The Blending of Poly(glycolic acid) with Polycaprolactone and Poly(l-lactide): Promising Combinations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blend Preparation
2.3. Material Characterization
3. Results
3.1. Blend Morphological Characterization
3.2. Wettability Tests
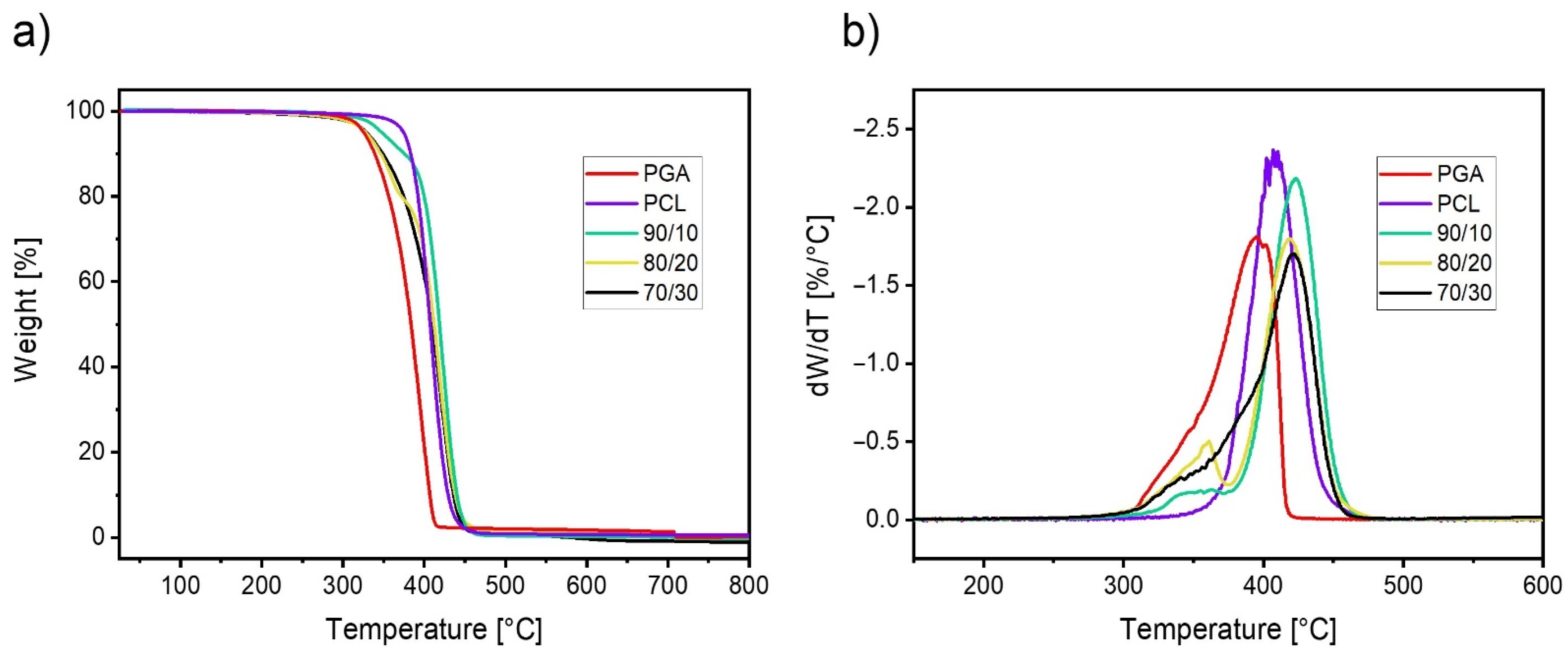
3.3. Study of the Hydrolytic Degradation
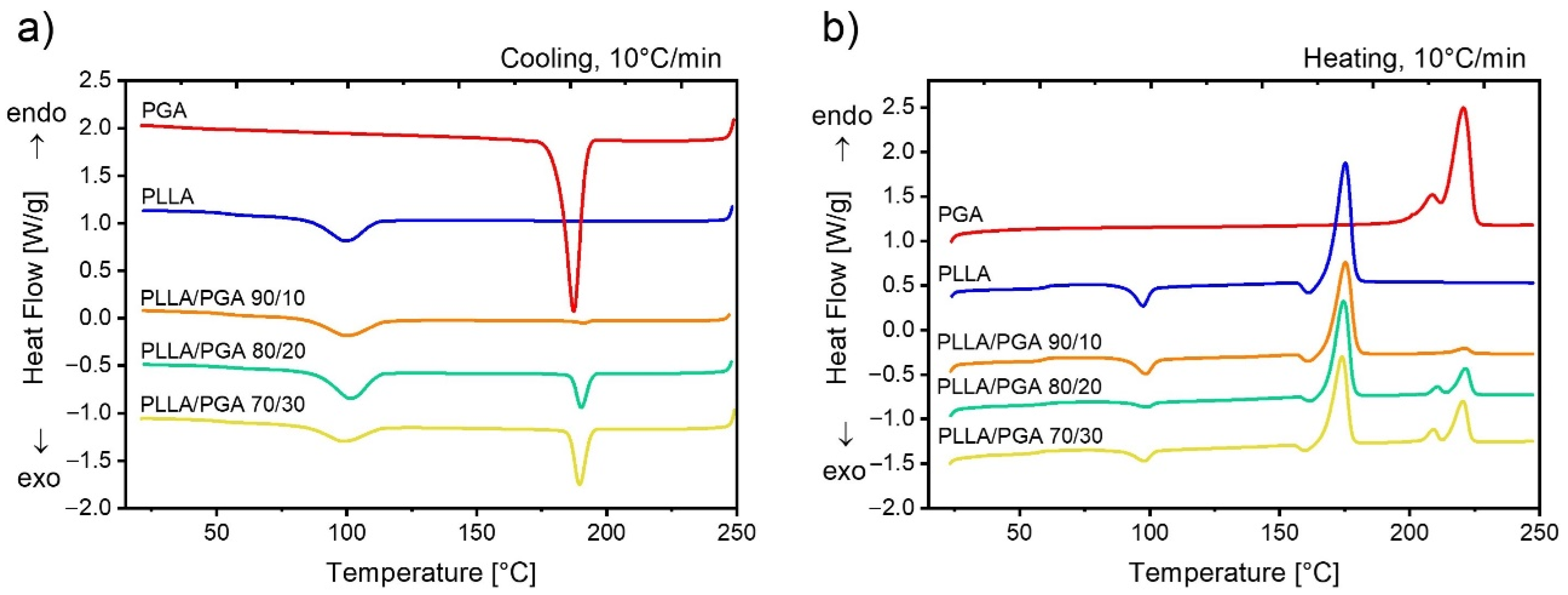
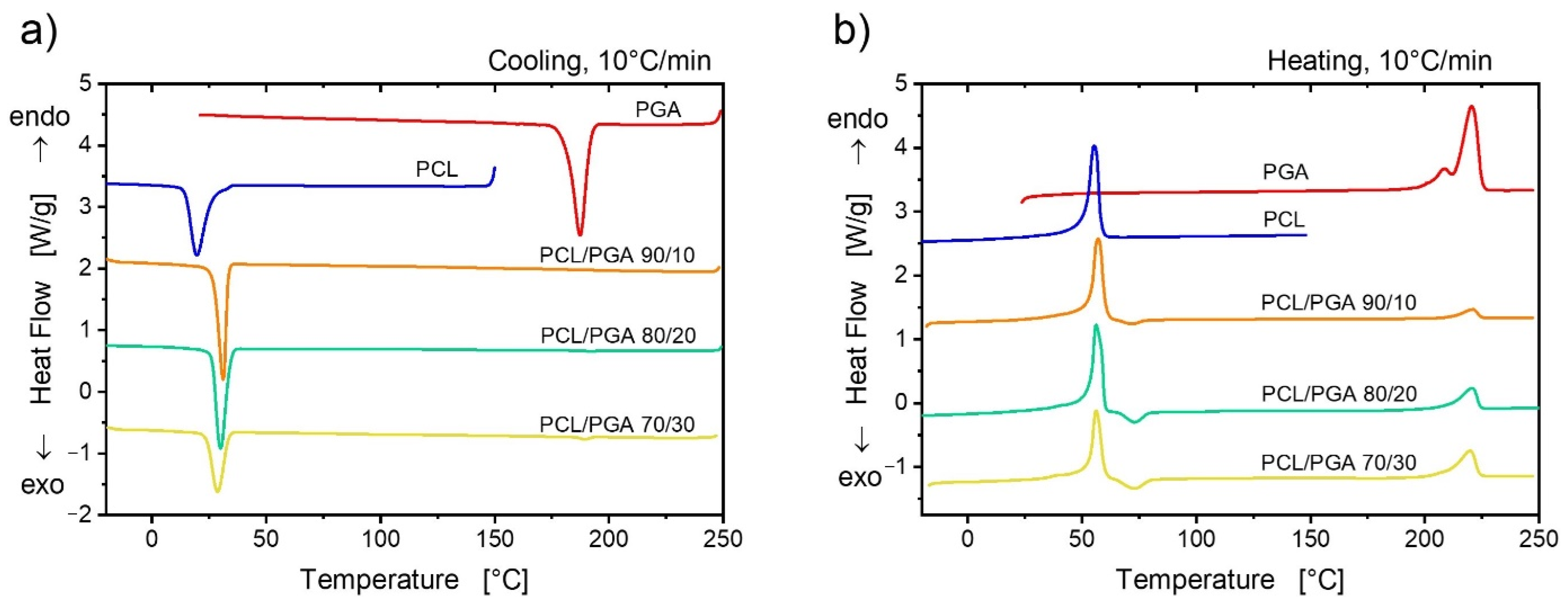
3.4. Study of the Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Di Bartolo, A.; Infurna, G.; Dintcheva, N. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymer 2021, 13, 1229. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Lee, S.; Hongo, C.; Nishino, T. Crystal Modulus of Poly(glycolic acid) and Its Temperature Dependence. Macromolecules 2017, 50, 5074–5079. [Google Scholar] [CrossRef]
- McKeen, L.W. Permeability Properties of Plastics and Elastomers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Yamane, K.; Sato, H.; Ichikawa, Y.; Sunagawa, K.; Shigaki, Y. Development of an industrial production technology for high-molecular-weight polyglycolic acid. Polym. J. 2014, 46, 769–775. [Google Scholar] [CrossRef]
- Wua, F.; Misraa, M.; Mohantya, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Progr. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Sato, H.; Miyada, M.; Yamamoto, S.; Reddy, K.R.; Ozaki, Y. C–H⋯O (ether) hydrogen bonding along the (110) direction in polyglycolic acid studied by infrared spectroscopy, wide-angle X-ray diffraction, quantum chemical calculations and natural bond orbital calculations. RSC Adv. 2016, 6, 16817–16823. [Google Scholar] [CrossRef]
- Nishimura, F.; Hoshina, H.; Ozaki, Y.; Sato, H. Isothermal crystallization of poly(glycolic acid) studied by terahertz and infrared spectroscopy and SAXS/WAXD simultaneous measurements. Polym. J. 2018, 51, 237–245. [Google Scholar] [CrossRef]
- Báez, J.E.; Marcos-Fernández, A. A Comparison of Three Different Biodegradable Aliphatic Oligoesters (PGA, PLLA, and PCL) with Similar Linear Alkyl End Groups by DSC and SAXS. Int. J. Polym. Anal. Charact. 2015, 20, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Tiwari, M. Structure-Processing-Property Relationship of Poly(Glycolic Acid) for Drug Delivery Systems 1: Synthesis and Catalysis. Int. J. Polym. Sci. 2010, 2010, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Shawe, S.; Buchanan, F.; Harkin-Jones, E.; Farrar, D. A study on the rate of degradation of the bioabsorbable polymer polyglycolic acid (PGA). J. Mater. Sci. 2006, 41, 4832–4838. [Google Scholar] [CrossRef]
- Hurrell, S.; Milroy, G.E.; Cameron, R.E. The Degradation of Polyglycolide in Water and Deuterium Oxide. Part I: The Effect of Reaction Rate. Polymer 2003, 44, 1421–1424. [Google Scholar] [CrossRef]
- De Oca, H.M.; Farrar, D.; Ward, I.M. Degradation studies on highly oriented poly(glycolic acid) fibres with different lamellar structures. Acta Biomater. 2011, 7, 1535–1541. [Google Scholar] [CrossRef]
- Milroy, G.E.; Smith, R.W.; Hollands, R.; Clough, A.S.; Mantle, M.D.; Gladden, L.F.; Huatan, H.; Cameron, R.E. The Degradation of Polyglycolide in Water and Deuterium Oxide. Part II: Nuclear Reaction Analysis and Magnetic Resonance Imaging of Water Distribution. Polymer 2003, 44, 1425–1435. [Google Scholar] [CrossRef]
- Abou-Zeid, D.-M.; Müller, R.-J.; Deckwer, W.-D. Degradation of natural and synthetic polyesters under anaerobic conditions. J. Biotechnol. 2001, 86, 113–126. [Google Scholar] [CrossRef]
- Matsuda, Y.; Karino, M.; Okui, T.; Kanno, T. Complications of poly-l-lactic acid and polyglycolic acid (PLLA/PGA) osteosynthesis systems for maxillofacial surgery: A retrospective clinical investigation. Polymers 2021, 13, 889. [Google Scholar] [CrossRef]
- Fujimaki, H.; Uchida, K.; Inoue, G.; Matsushita, O.; Nemoto, N.; Miyagi, M.; Inage, K.; Takano, S.; Orita, S.; Ohtori, S.; et al. Polyglycolic acid-collagen tube combined with collagen-binding basic fibroblast growth factor accelerates gait recovery in a rat sciatic nerve critical-size defect model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 326–332. [Google Scholar] [CrossRef]
- Kim, B.N.; Ko, Y.-G.; Yeo, T.; Kim, E.J.; Kwon, O.K.; Kwon, O.H. Guided Regeneration of Rabbit Calvarial Defects Using Silk Fibroin Nanofiber–Poly(glycolic acid) Hybrid Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 5266–5272. [Google Scholar] [CrossRef]
- DiCosimo, R.; Payne, M.S.; Panova, A.; Thompson, J.; O’Keefe, D.P. Enzymatic Production of Glycolic Acid. U.S. Patent 7,198,927, 29 June 2006. [Google Scholar]
- Spearman, S.S.; Irin, F.; Ramesh, S.; Rivero, I.; Green, M.J.; Harrysson, O.L.A. Effect of pseudomonas lipase enzyme on the degradation of polycaprolactone/polycaprolactone-polyglycolide fiber blended nanocomposites. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 360–367. [Google Scholar] [CrossRef]
- Vieira, A.; Vieira, J.; Guedes, R.; Marques, A. Degradation and Viscoelastic Properties of PLA-PCL, PGA-PCL, PDO and PGA Fibres. Mater. Sci. Forum 2010, 636, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Ajioka, M.; Suizu, H.; Higuchi, C.; Kashima, T. Aliphatic polyesters and their copolymers synthesized through direct condensation polymerization. Polym. Degrad. Stab. 1998, 59, 137–143. [Google Scholar] [CrossRef]
- Li, J.; Stayshich, R.M.; Meyer, T. Exploiting Sequence to Control the Hydrolysis Behavior of Biodegradable PLGA Copolymers. J. Am. Chem. Soc. 2011, 133, 6910–6913. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Ramdhanie, L.I.; AuBuchon, S.R.; Boland, E.D.; Knapp, D.C.; Barnes, C.P.; Simpson, D.G.; E Wnek, G.; Bowlin, G.L. Thermal and Mechanical Characterization of Electrospun Blends of Poly(lactic acid) and Poly(glycolic acid). Polym. J. 2006, 38, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Youk, J.H.; Lee, S.W.; Min, B.-M.; Lee, S.J.; Park, W.H. Preparation of porous ultrafine PGA fibers via selective dissolution of electrospun PGA/PLA blend fibers. Mater. Lett. 2006, 60, 757–760. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, N.; Xiong, C. Degradation and miscibility of poly(DL-lactic acid)/poly(glycolic acid) composite films: Effect of poly(DL-lactic-co-glycolic acid). Bull. Mater. Sci. 2012, 35, 575–578. [Google Scholar] [CrossRef] [Green Version]
- Takayama, T.; Daigaku, Y.; Ito, H.; Takamori, H. Mechanical properties of bio-absorbable PLA/PGA fiber-reinforced composites. J. Mech. Sci. Technol. 2014, 28, 4151–4154. [Google Scholar] [CrossRef]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the crystallinity and mechanical properties of PLA by nucleation and annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Gardella, L.; Forouharshad, M.; Pellegrino, T.; Monticelli, O. Star poly(ε-caprolactone)-based electrospun fibers as biocompatible scaffold for doxorubicin with prolonged drug release activity. Colloids Surf. B Biointerfaces 2018, 161, 488–496. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, K.; He, Y.; Hu, W. Fast-Scanning Chip-Calorimetry Measurement of Crystallization Kinetics of Poly(Glycolic Acid). Polymers 2021, 13, 891. [Google Scholar] [CrossRef]
- Thomas, S.; Grohens, Y.; Jyotishkumar, P. Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Li, K.; Fina, A.; Marrè, D.; Carosio, F.; Monticelli, O. Graphite oxide nanocoatings as a sustaibale route to extend the applicability of biopolymer-based film. Appl. Surf. Sci. 2020, 522, 146471. [Google Scholar] [CrossRef]
- Tiaw, K.; Goh, S.; Hong, M.; Wang, Z.; Lan, B.; Teoh, S. Laser surface modification of poly(ε-caprolactone) (PCL) membrane for tissue engineering applications. Biomaterials 2005, 26, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Vargha-Butler, E.I.; Kiss, E.; Lam, C.N.C.; Keresztes, Z.; Kálmán, E.; Zhang, L.; Neumann, A.W. Wettability of biodegradable surfaces. Colloid Polym. Sci. 2001, 279, 1160–1168. [Google Scholar] [CrossRef]
- Elsawya, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Más-Estellés, J.; Meseguer-Dueñas, J.M.; Ivirico, J.E.; Marí, B.; Vidaurre, A. Hydrolytic and enzymatic degradation of a poly(ε-caprolactone) network. Polym. Degrad. Stab. 2012, 97, 1241–1248. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Hurrell, S.; Cameron, R.E. Polyglycolide: Degradation and drug release. Part I: Changes in morphology during degradation. J. Mater. Sci. Mater. Med. 2001, 12, 811–816. [Google Scholar] [CrossRef]
- Hurrell, S.; Milroy, G.E.; Cameron, R.E. The distribution of water in degrading polyglycolide. Part I: Sample size and drug release. J. Mater. Sci. Mater. Med. 2003, 14, 457–464. [Google Scholar] [CrossRef]
- Righetti, M.C.; Marchese, P.; Vannini, M.; Celli, A.; Lorenzetti, C.; Cavallo, D.; Ocando, C.; Müller, A.J.; Androsch, R. Polymorphism and Multiple Melting Behavior of Bio-Based Poly(propylene 2,5-furandicarboxylate). Biomacromolecules 2020, 21, 2622–2634. [Google Scholar] [CrossRef]
- Sangroniz, L.; Wang, B.; Su, Y.; Liu, G.; Cavallo, D.; Wang, D.; Müller, A.J. Fractionated crystallization in semicrystalline polymers. Prog. Polym. Sci. 2021, 115, 101376. [Google Scholar] [CrossRef]




| Sample Code | Water | Diiodomethane | γs | ||
|---|---|---|---|---|---|
| Contact Angle | Contact Angle | ||||
| [°] | [°C] | [mN/m] | [mN/m] | [mN/m] | |
| PLLA | 80 ± 1.2 | 44 ± 2.8 | 41.9 | 8.8 | 33.1 |
| PCL | 75 ± 1.3 | 26 ± 2.5 | 46.8 | 4.6 | 42.2 |
| PGA | 50 ± 1.3 | 20 ± 2.1 | 58.1 | 18.2 | 39.9 |
| PLLA70/PGA30 | 63 ± 3.8 | 41 ± 2.2 | 47.1 | 13.5 | 33.6 |
| PCL70/PGA30 | 64 ± 1.4 | 22 ± 2.4 | 49.7 | 7.3 | 42.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magazzini, L.; Grilli, S.; Fenni, S.E.; Donetti, A.; Cavallo, D.; Monticelli, O. The Blending of Poly(glycolic acid) with Polycaprolactone and Poly(l-lactide): Promising Combinations. Polymers 2021, 13, 2780. https://doi.org/10.3390/polym13162780
Magazzini L, Grilli S, Fenni SE, Donetti A, Cavallo D, Monticelli O. The Blending of Poly(glycolic acid) with Polycaprolactone and Poly(l-lactide): Promising Combinations. Polymers. 2021; 13(16):2780. https://doi.org/10.3390/polym13162780
Chicago/Turabian StyleMagazzini, Luca, Sara Grilli, Seif Eddine Fenni, Alessandro Donetti, Dario Cavallo, and Orietta Monticelli. 2021. "The Blending of Poly(glycolic acid) with Polycaprolactone and Poly(l-lactide): Promising Combinations" Polymers 13, no. 16: 2780. https://doi.org/10.3390/polym13162780







