi, K.; Fukushima, K.; Yukami, S.; Maezawa, R.; Takeda, Y.; Tatsumi, K.; Ohya, Y.; Kuzuya, A. Intelligent, Biodegradable, and Self-Healing Hydrogels Utilizing DNA Quadruplexes. Chem. Asian J. 2017, 12, 2388–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Zeng, Q.; Desai, M.S.; Jin, H.-E.; Lee, J.H.; Chang, J.; Lee, S.-W. Self-healing elastin–bioglass hydrogels. Biomacromolecules 2016, 17, 2619–2625. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Shao, Y.; Zhou, X.; Wu, Y.; Yang, Z.; Li, Z.; Weil, T.; Liu, D. A Writable Polypeptide-DNA Hydrogel with Rationally Designed Multi-modification Sites. Small 2014, 11, 1138–1143. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Paul, A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Hsu, E.L.; Mendoza, M.; Ghodasra, J.; Nickoli, M.S.; Ashtekar, A.; Polavarapu, M.; Babu, J.; Riaz, R.M.; Nicolas, J.D.; et al. Gel Scaffolds of BMP-2-Binding Peptide Amphiphile Nanofibers for Spinal Arthrodesis. Adv. Health Mater. 2015, 4, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Colino, A.; Arias, F.J.; Alonso, M.; Rodríguez-Cabello, J.C. Self-Organized ECM-Mimetic Model Based on an Amphiphilic Multiblock Silk-Elastin-Like Corecombinamer with a Concomitant Dual Physical Gelation Process. Biomacromolecules 2014, 15, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A Customized Self-Assembling Peptide Hydrogel for Dental Pulp Tissue Engineering. Tissue Eng. Part A 2012, 18, 176–184. [Google Scholar] [CrossRef]
- Phipps, M.C.; Monte, F.; Mehta, M.; Kim, H.K.W. Intraosseous delivery of bone morphogenic protein-2 using a self-assembling peptide hydrogel. Biomacromolecules 2016, 17, 2329–2336. [Google Scholar] [CrossRef]
- Gačanin, J.; Kovtun, A.; Fischer, S.; Schwager, V.; Quambusch, J.; Kuan, S.L.; Liu, W.; Boldt, F.; Ling, K.S.; Yang, Z.; et al. Spatiotemporally Controlled Release of Rho-Inhibiting C3 Toxin from a Protein-DNA Hybrid Hydrogel for Targeted Inhibition of Osteoclast Formation and Activity. Adv. Health Mater. 2017, 6. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Zhu, W.; Xu, Z.; Fuchs, S.; Hilborn, J.; Zhu, L.; Ma, Q.; Wang, Y.; Weng, X.; et al. Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-Healing Conductive Injectable Hydrogels with Antibacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef]
- Bastings, M.M.C.; Koudstaal, S.; Kieltyka, R.E.; Nakano, Y.; Pape, A.C.H.; Feyen, D.A.M.; van Slochteren, F.; Doevendans, P.A.; Sluijter, J.; Meijer, E.; et al. A Fast pH-Switchable and Self-Healing Supramolecular Hydrogel Carrier for Guided, Local Catheter Injection in the Infarcted Myocardium. Adv. Health Mater. 2014, 3, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuo, T.-W.; Wei, T.-C.; Liu, Y.-L. Electrically driven self-healing polymers based on reversible guest-host complexation of β-cyclodextrin and ferrocene. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3395–3403. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, Y.; Chung, J.J.; Wang, T.; Gaffey, A.C.; Lu, M.; Cavanaugh, C.A.; Zhou, S.; Kanade, R.; Atluri, P.; et al. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat. Biomed. Eng. 2017, 1, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Rodell, C.B.; MacArthur, J.W., Jr.; Dorsey, S.M.; Wade, R.J.; Wang, L.L.; Woo, Y.J.; Burdick, J.A. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 2015, 25, 636–644. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-nanotube-embedded hydrogel sheets for engi-neering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Chen, J.; Li, L.; Li, M.; Wei, X.; Tan, B.; Shang, Y.; Fan, G.; Wang, W.; Liu, W. Conductive Hydrogen Sulfide-Releasing Hydrogel Encapsulating ADSCs for Myocardial Infarction Treatment. ACS Appl. Mater. Interfaces 2019, 11, 14619–14629. [Google Scholar] [CrossRef]
- Wu, T.; Cui, C.; Huang, Y.; Liu, Y.; Fan, C.; Han, X.; Yang, Y.; Xu, Z.; Liu, B.; Fan, G.; et al. Coadministration of an adhesive conductive hydrogel patch and an in-jectable hydrogel to treat myocardial infarction. ACS Appl. Mater. Interfaces 2019, 12, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Chung, J.J.; Li, E.C.; Uman, S.; Atluri, P.; Burdick, J.A. Injectable and protease-degradable hydrogel for siRNA sequestra-tion and triggered delivery to the heart. J. Control. Release 2018, 285, 152–161. [Google Scholar] [CrossRef]
- Waters, R.; Alam, P.; Pacelli, S.; Chakravarti, A.R.; Ahmed, R.P.; Paul, A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 2018, 69, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Alves, Y.V.; Sperling, L.E.; Pranke, P. Nanotechnology for the Treatment of Spinal Cord Injury. Tissue Eng. Part B Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wong, C.-W.; Hsu, S.-H. An Injectable, Electroconductive Hydrogel/Scaffold for Neural Repair and Motion Sensing. Chem. Mater. 2020, 32, 10407–10422. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, Y.-H.; Huang, A.P.-H.; Hsu, S.-H. Semi-Interpenetrating Polymer Network of Hyaluronan and Chitosan Self-Healing Hydrogels for Central Nervous System Repair. ACS Appl. Mater. Interfaces 2020, 12, 40108–40120. [Google Scholar] [CrossRef]
- Jacob, R.S.; Ghosh, D.; Singh, P.K.; Basu, S.K.; Jha, N.N.; Das, S.; Sukul, P.K.; Patil, S.; Sathaye, S.; Kumar, A.; et al. Self healing hydrogels composed of amyloid nano fibrils for cell culture and stem cell differentiation. Biomaterials 2015, 54, 97–105. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Huang, C.-F.; Wei, Y.; Hsu, S.-H. Novel chitosan–cellulose nanofiber self-healing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater. 2019, 11, 25. [Google Scholar] [CrossRef]
- Kornev, V.A.; Grebenik, E.A.; Solovieva, A.B.; Dmitriev, R.I.; Timashev, P.S. Hydrogel-assisted neuroregeneration approaches to-wards brain injury therapy: A state-of-the-art review. Comput. Struct. Biotechnol. J. 2018, 16, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Hoban, D.B.; Newland, B.; Moloney, T.C.; Howard, L.; Pandit, A.; Dowd, E. The reduction in immunogenicity of neurotrophin overexpressing stem cells after intra-striatal transplantation by encapsulation in an in situ gelling collagen hydrogel. Biomaterials 2013, 34, 9420–9429. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.Y.; Zhao, W.; Zhao, J.; Zhang, P.; Zhang, Q. (Invited) Self-healing Polysaccharide-based Hydrogels as Injectable Carriers for Neural Stem Cells. ECS Meet. Abstr. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Hou, C.; Duan, Y.; Zhang, Q.; Wang, H.; Li, Y. Bio-applicable and electroactive near-infrared laser-triggered self-healing hydrogels based on graphene networks. J. Mater. Chem. 2012, 22, 14991–14996. [Google Scholar] [CrossRef]
- Qiao, S.; Liu, Y.; Han, F.; Guo, M.; Hou, X.; Ye, K.; Deng, S.; Shen, Y.; Zhao, Y.; Wei, H.; et al. An intelligent neural stem cell delivery system for neurodegenerative dis-eases treatment. Adv. Healthc. Mater. 2018, 7, 1800080. [Google Scholar] [CrossRef]
- Dunphy, S.E.; Bratt, J.A.; Akram, K.M.; Forsyth, N.R.; El Haj, A.J. Hydrogels for lung tissue engineering: Biomechanical properties of thin collagen–elastin constructs. J. Mech. Behav. Biomed. Mater. 2014, 38, 251–259. [Google Scholar] [CrossRef]
- Tebyanian, H.; Karami, A.; Nourani, M.R.; Motavallian, E.; Barkhordari, A.; Yazdanian, M.; Seifalian, A. Lung tissue engineering: An update. J. Cell. Phys. 2019, 234, 19256–19270. [Google Scholar] [CrossRef]
- Pouliot, R.A.; Young, B.; Link, P.; Park, H.E.; Kahn, A.R.; Shankar, K.; Schneck, M.B.; Weiss, D.J.; Heise, R.L. Porcine Lung-Derived Extracellular Matrix Hydrogel Properties Are Dependent on Pepsin Digestion Time. Tissue Eng. Part C Methods 2020, 26, 332–346. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Akhtar, S.R.; Caldwell, E.; Rubenfeld, G.D. Incidence and outcomes of pediatric acute lung injury. Pediatrics 2009, 124, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and Outcomes of Acute Lung Injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [Green Version]
- Laffey, J.G.; Matthay, M.A. FiftyYearsofResearchinARDS.Cell-based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am. J. Respir. Crit. Care Med. 2017, 196, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Levy, M.; Artigas, A. Management of severe sepsis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2015, 9, 2079–2088. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.L.; Soeder, Y.; Dahlke, M.H. Concise Review: Mesenchymal Stromal Cell-Based Approaches for the Treatment of Acute Respiratory Distress and Sepsis Syndromes. STEM CELLS Transl. Med. 2017, 6, 1141–1151. [Google Scholar] [CrossRef]
- Saini, P.; Atina, J. Ventilator induced lung injury (vili) in acute respiratory distress syndrome (ards).“barotrauma” to “bio-trauma”: Case report. East Afr. Med. J. 2017, 94, 391–397. [Google Scholar]
- Horie, S.; Gonzalez, H.E.; Laffey, J.G.; Masterson, C.H. Cell therapy in acute respiratory distress syndrome. J. Thorac. Dis. 2018, 10, 5607–5620. [Google Scholar] [CrossRef]
- Le Blanc, K.; Ringden, O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med. 2007, 262, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Link, P.A.; Pouliot, R.A.; Mikhaiel, N.S.; Young, B.; Heise, R.L. Tunable Hydrogels from Pulmonary Extracellular Matrix for 3D Cell Culture. J. Vis. Exp. 2017, 2017, 55094. [Google Scholar] [CrossRef]
- Shulimzon, T.R.; Giladi, S.; Zilberman, M. Catheter Injectable Hydrogel-Based Scaffolds for Tissue Engineering Applications in lung disease. Isr. Med. Assoc. J. IMAJ 2020, 22, 736–740. [Google Scholar]
- Shafiee, A.; Moradi, L.; Lim, M.; Brown, J. Coronavirus disease 2019, A tissue engineering and regenerative medicine perspective. STEM CELLS Transl. Med. 2021, 10, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.C.; Powell, A.E.; Roth, G.A.; Ou, B.S.; Meany, E.L.; Grosskopf, A.K.; Adamska, J.; Picece, V.C.; d’Aquino, A.I.; Pulendran, B.; et al. Hydrogel-based slow release of a receptor-binding domain subunit vaccine elicits neutralizing antibody responses against SARS-CoV-2. bioRxiv 2021. [Google Scholar]
- Wu, Y.; Wei, W.; Zhou, M.; Wang, Y.; Wu, J.; Ma, G.; Su, Z. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials 2012, 33, 2351–2360. [Google Scholar] [CrossRef]
- Huang, Z.; Kłodzińska, S.N.; Wan, F.; Nielsen, H.M. Nanoparticle-mediated pulmonary drug delivery: State of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv. Transl. Res. 2021, 1–21. [Google Scholar]
- González-Alvarez, M.; Gonzalez-Alvarez, I.; Bermejo, M. Hydrogels: An interesting strategy for smart drug delivery. Ther. Deliv. 2013, 4, 157–160. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, Z.; Zhu, C.; Song, X.; Qin, Y.; Zhu, M.; Zhang, T.; Cui, W.; Tang, H.; Zheng, H. Injectable and Self-Healing Hydrogel with Anti-Bacterial and Anti-Inflammatory Properties for Acute Bacterial Rhinosinusitis with Micro Invasive Treatment. Adv. Health Mater. 2020, 9. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Thambi, T.; Yin, Y.; Kim, S.H.; Nguyen, T.L.; Phan, V.G.; Kim, J.; Jeong, J.H.; Lee, D.S. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials 2020, 230, 119599. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.-G.; Tran, L.D.; Park, J.S. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Niu, B.; Li, W. Characterization, release, and antioxidant activity of curcu-min-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C 2021, 118, 111324. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin–streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Luo, D. A CRISPR Path to Cutting-Edge Materials. N. Engl. J. Med. 2020, 382, 85–88. [Google Scholar] [CrossRef]







| Bioimplant | Scaffold Fabrication | Materials | Application | Ref. |
|---|---|---|---|---|
| SUPPRELIN LA | Hydrogel fibers | 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, and 50 mg of histrelin | The treatment of CPP during 12 months | [6] |
| PHEMA | Hydrogel fibers | PHEMA/beta-CD hydrogels | Ocular drug admission | [8] |
| HS-TENG | Freeze drying | PVA/PDAP/MWCNT | Wearable application | [10] |
| TC-Gel | Hydrogel nanoparticles | TA@CNCs/PANI | Sensor for electronic skin devices | [11] |
| PDMS/PEDOT:PSS/PAA | 3D Printing | polyacrylamide and poly(3,4-ethylenedioxythiophene) polystyrene sulfonate hydrogel | Neural attachment | [12] |
| Self-Healing Mechanisms | Materials | Application | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Imine bond | CEC/PEGDA20 hydrogel | Kinetic release drug Dox (2.0 mg Dox/mL hydrogel) | Suitable gelation time and mechanical strength | - | [35] |
| Thiol bond | CSSH/Cur-Lip | Kinetic release drug Curcomin (200 μM) | Good cytocompatibility | - | [41] |
| Thiol bond | BSA hydrogel | Sustain release drug DOX 100 µg/mL of Dox in 200 µL hydrogel | Biocompatibility, biodegradable, viscoelasticity | Low mechanical strength | [43] |
| Imine bond | Chitosan-PEG | Sustain release TOX(0.37 g TOX) was dissolved in 1800 μL | Biocompatibility, biodegradable | Low mechanical strength | [44] |
| Imine bond | PEI/PDMAEMA/PDA | Sustain release DOX(1 mg/mL) | Biocompatibility, biodegradable, thermosensitive | - | [48] |
| Imine bond | CSMA/BPEI-GO | Loading efficiency 60% (DOX) | Biocompatibility, biodegradable, thermosensitive | - | [49] |
| Ionic interaction | (CAT-Gel)/Fe | DOX (5 mg/kg) and DTX (25 mg/kg) entrapped in CAT-Gel | Biocompatible, non-hemolytic nature | - | [51] |
| Ionic interaction | MDP | CDN (20 μg/30 μL) | Biocompatible, sustaine release drug | Low mechanical strength | [54] |
| Enzymatic reaction | Fibrin gel | aCD47-Cy5.5 (50 μ per mouse) | Biocompatible, in situ formation therapeutic gel at the tumor resection site, inducing systemic immunological responses | - | [55] |
| Imine bond | mPEG-b-PELG | Co-delivery IL-15 1 μg/mL and CDDP 10 μg/mL in vitro and hydrogel co-loaded with IL-15 (0.5 mg·kg−1) and CDDP (1 mg·kg−1) (Gel + IL-15/CDDP) in vivo | Biocompatibility, biodegradable, In-situ gelation process at body temperature | - | [56] |
| Self-Healing Mechanisms | Materials | Application(s) | Ref. |
|---|---|---|---|
| Imine bond | Nanocomposite oxidized alginate (OA) With DNA nucleotide | Inducing osteogenic differentiation and migration of human adipose-derived stem cells | [72] |
| Hydrogen bonds | Dextran polymer polysaccharide (DEX) with multiple pendant UPy | Bone regeneration in a nude mouse model | [39] |
| Electrostatic interactions | Polypeptide backbone derived from human serum albumin | Treatment osteoporosis | [75] |
| Electrostatic interactions | SF-HA | Bone regeneration as a carrier of cell and drug delivery in the rate model | [78] |
| Thiol-ene | HA-Pam-Mg | Bone regeneration as a Dex drug delivery in a rabbit model | [65] |
| Self-Healing Mechanisms | Materials | Application(s) | Ref. |
|---|---|---|---|
| Hydrogen bonds | UPy-10 k gel | Carrier of growth factors (HGF and IGF-1) for MI | [80] |
| Host-guest | HA-CD/AD | Delivery mi-RNA302 for MI ina mouse infarction model | [82] |
| Host-guest/Thiol | Adamantane-thiol-HA β-cyclodextrin-methacrylate-HA | Tissue repair in pig model of MI | [83] |
| Photo crosslink | CNT-GELMA | Enhancing CM and stable beatine rate for MI | [84] |
| Imine bond | ALG-CHO and amine gelatine hydrogel | Improving neovascularization in MI | [85] |
| Imine bond | Mixture patch of gelatin-dopamine (GelDA) with dopamine-modified polypyrrole (DA-PPy) in hyaluronic hydrogel | Mechanical support and promoting angiogenesis MI | [86] |
| Imine bond | CS-AT-PEG-DA | Improving inflammatory reaction MI | [79] |
| Hydrazone band | ALD-HA/HA-MMP-HYD | Carrier of siRNA (MMP2) for MI in a rat infarction model | [87] |
| Self-Healing Mechanisms | Materials | Application(s) | Ref. |
|---|---|---|---|
| Imine bond | CEC-DFPU/DCP | Neural repair in zebrafish brain injury model | [90] |
| Imine bond | SIPN | Neural repair in the zebrafish brain model | [91] |
| Imine bond | CS–CNF | Neural stem cells encapsulation and differentiation | [93] |
| Imine bond | collagen-4S-StarPEG | Stem cell therapy for the degenerated CNS | [95] |
| Ionic interaction | calcium alginate gel beads | Neural stem cells encapsulation and differentiation in a mouse model | [98] |
| Self-Healing Mechanisms | Materials | Application(s) | Ref. |
|---|---|---|---|
| Ionic interaction | HPMC-C12/PEG-PLA | COVID-19 vaccine for sustained release antigen RBD | [113] |
| Ionic interaction | HTCC hydrogel/split antigen | H5N1 influenza vaccine for sustained release H5N1 antigen | [114] |
| Ionic interaction | CAM@Hydrogel/silver | Antibacterial and anti-inflammatory properties | [117] |
| Imine bond | HA-PCLA | Delivery of pOVA to inhibit human lung carcinoma | [118] |
| Self-Healing Mechanisms | Materials | Application(s) | Ref. |
|---|---|---|---|
| Ionic interaction | Sodium alginate/ZnO | Atioxidant wound healing | [120] |
| Ionic interaction | GT-DA/CS/CNT/Doxy | Infected wounnds healing | [121] |
| Imine bond | N,O-CMC/OCS | Infected wounnds healing | [122] |
| Ionic interaction | PVA/Kaolin | Infected wounnds healing | [123] |
| Ionic interaction | QCSG/GM/GO | Infected wounnds healing | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pishavar, E.; Khosravi, F.; Naserifar, M.; Rezvani Ghomi, E.; Luo, H.; Zavan, B.; Seifalian, A.; Ramakrishna, S. Multifunctional and Self-Healable Intelligent Hydrogels for Cancer Drug Delivery and Promoting Tissue Regeneration In Vivo. Polymers 2021, 13, 2680. https://doi.org/10.3390/polym13162680
Pishavar E, Khosravi F, Naserifar M, Rezvani Ghomi E, Luo H, Zavan B, Seifalian A, Ramakrishna S. Multifunctional and Self-Healable Intelligent Hydrogels for Cancer Drug Delivery and Promoting Tissue Regeneration In Vivo. Polymers. 2021; 13(16):2680. https://doi.org/10.3390/polym13162680
Chicago/Turabian StylePishavar, Elham, Fatemeh Khosravi, Mahshid Naserifar, Erfan Rezvani Ghomi, Hongrong Luo, Barbara Zavan, Amelia Seifalian, and Seeram Ramakrishna. 2021. "Multifunctional and Self-Healable Intelligent Hydrogels for Cancer Drug Delivery and Promoting Tissue Regeneration In Vivo" Polymers 13, no. 16: 2680. https://doi.org/10.3390/polym13162680
APA StylePishavar, E., Khosravi, F., Naserifar, M., Rezvani Ghomi, E., Luo, H., Zavan, B., Seifalian, A., & Ramakrishna, S. (2021). Multifunctional and Self-Healable Intelligent Hydrogels for Cancer Drug Delivery and Promoting Tissue Regeneration In Vivo. Polymers, 13(16), 2680. https://doi.org/10.3390/polym13162680









