Novel High Flux Poly(m-phenylene isophtalamide)/TiO2 Membranes for Ultrafiltration with Enhanced Antifouling Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Porous Membrane Preparation
2.3. Structural Investigation of the Membranes
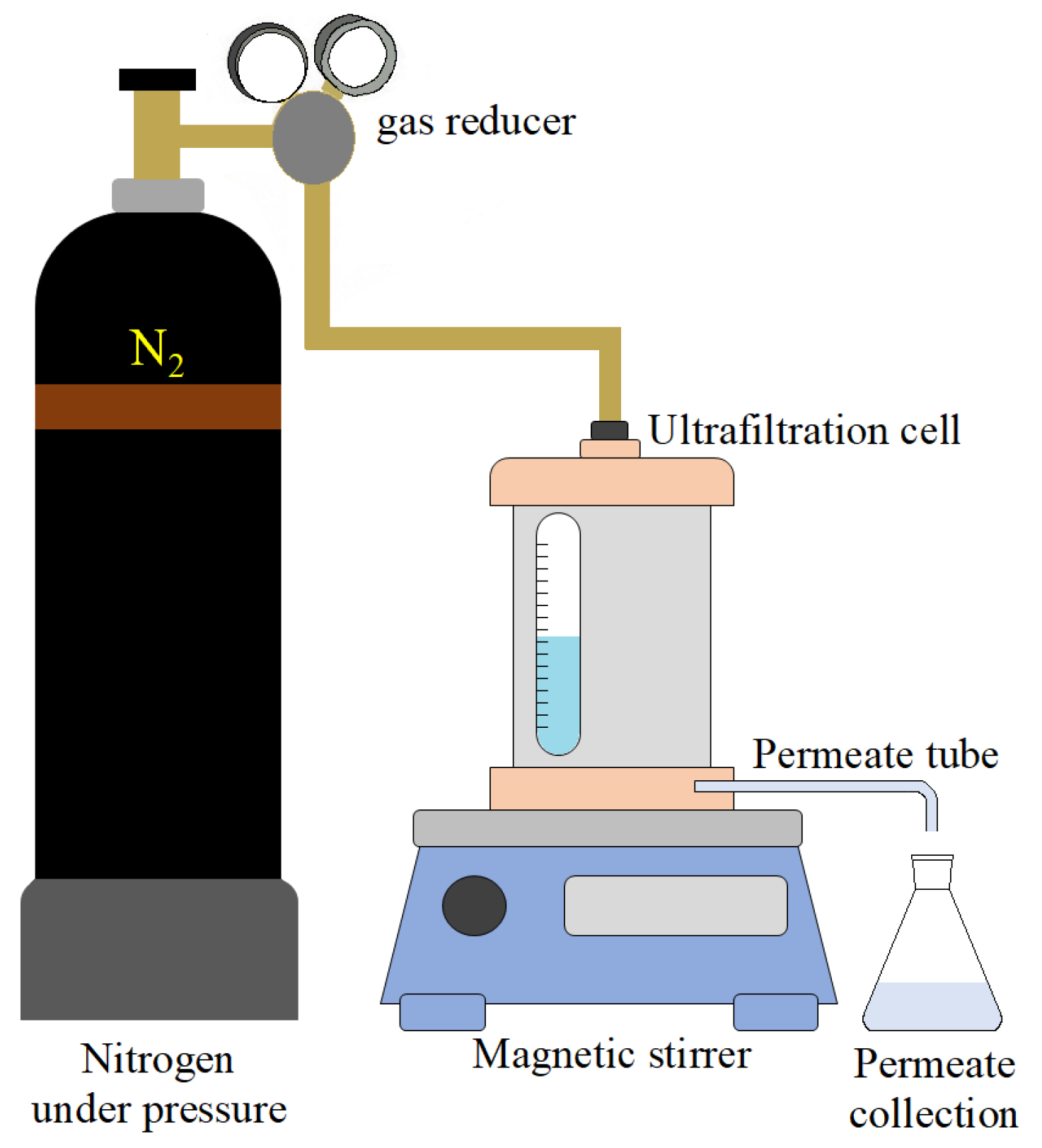
2.4. Determination of Separation and Antifouling Membrane Performance
3. Results and Discussions
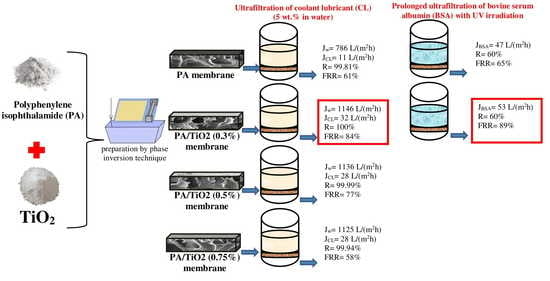
3.1. Characterization of PA and PA/TiO2 Membranes
3.2. Transport Properties of PA and PA/TiO2 Membranes in Ultrafiltration
3.2.1. Ultrafiltration of Coolant Lubricant Emulsion
3.2.2. Ultrafiltration of BSA Solution and Flux Recovery of Membranes by Photocatalysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-Doped Electrospun Nanofibrous Membrane for Photocatalytic Water Treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Hejase, C.A.; Tarabara, V.V. Nanofiltration of saline oil-water emulsions: Combined and individual effects of salt concentration polarization and fouling by oil. J. Memb. Sci. 2021, 617, 118607. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Ouzzine, M.; Maciá-Agulló, J.A.; Lillo-Ródenas, M.A.; Quijada, C.; Linares-Solano, A. Synthesis of high surface area TiO2 nanoparticles by mild acid treatment with HCl or HI for photocatalytic propene oxidation. Appl. Catal. B Environ. 2014, 154–155, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Srivastava, N.; Mishra, P.K.; Bhatiya, A.K.; Singh, N.L. Application of TiO2 Nanoparticle in Photocatalytic Degradation of Organic Pollutants. Mater. Sci. Forum 2016, 855, 20–32. [Google Scholar] [CrossRef]
- Al-Hobaib, A.S.; Al-Sheetan, K.M.; Shaik, M.R.; Al-Suhybani, M.S. Modification of thin-film polyamide membrane with multi-walled carbon nanotubes by interfacial polymerization. Appl. Water Sci. 2017, 7, 4341–4350. [Google Scholar] [CrossRef] [Green Version]
- Kotlhao, K.; Lawal, I.; Moutloali, R.; Klink, M. Antifouling Properties of Silver-Zinc Oxide Polyamide Thin Film Composite Membrane and Rejection of 2-Chlorophenol and 2,4-Dichlorophenol. Membranes 2019, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. Process Intensif. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Silva, C.G.; Silva, A.M.T.; Vilar, V.J.P.; Boaventura, R.A.R.; Faria, J.L. Photocatalytic activity of TiO2-coated glass raschig rings on the degradation of phenolic derivatives under simulated solar light irradiation. Chem. Eng. J. 2013, 224, 32–38. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, C.; Zhang, S.; Li, P.; Hou, D. Preparation of graphene oxide modified poly(m-phenylene isophthalamide) nanofiltration membrane with improved water flux and antifouling property. Appl. Surf. Sci. 2017, 394, 149–159. [Google Scholar] [CrossRef]
- Hua, D.; Japip, S.; Wang, K.Y.; Chung, T.-S. Green Design of Poly(m-Phenylene Isophthalamide)-Based Thin-Film Composite Membranes for Organic Solvent Nanofiltration and Concentrating Lecithin in Hexane. ACS Sustain. Chem. Eng. 2018, 6, 10696–10705. [Google Scholar] [CrossRef]
- Pramila, J.; Melbiah, J.S.B.; Rana, D.; Gandhi, N.N.; Nagendran, A.; Mohan, D. Permeation characteristics of tailored poly(m-phenylene isophthalamide) ultrafiltration membranes and probing its efficacy on bovine serum albumin separation. Polym. Test. 2018, 67, 218–227. [Google Scholar] [CrossRef]
- Sudareva, N.N.; Penkova, A.V.; Kostereva, T.A.; Polotskii, A.E.; Polotskaya, G.A. Properties of casting solutions and ultrafiltration membranes based on fullerene-polyamide nanocomposites. Express Polym. Lett. 2012, 6, 178–188. [Google Scholar] [CrossRef]
- Ruan, H.; Guo, C.; Yu, H.; Shen, J.; Gao, C.; Sotto, A.; Van der Bruggen, B. Fabrication of a MIL-53(Al) Nanocomposite Membrane and Potential Application in Desalination of Dye Solutions. Ind. Eng. Chem. Res. 2016, 55, 12099–12110. [Google Scholar] [CrossRef]
- Shawky, H.A. Performance of aromatic polyamide RO membranes synthesized by interfacial polycondensation process in a water–tetrahydrofuran system. J. Memb. Sci. 2009, 339, 209–214. [Google Scholar] [CrossRef]
- Joshi, S.V.; Rao, A.V. Synthesis and evaluation of poly(m-phenylene isophthalamide) as a reverse osmosis membrane. Desalination 1990, 78, 355–362. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, S.S.; Zo, S.M.; Choi, S.M. Cellulose/poly-(m-phenylene isophthalamide) porous film as a tissue-engineered skin bioconstruct. Results Phys. 2018, 9, 113–120. [Google Scholar] [CrossRef]
- Xiao, K.; Zhai, Y.; Yu, J.; Ding, B. Nanonet-structured poly(m-phenylene isophthalamide)–polyurethane membranes with enhanced thermostability and wettability for high power lithium ion batteries. RSC Adv. 2015, 5, 55478–55485. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, T.; Liu, L.; Du, W.; Wang, S. Ultra-fine SiO2 nanofilament-based PMIA: A double network membrane for efficient filtration of PM particles. Sep. Purif. Technol. 2018, 202, 357–364. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yu, J.; Luo, W.; Ding, B. Microwave structured polyamide-6 nanofiber/net membrane with embedded poly(m-phenylene isophthalamide) staple fibers for effective ultrafine particle filtration. J. Mater. Chem. A 2016, 4, 6149–6157. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jeon, G.W. Microstructure and Performance of Multiwalled Carbon Nanotube/m-Aramid Composite Films as Electric Heating Elements. ACS Appl. Mater. Interfaces 2013, 5, 6527–6534. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Atta, R.R.; Zolotarev, A.A.; Mazur, A.S.; Vezo, O.S.; Lahderanta, E.; Markelov, D.A.; Ermakov, S.S. Development and investigation of novel polyphenylene isophthalamide pervaporation membranes modified with various fullerene derivatives. Sep. Purif. Technol. 2019, 226, 241–251. [Google Scholar] [CrossRef]
- Kedchaikulrat, P.; Vankelecom, I.F.J.; Faungnawakij, K.; Klaysom, C. Effects of colloidal TiO2 and additives on the interfacial polymerization of thin film nanocomposite membranes. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 601, 125046. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Shabestari, M.E.; Agathopoulos, S. High flux thin film nanocomposite membrane incorporated with functionalized TiO2@reduced graphene oxide nanohybrids for organic solvent nanofiltration. Chem. Eng. Sci. 2019, 204, 99–109. [Google Scholar] [CrossRef]
- Urper-Bayram, G.M.; Bossa, N.; Warsinger, D.M.; Koyuncu, I.; Wiesner, M. Comparative impact of SiO2 and TiO2 nanofillers on the performance of thin-film nanocomposite membranes. J. Appl. Polym. Sci. 2020, 137, 49382. [Google Scholar] [CrossRef]
- Wei, S.; Chen, Y.; Hu, X.; Wang, C.; Huang, X.; Liu, D.; Zhang, Y. Monovalent/Divalent salts separation via thin film nanocomposite nanofiltration membrane containing aminated TiO2 nanoparticles. J. Taiwan Inst. Chem. Eng. 2020, 112, 169–179. [Google Scholar] [CrossRef]
- Ngo, T.H.A.; Nguyen, C.T.M.; Do, K.D.; Duong, Q.X.; Tran, N.H.; Nguyen, H.T.V.; Tran, D.T. Improvement of Hydrophilicity for Polyamide Composite Membrane by Incorporation of Graphene Oxide-Titanium Dioxide Nanoparticles. J. Anal. Methods Chem. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Habib, Z.; Khan, S.J.; Ahmad, N.M.; Shahzad, H.M.A.; Jamal, Y.; Hashmi, I. Antibacterial behaviour of surface modified composite polyamide nanofiltration (NF) membrane by immobilizing Ag-doped TiO2 nanoparticles. Environ. Technol. 2020, 41, 3657–3669. [Google Scholar] [CrossRef]
- Al-Gamal, A.Q.; Falath, W.S.; Saleh, T.A. Enhanced efficiency of polyamide membranes by incorporating TiO2-Graphene oxide for water purification. J. Mol. Liq. 2021, 323, 114922. [Google Scholar] [CrossRef]
- Gayed, H.M.; El Fadl, F.I.A.; Maziad, N.A.; El-Aassar, A.H.M.; Abdel Mottaleb, M.S.A. Surface modification of composite polyamide reverse osmosis membrane by irradiated chitosan and TiO2 nanoparticles. Desalin. WATER Treat. 2019, 160, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.A.; Goh, P.S.; Wong, K.C.; Zulhairun, A.K.; Ismail, A.F. Enhancing desalination performance of thin film composite membrane through layer by layer assembly of oppositely charged titania nanosheet. Desalination 2020, 476, 114167. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Mousavi, S.A.; Heydari, H.; Mousavi, D.V. Improvement of performance and fouling resistance of polyamide reverse osmosis membranes using acrylamide and TiO2 nanoparticles under UV irradiation for water desalination. J. Appl. Polym. Sci. 2020, 137, 48461. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Zulhairun, A.K.; Ismail, A.F. Antifouling Property of Oppositely Charged Titania Nanosheet Assembled on Thin Film Composite Reverse Osmosis Membrane for Highly Concentrated Oily Saline Water Treatment. Membranes 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Zarshenas, K.; Jiang, G.; Zhang, J.; Jauhar, M.A.; Chen, Z. Atomic scale manipulation of sublayer with functional TiO2 nanofilm toward high-performance reverse osmosis membrane. Desalination 2020, 480, 114342. [Google Scholar] [CrossRef]
- Liu, X.-W.; Cao, Y.; Li, Y.-X.; Xu, Z.-L.; Li, Z.; Wang, M.; Ma, X.-H. High-performance polyamide/ceramic hollow fiber TFC membranes with TiO2 interlayer for pervaporation dehydration of isopropanol solution. J. Memb. Sci. 2019, 576, 26–35. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Łożyńska, M.; Barszcz, W.; Sowa, S.; Wieciński, P.; Woskowicz, E. Microfiltration Membranes Modified with Composition of Titanium Oxide and Silver Oxide by Magnetron Sputtering. Polymers 2020, 13, 141. [Google Scholar] [CrossRef]
- Plisko, T.V.; Liubimova, A.S.; Bildyukevich, A.V.; Penkova, A.V.; Dmitrenko, M.E.; Mikhailovskii, V.Y.; Melnikova, G.B.; Semenov, K.N.; Doroshkevich, N.V.; Kuzminova, A.I. Fabrication and characterization of polyamide-fullerenol thin film nanocomposite hollow fiber membranes with enhanced antifouling performance. J. Memb. Sci. 2018, 551, 20–36. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Rajabi, L.; Derakhshan, A.A.; Seyedpour, F. PAA grafting onto new acrylate-alumoxane/PES mixed matrix nano-enhanced membrane: Preparation, characterization and performance in dye removal. Chem. Eng. J. 2013, 221, 111–123. [Google Scholar] [CrossRef]
- Ma, T.; Su, Y.; Li, Y.; Zhang, R.; Liu, Y.; He, M.; Li, Y.; Dong, N.; Wu, H.; Jiang, Z. Fabrication of electro-neutral nanofiltration membranes at neutral pH with antifouling surface via interfacial polymerization from a novel zwitterionic amine monomer. J. Memb. Sci. 2016, 503, 101–109. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Z.; Pan, Y.; Zeng, G.; Shi, H.; Yang, X.; Li, F.; Yang, S.; He, Y. Enhancing the photocatalytic and antibacterial property of polyvinylidene fluoride membrane by blending Ag–TiO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 3865–3874. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Kumar, M.; Villalobos, L.F.; Vovusha, H.; Shevate, R.; Schwingenschlögl, U.; Peinemann, K.-V. Polybenzimidazole-based mixed membranes with exceptionally high water vapor permeability and selectivity. J. Mater. Chem. A 2017, 5, 21807–21819. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Im, S.J.; Kim, J.H.; Kim, H.J.; Kim, J.P.; Min, B.R. Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination 2008, 219, 48–56. [Google Scholar] [CrossRef]
- Bai, L.; Bossa, N.; Qu, F.; Winglee, J.; Li, G.; Sun, K.; Liang, H.; Wiesner, M.R. Comparison of Hydrophilicity and Mechanical Properties of Nanocomposite Membranes with Cellulose Nanocrystals and Carbon Nanotubes. Environ. Sci. Technol. 2017, 51, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Li, X.; Zhao, X.; Liu, D.; He, C. Engineering a Highly Hydrophilic PVDF Membrane via Binding TiO2 Nanoparticles and a PVA Layer onto a Membrane Surface. ACS Appl. Mater. Interfaces 2015, 7, 8427–8436. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Atta, R.R.; Zolotarev, A.A.; Plisko, T.V.; Mazur, A.S.; Solovyev, N.D.; Ermakov, S.S. The development and study of novel membrane materials based on polyphenylene isophthalamide—Pluronic F127 composite. Mater. Des. 2019, 165, 107596. [Google Scholar] [CrossRef]
- Ravidhas, C.; Anitha, B.; Arivukarasan, D.; Venkatesh, R.; Christy, A.J.; Jothivenkatachalam, K.; Nithya, A.; Moses Ezhil Raj, A.; Ravichandran, K.; Sanjeeviraja, C. Tunable morphology with selective faceted growth of visible light active TiO2 thin films by facile hydrothermal method: Structural, optical and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2016, 27, 5020–5032. [Google Scholar] [CrossRef]
- Ben Chobba, M.; Messaoud, M.; Weththimuni, M.L.; Bouaziz, J.; Licchelli, M.; De Leo, F.; Urzì, C. Preparation and characterization of photocatalytic Gd-doped TiO2 nanoparticles for water treatment. Environ. Sci. Pollut. Res. 2019, 26, 32734–32745. [Google Scholar] [CrossRef] [PubMed]










| Membrane | Ra, nm | Rq, nm | Total Porosity, % | Contact Angle, ° |
|---|---|---|---|---|
| PA | 7.9 | 10.1 | 88 | 31 ± 2 |
| PA/TiO2 (0.3) | 8.5 | 11.0 | 90 | 25 ± 2 |
| PA/TiO2 (0.5) | 9.8 | 12.6 | 83 | 22 ± 2 |
| PA/TiO2 (0.75) | 14.0 | 18.9 | 79 | 20 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Liamin, V.; Plisko, T.; Burts, K.; Bildyukevich, A.; Ermakov, S.; Penkova, A. Novel High Flux Poly(m-phenylene isophtalamide)/TiO2 Membranes for Ultrafiltration with Enhanced Antifouling Performance. Polymers 2021, 13, 2804. https://doi.org/10.3390/polym13162804
Dmitrenko M, Kuzminova A, Zolotarev A, Liamin V, Plisko T, Burts K, Bildyukevich A, Ermakov S, Penkova A. Novel High Flux Poly(m-phenylene isophtalamide)/TiO2 Membranes for Ultrafiltration with Enhanced Antifouling Performance. Polymers. 2021; 13(16):2804. https://doi.org/10.3390/polym13162804
Chicago/Turabian StyleDmitrenko, Mariia, Anna Kuzminova, Andrey Zolotarev, Vladislav Liamin, Tatiana Plisko, Katsiaryna Burts, Alexandr Bildyukevich, Sergey Ermakov, and Anastasia Penkova. 2021. "Novel High Flux Poly(m-phenylene isophtalamide)/TiO2 Membranes for Ultrafiltration with Enhanced Antifouling Performance" Polymers 13, no. 16: 2804. https://doi.org/10.3390/polym13162804
APA StyleDmitrenko, M., Kuzminova, A., Zolotarev, A., Liamin, V., Plisko, T., Burts, K., Bildyukevich, A., Ermakov, S., & Penkova, A. (2021). Novel High Flux Poly(m-phenylene isophtalamide)/TiO2 Membranes for Ultrafiltration with Enhanced Antifouling Performance. Polymers, 13(16), 2804. https://doi.org/10.3390/polym13162804