Destruction of Chitosan and Its Complexes with Cobalt(II) and Copper(II) Tetrasulphophthalocyanines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
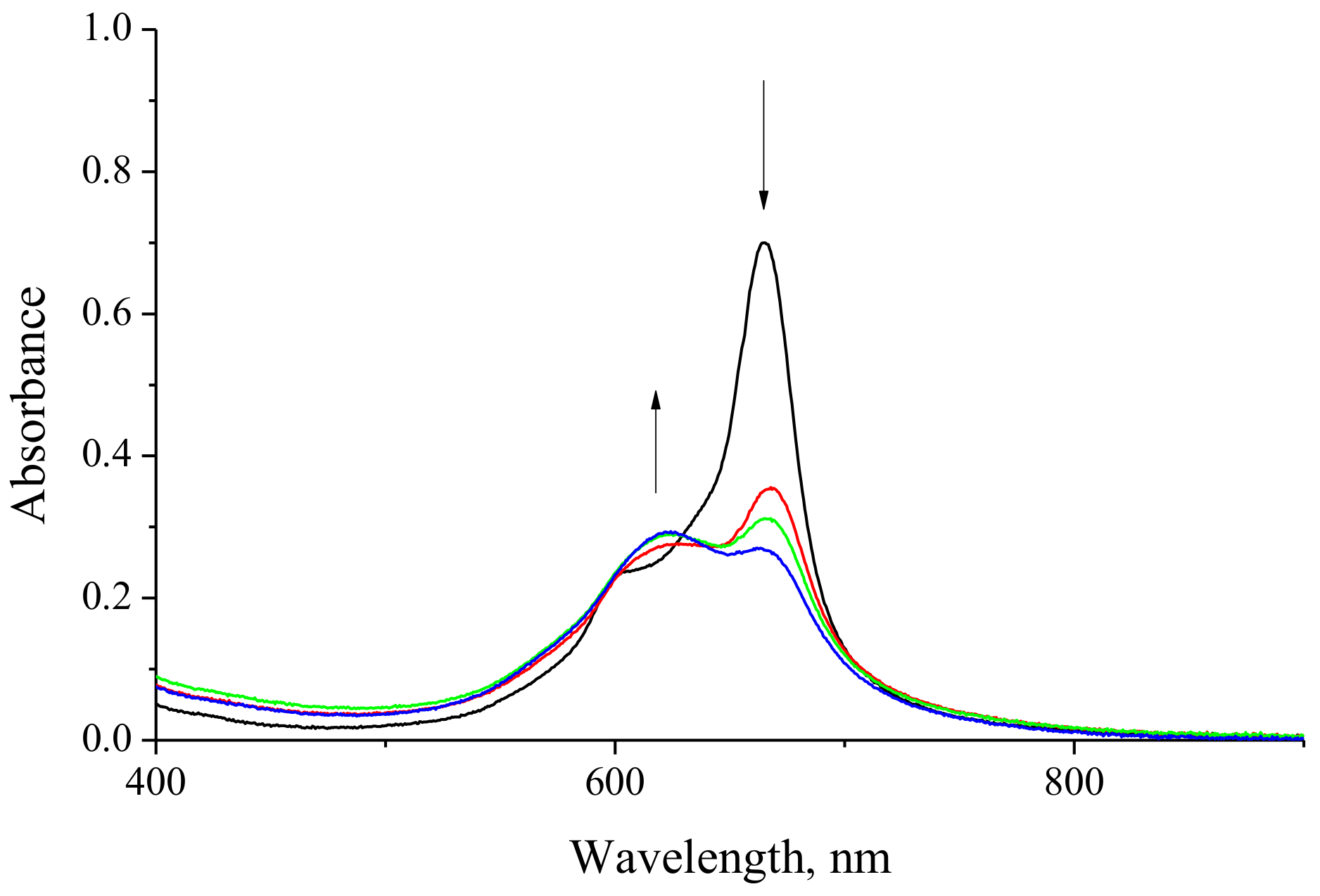
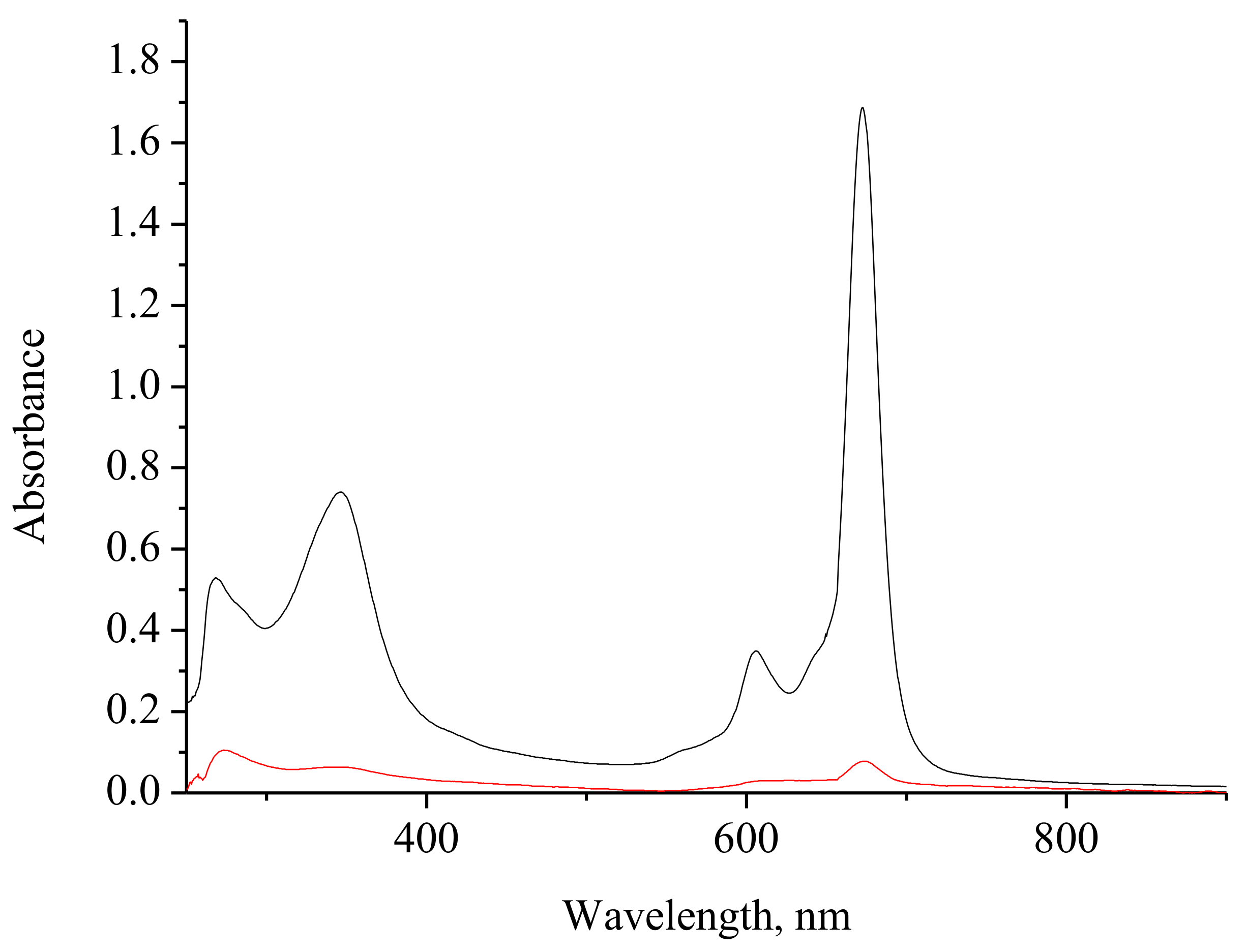
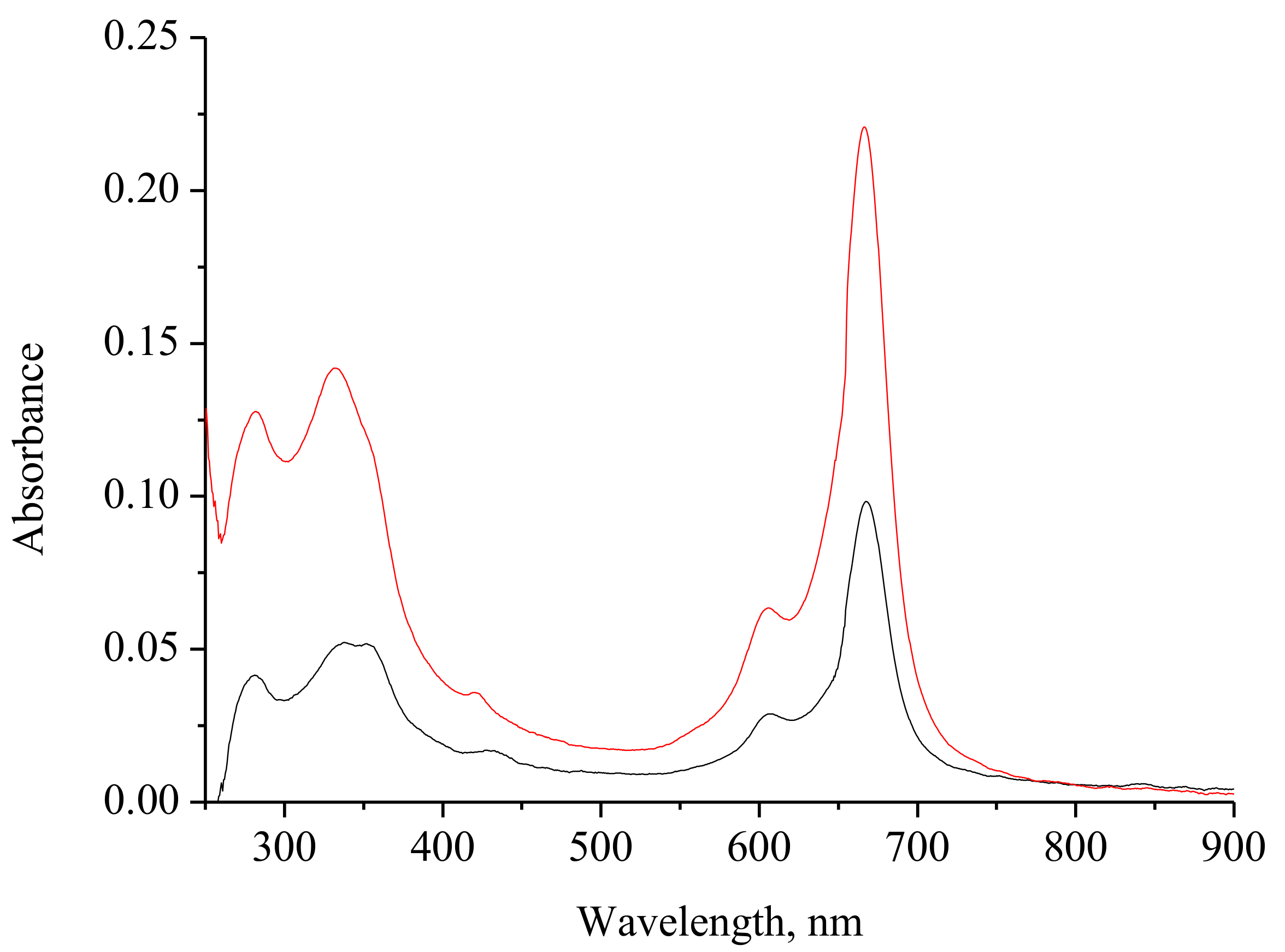
2.2. Spectral Investigation
2.3. TG/DTG
2.4. Mass Spectrometric Analysis
3. Results and Discussion
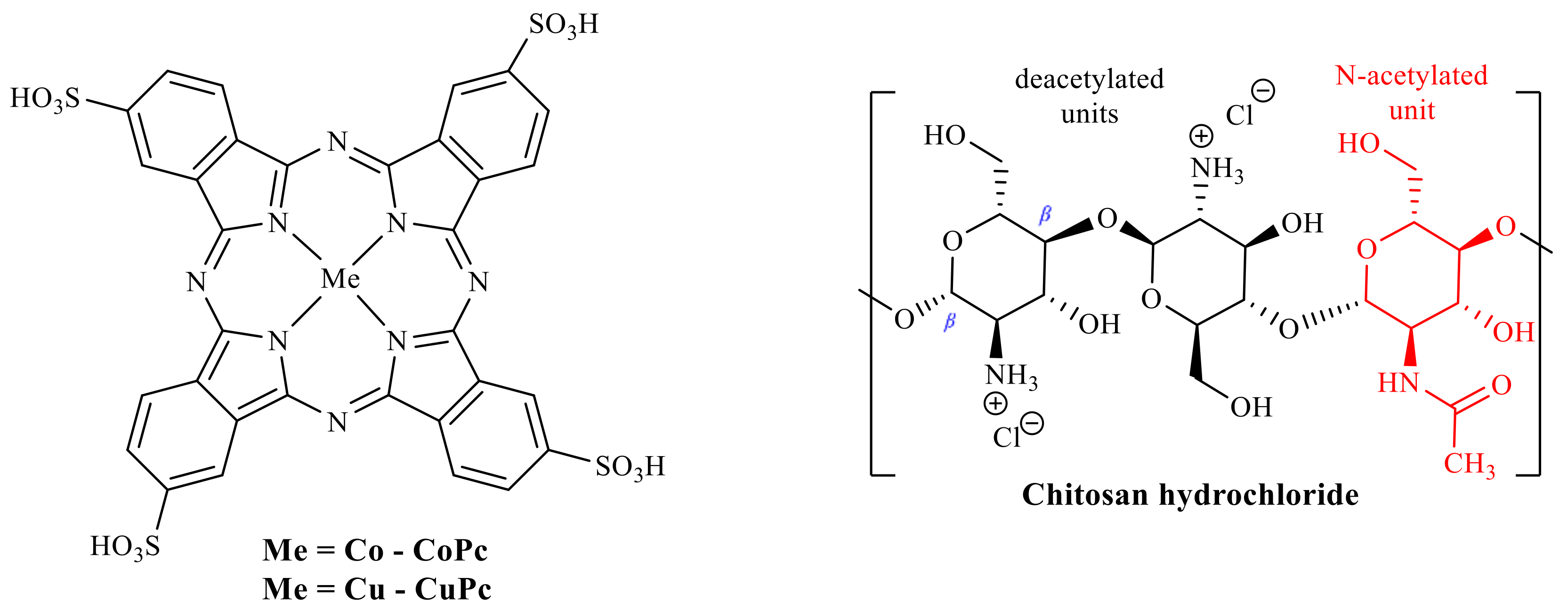
3.1. Preparation of MPc-Chitosan Complexes
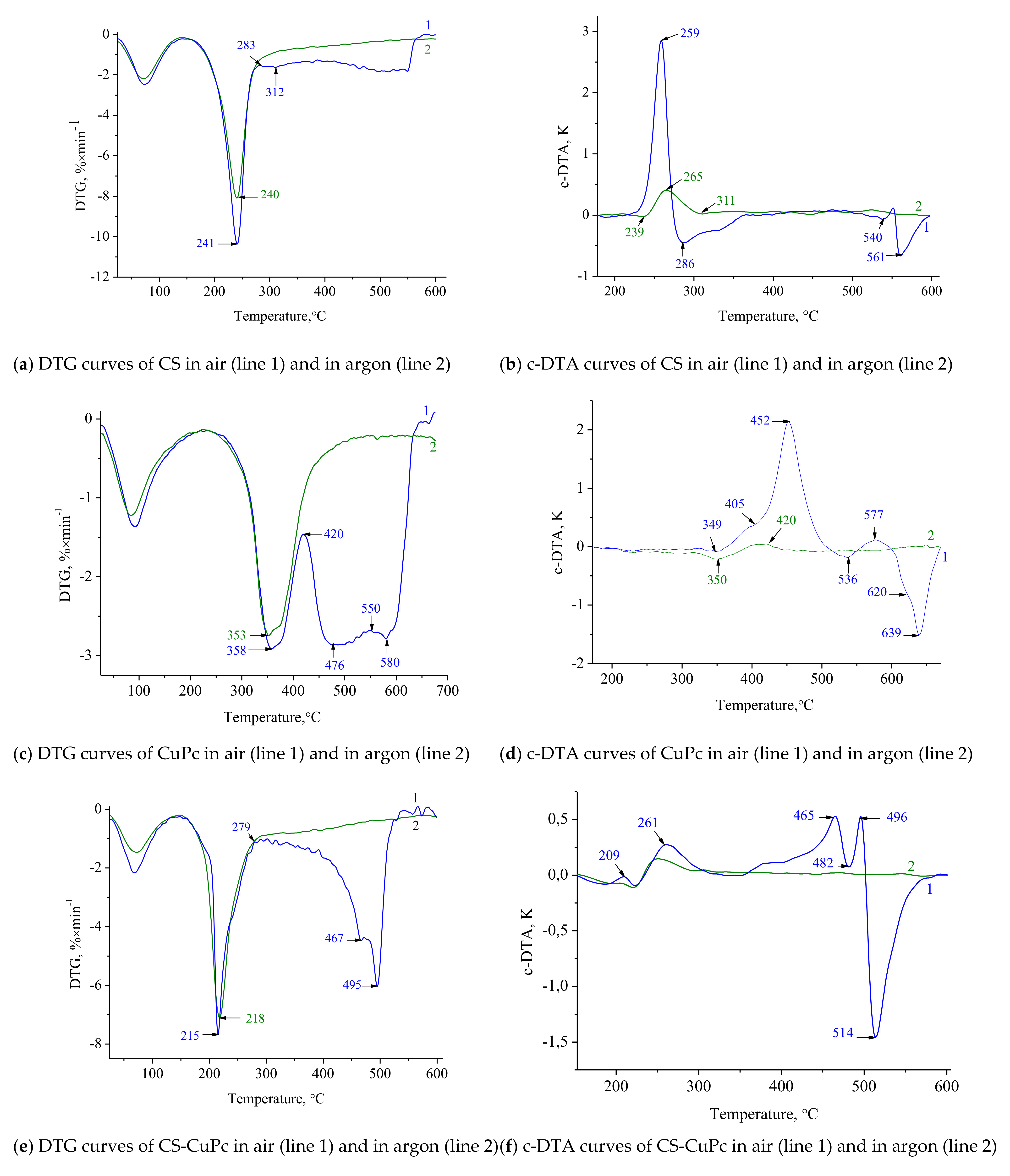
3.2. Thermochemical Study
3.2.1. Pyrolysis and Thermo-Oxidative Destruction of Chitosan
3.2.2. Pyrolysis and Thermal Oxidative Destruction of MPc
3.2.3. Pyrolysis and Thermal Oxidative Destruction of CS-CuPc
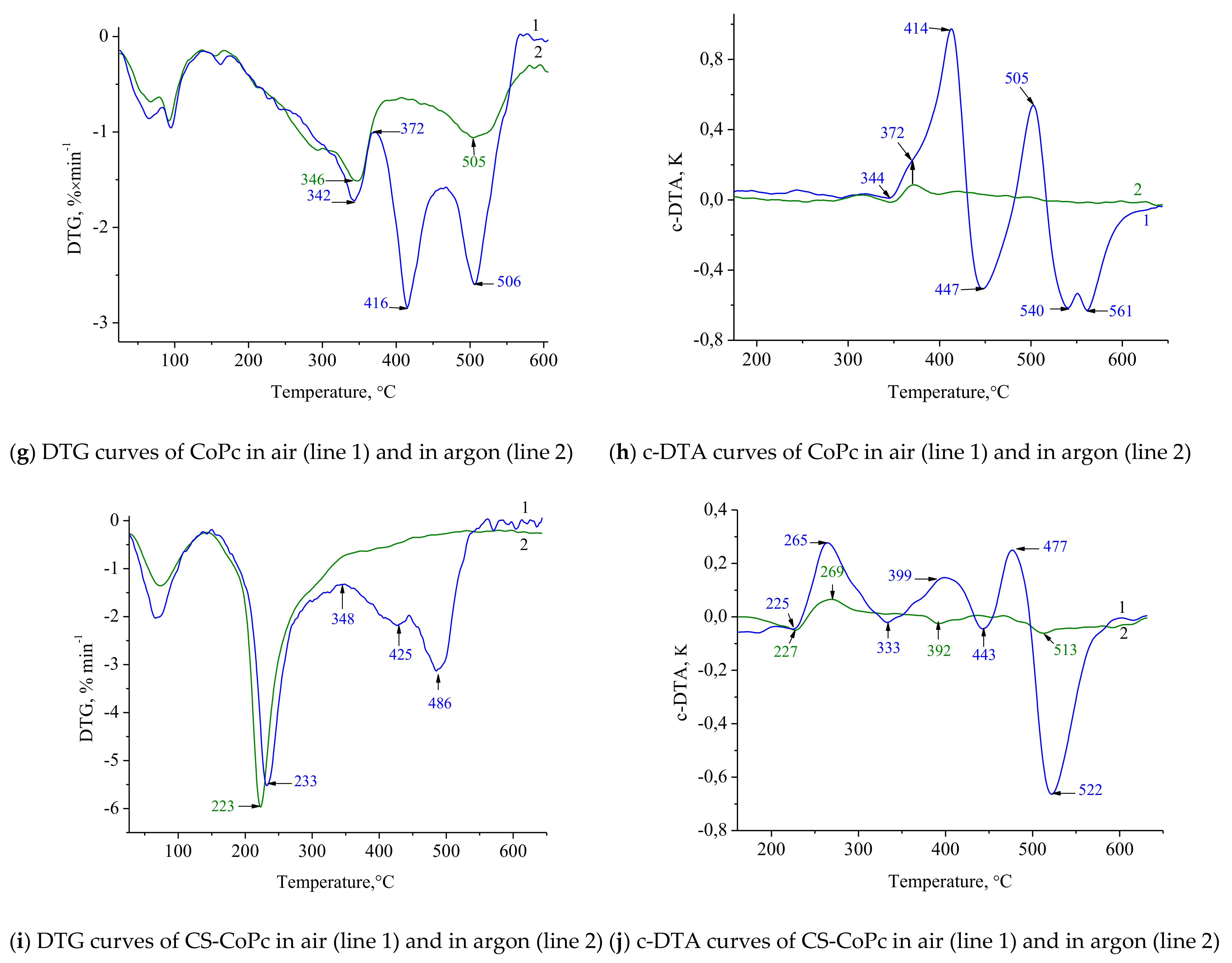
3.2.4. Pyrolysis and Thermal Oxidative Destruction of CS-CoPc
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheidt, W.R.; Dow, W. Molecular Stereochemistry of Phthalocyanatozinc (II). J. Am. Chem. Soc. 1977, 99, 1101–1104. [Google Scholar] [CrossRef]
- Brunet, J.; Pauly, A.; Varenne, C.; Lauron, B. On-Board Phthalocyanine Gas Sensor Microsystem Dedicated to the Monitoring of Oxidizing Gases Level in Passenger Compartments. Sens. Actuators B Chem. 2008, 130, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Gregory, P. Industrial Applications of Phthalocyanines. J. Porphyr. Phthalocyanines 2012, 4, 432–437. [Google Scholar] [CrossRef]
- Bonnett, R. Photosensitizers of the Porphyrin and Phthalocyanine Series for Photodynamic Therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Meunier, B. Metalloporphyrins as Versatile Catalysts for Oxidation Reactions and Oxidative DNA Cleavage. Chem. Rev. 1992, 92, 1411–1456. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, J.; Chen, Y.; Cui, A.; He, M.; Xu, Z.; Chen, Q. Metallophthalocyanine Intercalated Layered Double Hydroxides as an Efficient Catalyst for the Selective Epoxidation of Olefin with Oxygen. Appl. Catal. A General 2017, 542, 191–200. [Google Scholar] [CrossRef]
- Ventura, D.L.; Heller, S.J.; Noworyta, T.D.; Kijanka, K.C.; Belz, B.M. Metallophthalocyanine Catalyzed Olefination of Aldehydes. Tetrahedron Lett. 2018, 60, 302–305. [Google Scholar] [CrossRef]
- Li, F.; Tang, S.; Tang, Z.; Ye, L.; Li, H.; Niu, F.; Sun, X. Synergistic Catalytic Effect of N-Hydroxyphthalimide/Cobalt Tetraamide Phthalocyanine and Its Application for Aerobic Oxidation of Hydrocarbons and Alcohols. Catal. Lett. 2020, 151, 17–26. [Google Scholar] [CrossRef]
- Acar, I.; Bayrak, R.; Saka, E.T.; Bıyıklıoğlu, Z.; Kantekin, H. Novel Metal-Free, Metallophthalocyanines and their Quaternized Derivatives: Synthesis, Spectroscopic Characterization and Catalytic Activity of Cobalt Phthalocyanine in 4-Nitrophenol Oxidation. Polyhedron 2013, 50, 345–353. [Google Scholar] [CrossRef]
- Aktaş, A.; Saka, E.T.; Bıyıklıoğlu, Z.; Acar, I.; Kantekin, H. Investigation of Catalytic Activity of New Co (II) Phthalocya-nine Complexes in Cyclohexene Oxidation using Different Type of Oxidants. J. Organomet. Chem. 2013, 745, 18–24. [Google Scholar] [CrossRef]
- Türk, H.; Çimen, Y. Oxidation of 2, 6-di-tert-Butylphenol with Tert-Butylhydroperoxide Catalyzed by Cobalt (II) Phthalocyanine Tetrasulfonate in a Methanol–Water Mixture and Formation of an Unusual Product 4, 4′-Dihydroxy-3, 3′, 5, 5′-Tetra-Tert-Butylbiphenyl. J. Mol. Catal. A Chem. 2005, 234, 19–24. [Google Scholar] [CrossRef]
- Rao, T.V.; Rao, K.N.; Jain, S.L.; Sain, B. Cobalt Phthalocyanine Mediated Aerobic Oxidation of Thiols: A Simple and Convenient Preparation of Disulphides. Synth. Commun. 2002, 32, 1151–1157. [Google Scholar] [CrossRef]
- Saka, E.T.; Çelik, G.; Sarkı, G.; Kantekin, H. An Efficient Method for the Oxidation of Phenolic Compounds Using New Co(II) and Fe(II) Phthalocyanines. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 161–168. [Google Scholar] [CrossRef]
- Ba Saka, E.T.; Acar, İ.; Kantekin, H.; Kani, İ. Synthesis and Characterization of Peripheral and Non-Peripheral Substituted Co (II) Phthalocyanines and their Catalytic Activity in Styrene Oxidation. Synth. Met. 2013, 169, 12–17. [Google Scholar] [CrossRef]
- Kothari, V.M.; Tazuma, J.J. Selective Autoxidation of Some Phenols Using Salcomines and Metal Phthalocyanines. J. Catal. 1976, 41, 180–189. [Google Scholar] [CrossRef]
- Ağırtaş, M.S.; Karataş, C.; Gümüş, S.; Okumuş, V. Synthesis of Some Novel Phthalocyanines with Methyl 2-(oxy)-2, 2-diphenylacetate Substituents, Evaluation of Their Antioxidant-Antibacterial Activities and Electronic Properties. Z. Anorg. Allg. Chem. 2015, 641, 442–447. [Google Scholar] [CrossRef]
- Yıldırım, N.; Bilgiçli, A.T.; Alici, E.H.; Arabacı, G.; Yarasir, M.N. Formation, Characterization, Aggregation, Fluores-Cence and Antioxidant Properties of Novel Tetrasubstituted Metal-Free and Metallophthalocyanines Bearing (4-(Methylthio) Phenoxy) Moieties. J. Mol. Struct. 2017, 1144, 66–79. [Google Scholar] [CrossRef]
- Zel, A.; Barut, B.; Demirbaş, Ü.; Biyiklioglu, Z. Investigation of DNA Binding, DNA Photocleavage, Topoisomerase I Inhibition and Antioxidant Activities of Water Soluble Titanium (IV) Phthalocyanine Compounds. J. Photochem. Photobiol. B Biol. 2016, 157, 32–38. [Google Scholar]
- Ağırtaş, M.S.; Çelebi, M.; Gümüş, S.; Özdemir, S.; Okumuş, V. New Water Soluble Phenoxy Phenyl Diazenyl Benzoic Acid Substituted Phthalocyanine Derivatives: Synthesis, Antioxidant Activities, Atypical Aggregation Behavior and Electronic Properties. Dye. Pigment. 2013, 99, 423–431. [Google Scholar] [CrossRef]
- Aydın, M.; Alıcı, E.H.; Bilgiçli, A.T.; Yarasir, M.N.; Arabaci, G. Synthesis, Characterization, Aggregation, Fluorescence and Antioxidant Properties of Bearing (4-(Methylthio) Phenylthio) Tetra Substituted Phthalocyanines. Inorg. Chim. Acta 2017, 464, 1–10. [Google Scholar] [CrossRef]
- Amaral, G.P.; Puntel, G.O.; Corte, C.L.D.; Dobrachinski, F.; Barcelos, R.P.; Bastos, L.L.; Ávila, D.S.; Rocha, J.B.T.; da Silva, E.O.; Puntel, R.L.; et al. The Antioxidant Properties of Different Phthalocyanines. Toxicol. Vitr. 2012, 26, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantar, C.; Akal, H.; Kaya, B.; Islamoğlu, F.; Türk, M.; Şaşmaz, S. Novel Phthalocyanines Containing Resorcinol azo Dyes; synthesis, Determination of pKa Values, Antioxidant, Antibacterial and Anticancer Activity. J. Organomet. Chem. 2015, 783, 28–39. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H.; Zulkifli, R.M. Chemical Composition and Biological Activities of Essential Oil of Beilschmiedia Pulverulenta. Pharm. Biol. 2016, 54, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, N.A.; Ahmed, E.M.; El-Nassan, H.B.; Ahmed, O.K.; Al-Abd, A.M. Synthesis and Biological Evaluation of Novel Pyrazoline Derivatives as Anti-Inflammatory and Antioxidant Agents. Arch. Pharmacal Res. 2012, 35, 995–1002. [Google Scholar] [CrossRef]
- Boroujeni, M.B.; Laeini, M.S.; Nazeri, M.T.; Shaabani, A. A Novel and Green in Situ Strategy for the Synthesis of Met-Allophthalocyanines on Chitosan and Investigation Their Catalytic Activity in the CO2 Fixation. Catal. Lett. 2019, 149, 2089–2097. [Google Scholar] [CrossRef]
- Ebani, P.R.; Stefanello, L.; Kuhn, B.L.; Frizzo, C.P.; Burgo, T.A.L.; Kloster, C.L.; Villetti, M.A. Carboxymethyl chi-tosan/ionic Liquid Imidazolium-Based Nanoparticles as Nanocarriers for Zinc Phthalocyanine and its Photodynamic Activity. J. Mol. Liq. 2021, 336, 116874. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Chuang, W.-C.; Yu, K.-H.; Jheng, C.-P.; Lee, C.-I. Sequential Photodynamic Therapy with Phthalocyanine Encapsulated Chitosan-Tripolyphosphate Nanoparticles and Flucytosine Treatment against Candida tropicalis. Pharmaceutics 2019, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Kardumyan, V.; Aksenova, N.; Timofeeva, V.; Krivandin, A.; Shatalova, O.; Dubovik, A.; Plashchina, I.; Timashev, P.; Solovieva, A. Effect of Chitosan on the Activity of Water-Soluble and Hydrophobic Porphyrin Photosensitizers Solubilized by Amphiphilic Polymers. Polymers 2021, 13, 1007. [Google Scholar] [CrossRef]
- Wang, W.; Bo, S.Q.; Li, S.Q.; Qin, W. Determination of the Mark-Houwink Equation for Chitosans with Different Degrees of Deacetylation. Int. J. Biol. Macromol. 1991, 13, 281–285. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of Degree of Deacetylation of Chitosan by 1H NMR Spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Kadish, K.; Smith, K.M.; Guilard, R. The Porphyrin Handbook: Phthalocyanines: Synthesis; Academic Press: Cambridge, MA, USA, 2000; Volume 15. [Google Scholar]
- Schindler, A.; Doedt, M.; Gezgin, Ş.; Menzel, J.; Schmölzer, S. Identification of Polymers by Means of DSC, TG, STA and Computer-Assisted Database Search. J. Therm. Anal. Calorim. 2017, 129, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Lebedeva, N.S.; Gubarev, Y.A.; Yurina, E.S.; Vyugin, A.I.; Lipatova, I.M. Features of Chitosan Interaction with Copper(II) and Cobalt(II) Tetrasulfophthalocyanines. Russ. J. Gen. Chem. 2017, 87, 2327–2331. [Google Scholar] [CrossRef]
- Lebedeva, N.S.; Guseinov, S.S.; Yurina, E.S.; Gubarev, Y.A.; Koifman, O.I. Thermochemical Research of Chitosan Complexes with Sulfonated Metallophthalocyanines. Int. J. Biol. Macromol. 2019, 137, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, N.S.; Guseynov, S.S.; Yurina, E.S.; Gubarev, Y.A.; V’yugin, A.I. Pyrolysis of Complexes of Metallosul-Phophthalocyanines with Chitosan for Obtaining Graphite-Like Structures. J. Inorg. Organomet. Polym. Mater. 2021. [Google Scholar] [CrossRef]
- Mucha, M.; Pawlak, A. Complex Study on Chitosan Degradability. Polim. Warsaw 2002, 47, 509–516. [Google Scholar] [CrossRef]
- Ou, C.; Chen, S.; Liu, Y.; Shao, J.; Li, S.; Fu, T.; Fan, W.; Zheng, H.; Lu, Q.; Bi, X. Study on the Thermal Degradation Kinetics and Pyrolysis Characteristics of Chitosan-Zn Complex. J. Anal. Appl. Pyrolys. 2016, 122, 268–276. [Google Scholar] [CrossRef]


| Stage | Temperature | Sample | ||||
|---|---|---|---|---|---|---|
| CS | CoPc | CS-CoPc | CuPc | CS-CuPc | ||
| Initial mass loss (dehydration) | Ton (°C) | 52.1 | 49.9 | 50.0 | 62.1 | 49.9 |
| Tmax (°C) | 71.6 | 65.9 94.3 | 68.7 | 92.6 | 68.0 | |
| Tend (°C) | 101.1 | 109.4 | 97.9 | 134.9 | 97.0 | |
| mass loss, (%) | 13.6 | 6.1 | 11.5 | 11.6 | 11.9 | |
| First stage degradation | Ton (°C) | 215.2 | 270.1 | 210.3 | 320.7 | 204.0 |
| Tmax (°C) | 240.7 | 341.7 | 232.7 | 358.8 | 215.0 | |
| Tend (°C) | 254.4 | 359.6 | 259.6 | 393.6 | 259.3 | |
| mass loss, (%) | 43.1 | 18.2 | 40.1 | 26.2 | 34.1 | |
| Second stage degradation | Ton (°C) | 292.1 | 399.6 | 389.7 | 447.4 | 430.8 |
| Tmax (°C) | 311.9 | 416.3 | 425.0 | 475.7 | 467.1 | |
| Tend (°C) | 332.2 | 434.1 | 440.0 | 513.0 | 474.4 | |
| mass loss, (%) | 15.5 | 17.8 | 18.3 | 33.9 | 35.1 | |
| Third stage degradation | Ton (°C) | 454.0 | 491.4 | 470.3 | 564.2 | 490.3 |
| Tmax (°C) | 514.5 | 505.7 | 485.5 | 578.5 | 495.1 | |
| Tend (°C) | 557.9 | 537.0 | 516.5 | 618.6 | 509.4 | |
| mass loss, (%) | 26.4 | 16.1 | 19.8 | 19.0 | 17.3 | |
| Sample | Δm, % | ΔΔm = ΔmAir − ΔmAr | T2, °C | |
|---|---|---|---|---|
| Ar | Air | |||
| CS | 39.1 | 43.1 | 4.0 | 277 |
| CoPc | 17.6 | 17.6 | 0.0 | 366 |
| CS-CoPc | 39.3 | 39.7 | 0.4 | 342 |
| CuPc | 25.3 | 26.3 | 1.0 | 420 |
| CS-CuPc | 35.0 | 32.8 | −2.2 | 290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedeva, N.S.; Yurina, E.S.; Guseinov, S.S.; Gubarev, Y.A.; V’yugin, A.I. Destruction of Chitosan and Its Complexes with Cobalt(II) and Copper(II) Tetrasulphophthalocyanines. Polymers 2021, 13, 2781. https://doi.org/10.3390/polym13162781
Lebedeva NS, Yurina ES, Guseinov SS, Gubarev YA, V’yugin AI. Destruction of Chitosan and Its Complexes with Cobalt(II) and Copper(II) Tetrasulphophthalocyanines. Polymers. 2021; 13(16):2781. https://doi.org/10.3390/polym13162781
Chicago/Turabian StyleLebedeva, Natalia Sh., Elena S. Yurina, Sabir S. Guseinov, Yury A. Gubarev, and Anatoly I. V’yugin. 2021. "Destruction of Chitosan and Its Complexes with Cobalt(II) and Copper(II) Tetrasulphophthalocyanines" Polymers 13, no. 16: 2781. https://doi.org/10.3390/polym13162781
APA StyleLebedeva, N. S., Yurina, E. S., Guseinov, S. S., Gubarev, Y. A., & V’yugin, A. I. (2021). Destruction of Chitosan and Its Complexes with Cobalt(II) and Copper(II) Tetrasulphophthalocyanines. Polymers, 13(16), 2781. https://doi.org/10.3390/polym13162781