An N-Halamine/Graphene Oxide-Functionalized Electrospun Polymer Membrane That Inactivates Bacteria on Contact and by Releasing Active Chlorine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide
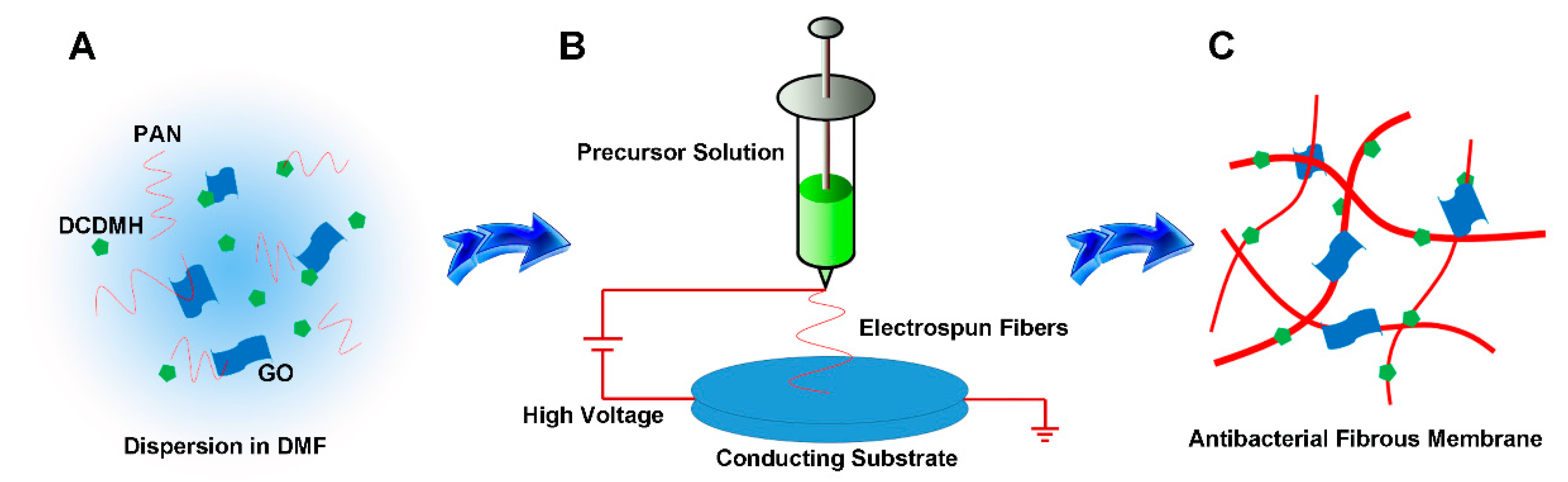
2.3. Synthesis of PAN/GO/DCDMH Fibrous Membrane
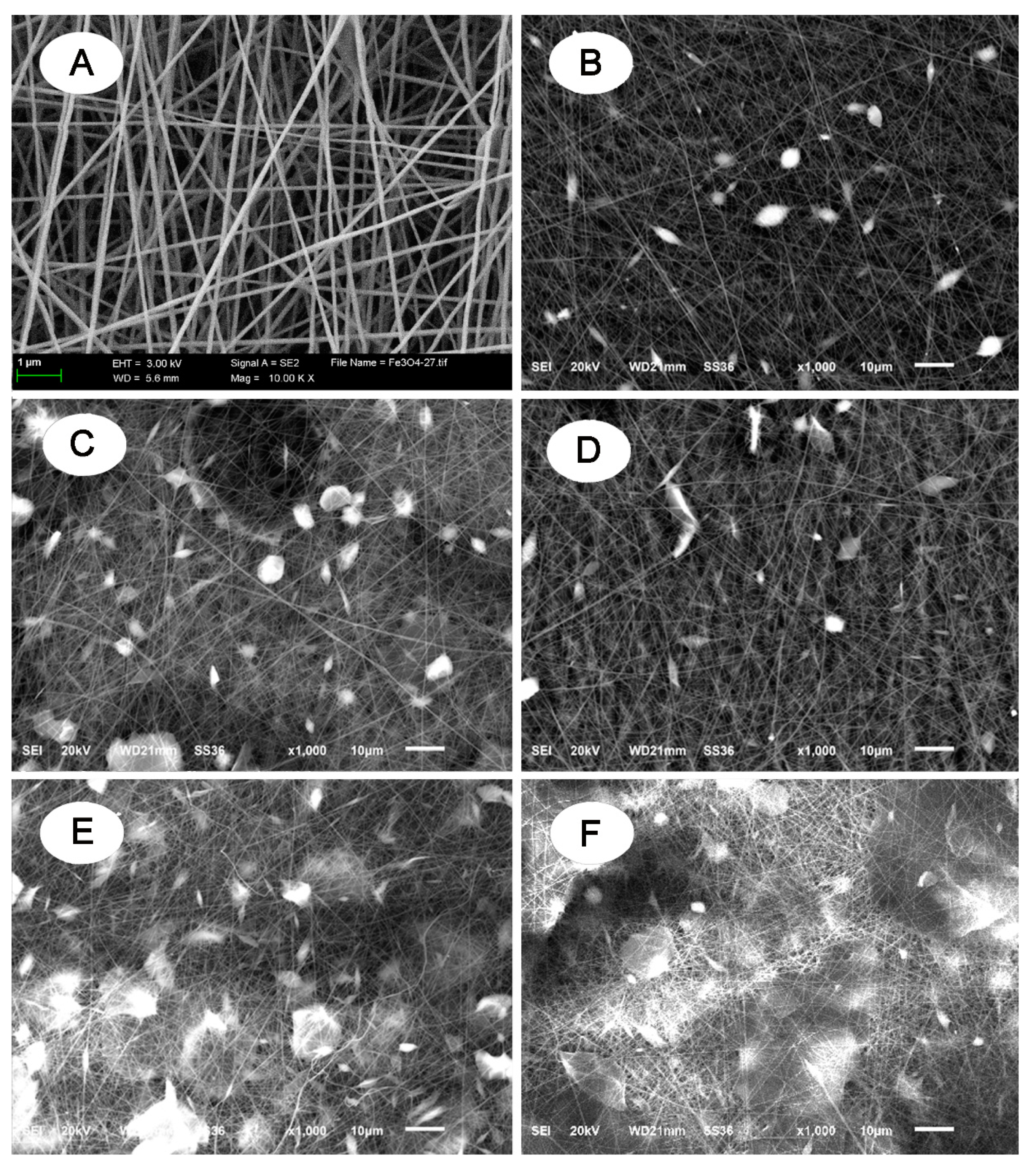
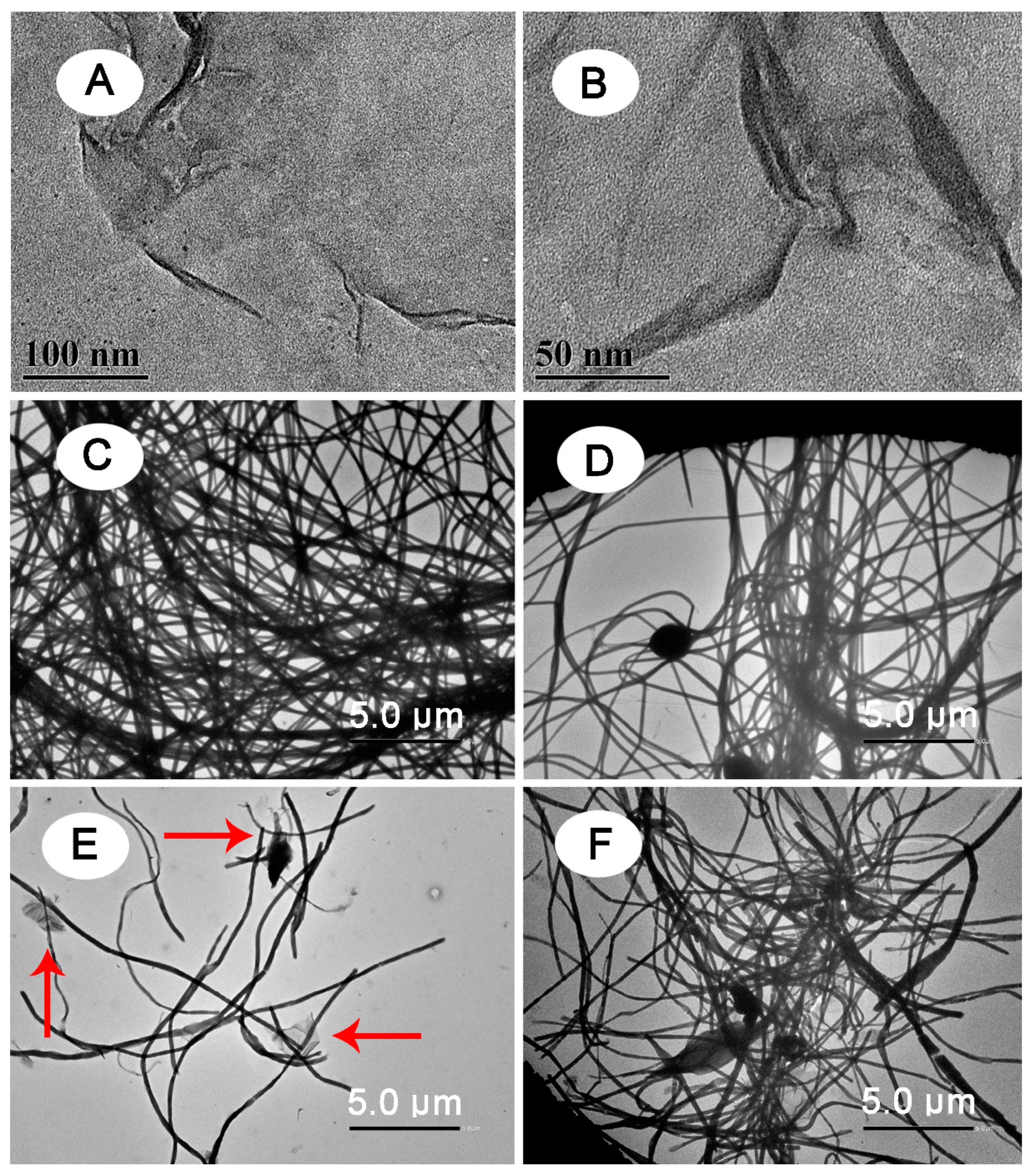
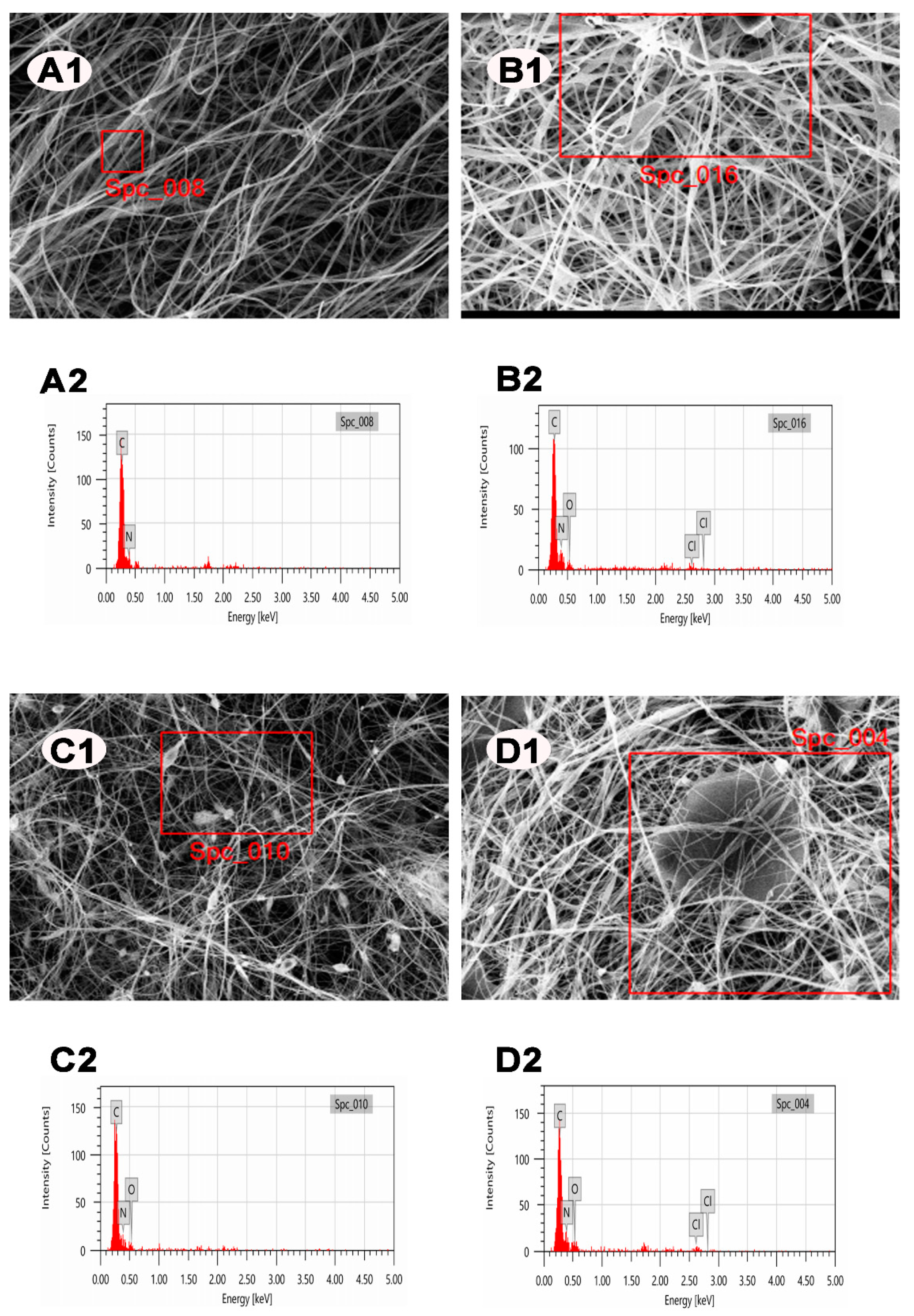
2.4. Characterization
2.5. Antibacterial Assay
2.6. Dialysis Test
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of nanozyme-hydrogel for enhanced capture and elimination of bacteria. Adv. Funct. Mater. 2019, 29, 1900518. [Google Scholar] [CrossRef]
- Zhao, J.; Qu, Y.; Chen, H.; Xu, R.; Yu, Q.; Yang, P. Self-assembled proteinaceous wound dressings attenuate secondary trauma and improve wound healing in vivo. J. Mater. Chem. B 2018, 6, 4645–4655. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Chen, H. Responsive and synergistic antibacterial coatings: Fighting against bacteria in a smart and effective way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef]
- Almeida, A.; Alves, M.; Domingues, I.; Henriques, I. The impact of antibiotic exposure in water and zebrafish gut microbiomes: A 16S rRNA gene-based metagenomic analysis. Ecotoxicol. Environ. Saf. 2019, 186, 109771–109781. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.; Zhang, Y.; Dong, A. Black phosphorus nanosheets counteract bacteria without causing antibiotic resistance. Chem. Eur. J. 2020, 26, 2478–2485. [Google Scholar] [CrossRef]
- Xu, M.; Song, Q.; Gao, L.; Liu, H.; Feng, W.; Huo, J.; Jin, H.; Huang, L.; Chai, J.; Pei, Y.; et al. Single-step fabrication of catechol-ε-poly-L-lysine antimicrobial paint that prevents superbug infection and promotes osteoconductivity of titanium implants. Chem. Eng. J. 2020, 396, 125240. [Google Scholar] [CrossRef]
- Wang, S.; Xu, M.; Huang, K.; Zhi, J.; Sun, C.; Wang, K.; Zhou, Q.; Gao, L.; Jia, Q.; Shi, H.; et al. Biocompatible metal-free organic phosphorescent nanoparticles for efficiently multidrug-resistant bacteria eradication. Sci. China Mater. 2020, 63, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Li, P.; Zhao, H.; Chen, Y.; Jiang, L.; Ma, P. Methacrylate-ended polypeptides and polypeptoids for antimicrobial and antifouling coatings. Polym. Chem. 2017, 8, 6386–6397. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Yang, X.; Lei, K.; Wang, L. The innovative fabrication of nano-natural antimicrobial agent@polymeric microgels-TiO2 hybrid films capable of absorbing UV and antibacterial on touch screen panel. Colloids Surf. B 2020, 197, 111410–111420. [Google Scholar] [CrossRef]
- Zhou, M.; Qian, Y.; Xie, J.; Zhang, W.; Jiang, W.; Xiao, X.; Chen, S.; Dai, C.; Cong, Z.; Ji, Z.; et al. Poly(2-oxazoline)-based functional peptide mimics: Eradicating MRSA infections and persisters while alleviating antimicrobial resistance. Angew. Chem. Int. Ed. 2020, 59, 6412–6419. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Peng, X.; He, Y.; Wei, L.; Su, W.; Wu, J.; Cui, L.; Liu, Z.; Guo, X. Photocatalytic antibacterial agent incorporated double-network hydrogel for wound healing. Colloids Surf. B 2019, 180, 237–244. [Google Scholar] [CrossRef]
- Dong, A.; Sun, Y.; Lan, S.; Wang, Q.; Cai, Q.; Qi, X.; Zhang, Y.; Gao, G.; Liu, F.; Harnoode, C. Barbituric acid-based magnetic N-halamine nanoparticles asrecyclable antibacterial agents. ACS Appl. Mater. Interfaces 2013, 5, 8125–8133. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Lan, S.; Huang, J.; Wang, T.; Zhao, T.; Xiao, L.; Wang, W.; Zheng, X.; Liu, F.; Gao, G.; et al. Modifying Fe3O4-functionalized nanoparticles with N-halamine and their magnetic/antibacterial properties. ACS Appl. Mater. Interfaces 2011, 3, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Wang, Y.; Gao, Y.; Gao, T.; Gao, G. Chemical insights into antibacterial N-halamines. Chem. Rev. 2017, 117, 4806–4862. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Huang, Z.; Lan, S.; Wang, Q.; Bao, S.; Siriguleng, S.; Zhang, Y.; Gao, G.; Liu, F.; Harnoode, C. N-Halamine-decorated polystyrene nanoparticles based on 5-allylbarbituricacid: From controllable fabrication to bactericidal evaluation. J. Colloid Interface Sci. 2014, 413, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Natan, M.; Gutman, O.; Lavi, R.; Margel, S.; Banin, E. Killing mechanism of stable N-halamine cross-linked polymethacrylamide nanoparticles that selectively target bacteria. ACS Nano 2015, 9, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, Q.; Li, L.; Li, P.; Wang, Y.; Simalou, O.; Zhang, Y.; Gao, G.; Dong, A. N-Halamine-containing electrospun fibers kill bacteria via a contact/release co-determined antibacterial pathway. ACS Appl. Mater. Interfaces 2016, 8, 31530–31540. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Bodula, R.; Guo, B.; et al. Antibacterial carbon-based nanomaterials. Adv. Mater. 2018, 31, e1804838. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selim, M.S.; Mo, P.J.; Hao, Z.; Fatthallah, N.A.; Chen, X. Blade-like structure of graphene oxide sheets decorated with cuprous oxide and silicon carbide nanocomposites as bactericidal materials. J. Colloid Interface Sci. 2020, 578, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhu, G.; Yu, C.; Chen, G.; Zhang, C.; Zeng, X.; Chen, Q.; Peng, Q. Functionalized graphene oxide nanosheets with unique three-in-one properties for efficient and tunable antibacterial applications. Nano Res. 2002, 14, 185–190. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Khodi, S.; Xu, X.; Agathpoulos, S. Multifunctional thin-film nanofiltration membrane incorporated with reduced graphene oxide@TiO2@Ag nanocomposites for high desalination performance, dye retention, and antibacterial properties. ACS Appl. Mater. Interfaces 2019, 11, 23535–23545. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Goudar, V.S.; Padmalekha, K.G.; Bhat, S.V.; Indi, S.S.; Vasan, H.N. ZnO/Ag nanohybrid: Synthesis, characterization, synergistic antibacterial activity and its mechanism. RSC Adv. 2012, 2, 930–940. [Google Scholar] [CrossRef]
- Aksoy, I.; Küçükkeçeci, H.; Sevgi, F.; Metin, Ö.; Patir, I.H. Photothermal antibacterial and antibiofilm activity of black phosphorus/gold nanocomposites against pathogenic bacteria. ACS Appl. Mater. Interfaces 2020, 12, 26822–26831. [Google Scholar] [CrossRef] [PubMed]
- Borjihan, Q.; Dong, A. Design of nanoengineered antibacterial polymers for biomedical applications. Biomater. Sci. 2020, 8, 6867–6882. [Google Scholar] [CrossRef] [PubMed]
- Robert, B.; Nallathambi, G. A concise review on electrospun nanofibers/nanonets for filtration of gaseous and solid constituents (PM2.5) from polluted air. Colloid Interface Sci. Commun. 2020, 37, 100275. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and antibacterial performance of novel polylactic acid-graphene oxide-silver nanoparticle hybrid nanocomposite mats prepared by electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Awasthi, G.P.; Lee, J.; Park, C.H.; Kim, C.S. Synthesis, characterization, organic compound degradation activity and antimicrobial performance of g-C3N4 sheets customized with metal nanoparticles-decorated TiO2 nanofibers. RSC Adv. 2016, 6, 55079–55091. [Google Scholar] [CrossRef]
- Choi, J.; Yang, B.Y.; Bae, G.; Jung, J.H. Heral extract incorporated nanofiber fabricated by an electrospinning technique and its application to antimicrobial air filtration. ACS Appl. Mater. Interfaces 2015, 7, 25313–25320. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A review of the functional principles and applications of solution blow spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Du, Y.; Su, Y.; Guo, Y.; Zhu, Z.; Dong, A. A review on recent achievements and current challenges in antibacterial electrospun N-halamines. Colloid Interface Sci. Commun. 2018, 24, 24–34. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing-a review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [Green Version]
- Mukhejee, M.; De, S. Antibacterial polymeric membranes: A short review. Environ. Sci. Water Res. Technol. 2018, 4, 1078–1104. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured electrospun nanofibers for air filtration: A review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, Y.; Sun, Z.; Chang, D.; Gao, G.; Dong, A. Synthesis, Characterization, and bactericidal evaluation of chitosan/guanidine functionalized graphene oxide composites. Molecules 2017, 22, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liu, K.; Williams, G.R.; Wu, J.; Wu, J.; Wang, H.; Niu, S.; Zhu, L. Dual temperature and pH responsive fibrous formulations prepared by electrospinning. Colloid Surf. B 2018, 171, 142–149. [Google Scholar]
- Lan, S.; Sheng, X.; Lu, Y.; Li, C.; Zhao, S.; Liu, N. Modification of antibacterial ZnO nanorods with CeO2 nanoparticles: Role of CeO2 in impacting morphology and antibacterial activity. Colloid Interface Sci. Commun. 2018, 26, 32–38. [Google Scholar] [CrossRef]
- Ahmed, A.E.I.; Hay, J.N.; Bushell, M.E.; Wardell, J.N.; Cavalli, G. Biocidal polymers (II): Determination of biological activity of novel N-halamine biocidal polymers and evaluation for use in water filters. React. Funct. Polym. 2008, 68, 1448–1458. [Google Scholar] [CrossRef] [Green Version]
- Lan, S.; Lu, Y.; Li, C.; Zhao, S.; Liu, N.; Sheng, X. Sesbania gum-supported hydrophilic electrospun fibers containing nanosilver with superior antibacterial activity. Nanomaterials 2019, 9, 592. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Song, N.; Liu, W.; Dong, A.; Wang, Y.; Yang, Y. Construction of antibacterial N-halamine polymer nanomaterials capable of bacterial membrane disruption for efficient anti-infective wound therapy. Macromol. Biosci. 2019, 19, 1800453. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Kumar, A. Effect of particle weight concentration on the lubrication properties of graphene based epoxy composites. Colloid Interface Sci. Commun. 2019, 33, 100206. [Google Scholar] [CrossRef]
- Borjihan, Q.; Zhang, Z.; Zi, X.; Huang, M.; Chen, Y.; Zhang, Y.; Dong, A. Pyrrolidone-based polymers capable of reversible iodine capture for reuse in antibacterial applications. J. Hazard. Mater. 2020, 384, 121305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, Y. N-Halamine-based antimicrobial additives for polymers: Preparation, characterization, and antimicrobial activity. Ind. Eng. Chem. Res. 2006, 45, 2634–2640. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Kang, J.; Simalou, O.; Liu, W.; Ren, H.; Gao, T.; Gao, Y.; Chen, W.; Dong, A.; Jia, R. Nover N-Br bond-containing N-halamine nanofibers with antibacterial activities. ACS Biomater. Sci. Eng. 2018, 4, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Borjihan, Q.; Yang, J.; Song, Q.; Gao, L.; Xu, M.; Gao, T.; Liu, W.; Li, P.; Li, Q.; Dong, A. Povidone-iodine-functionalized fluorinated copolymers with dual-functional antibacterial and antifouling activities. Biomater. Sci. 2019, 7, 3334. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Kumar, A. Enzyme-mimetic activity of sugar cane juice stabilized CuO nanospheres and CuO/GO nanocomposite: Green synthesis and applications. Colloid Interface Sci. Commun. 2020, 35, 100239. [Google Scholar]
- Jie, Z.; Yan, X.; Zhao, L.; Worley, S.D.; Liang, J. Eco-friendly synthesis of regenerable antimicrobial polymeric resin with N-halamine and quaternary ammonium salt groups. RSC Adv. 2014, 4, 6048–6054. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Sun, Y. Biocidal efficacy, biofilm-controlling function, and controlled release effect of chloromelamine-based bioresponsive fibrous Materials. Biomaterials 2007, 28, 1597–1609. [Google Scholar] [CrossRef] [Green Version]
- Badrossamay, M.R.; Sun, G. Graft polymerization of N-tert-butylacrylamide onto polypropylene during melt extrusion andbiocidal properties of its products. Polym. Eng. Sci. 2009, 49, 359–368. [Google Scholar] [CrossRef]
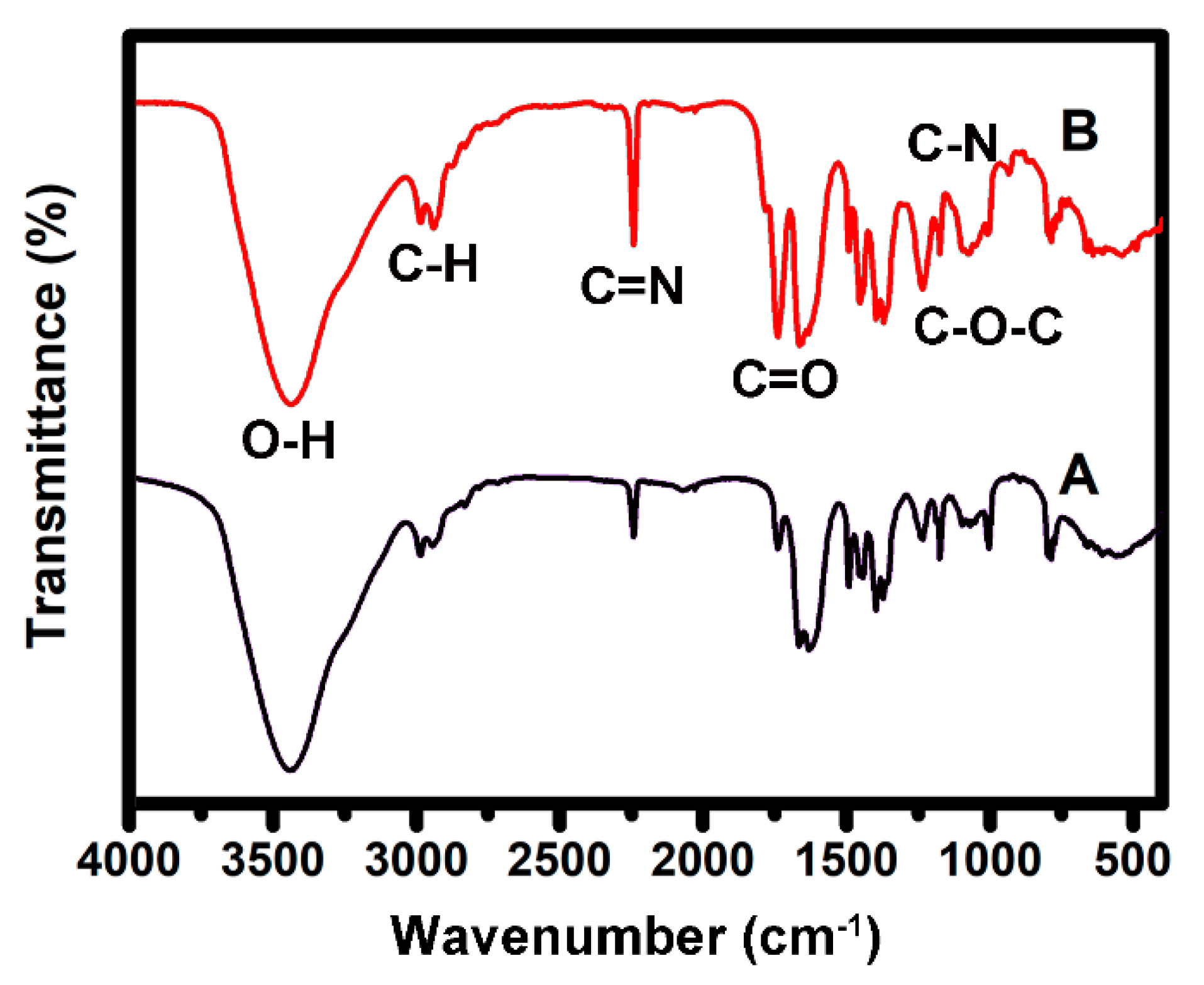


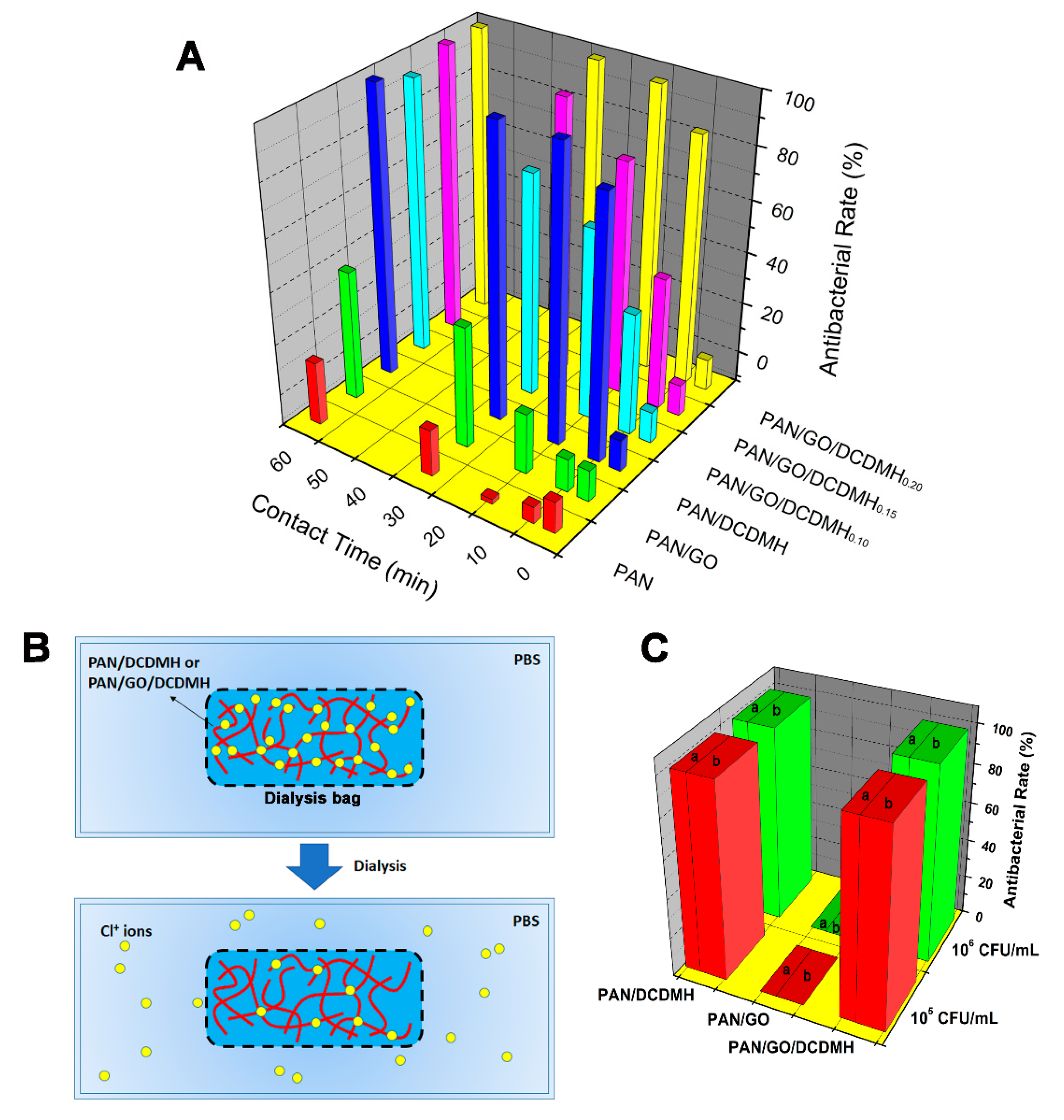
| Types of Fibrous Membrane | Feed Ratios | |||
|---|---|---|---|---|
| PAN (g) | GO (g) | DCDMH (g) | DMF (mL) | |
| PAN | 1.15 | - | - | 10 |
| PAN/GO | 1.15 | 0.05 | - | 10 |
| PAN/DCDMH | 1.15 | - | 0.15 | 10 |
| PAN/GO0.05/DCDMH | 1.15 | 0.05 | 0.15 | 10 |
| PAN/GO0.1/DCDMH | 1.15 | 0.10 | 0.15 | 10 |
| PAN/GO0.15/DCDMH | 1.15 | 0.15 | 0.15 | 10 |
| PAN/GO/DCDMH0.1 | 1.15 | 0.05 | 0.10 | 10 |
| PAN/GO0.1/DCDMH0.15 | 1.15 | 0.05 | 0.15 | 10 |
| PAN/GO0.1/DCDMH0.20 | 1.15 | 0.05 | 0.20 | 10 |
| Elements | PAN | PAN/DCDMH | PAN/GO | PAN/GO/DCDMH |
|---|---|---|---|---|
| Carbon | + | + | + | + |
| Oxygen | − | + | + | + |
| Nitrogen | + | + | + | + |
| Chlorine | − | + | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, S.; Zhang, J.; Li, J.; Guo, Y.; Sheng, X.; Dong, A. An N-Halamine/Graphene Oxide-Functionalized Electrospun Polymer Membrane That Inactivates Bacteria on Contact and by Releasing Active Chlorine. Polymers 2021, 13, 2784. https://doi.org/10.3390/polym13162784
Lan S, Zhang J, Li J, Guo Y, Sheng X, Dong A. An N-Halamine/Graphene Oxide-Functionalized Electrospun Polymer Membrane That Inactivates Bacteria on Contact and by Releasing Active Chlorine. Polymers. 2021; 13(16):2784. https://doi.org/10.3390/polym13162784
Chicago/Turabian StyleLan, Shi, Jinghua Zhang, Jie Li, Yanan Guo, Xianliang Sheng, and Alideertu Dong. 2021. "An N-Halamine/Graphene Oxide-Functionalized Electrospun Polymer Membrane That Inactivates Bacteria on Contact and by Releasing Active Chlorine" Polymers 13, no. 16: 2784. https://doi.org/10.3390/polym13162784
APA StyleLan, S., Zhang, J., Li, J., Guo, Y., Sheng, X., & Dong, A. (2021). An N-Halamine/Graphene Oxide-Functionalized Electrospun Polymer Membrane That Inactivates Bacteria on Contact and by Releasing Active Chlorine. Polymers, 13(16), 2784. https://doi.org/10.3390/polym13162784







