Hybrid Microporous Polymeric Materials with Outstanding Permeability and Increased Gas Transport Stability: PTMSP Aging Prevention by Sorption of the Polymerization Catalyst on HCPS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of PTMSP and PTMSP/HCPS Membranes
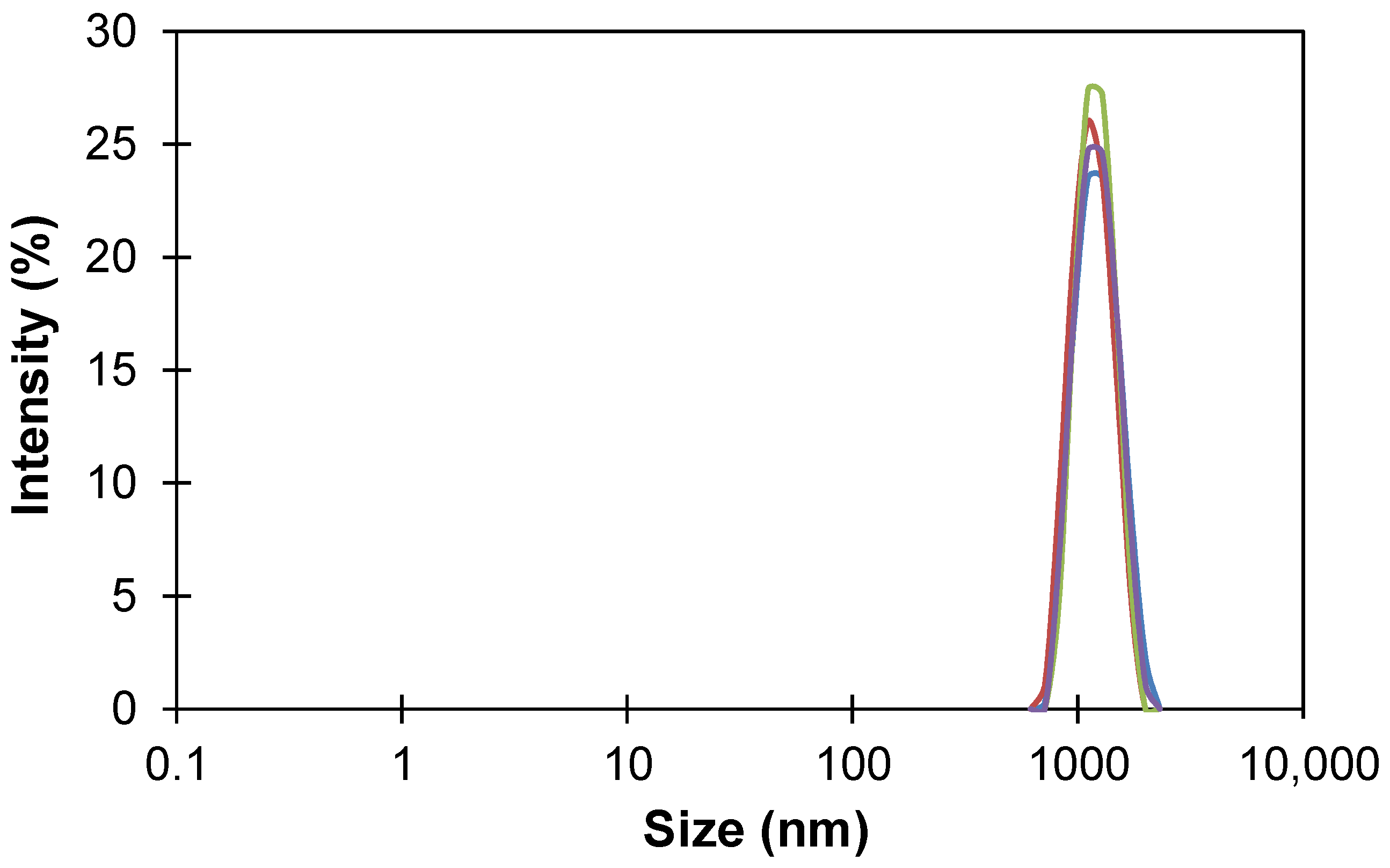
2.3. Particle Size Distribution
2.4. PTMSP Molecular Weight
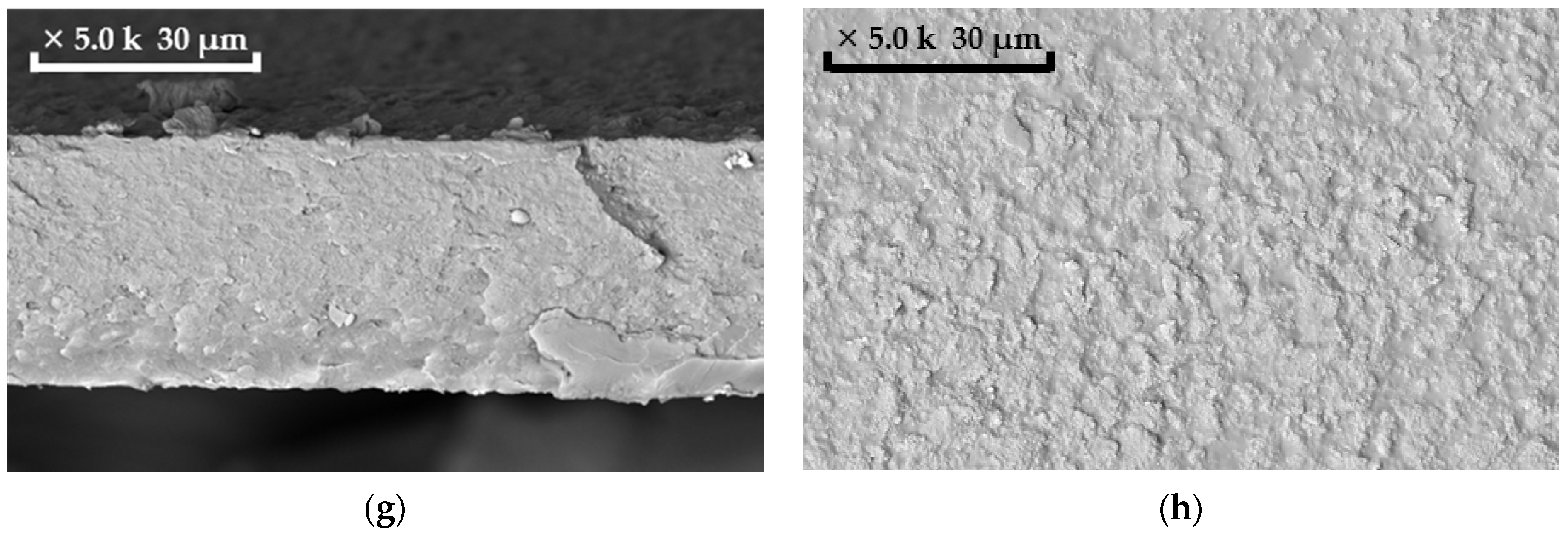
2.5. Membrane Characterization
2.6. Gas Transport Parameters
2.7. Sorption of the Polymerization Catalyst
3. Results and Discussion
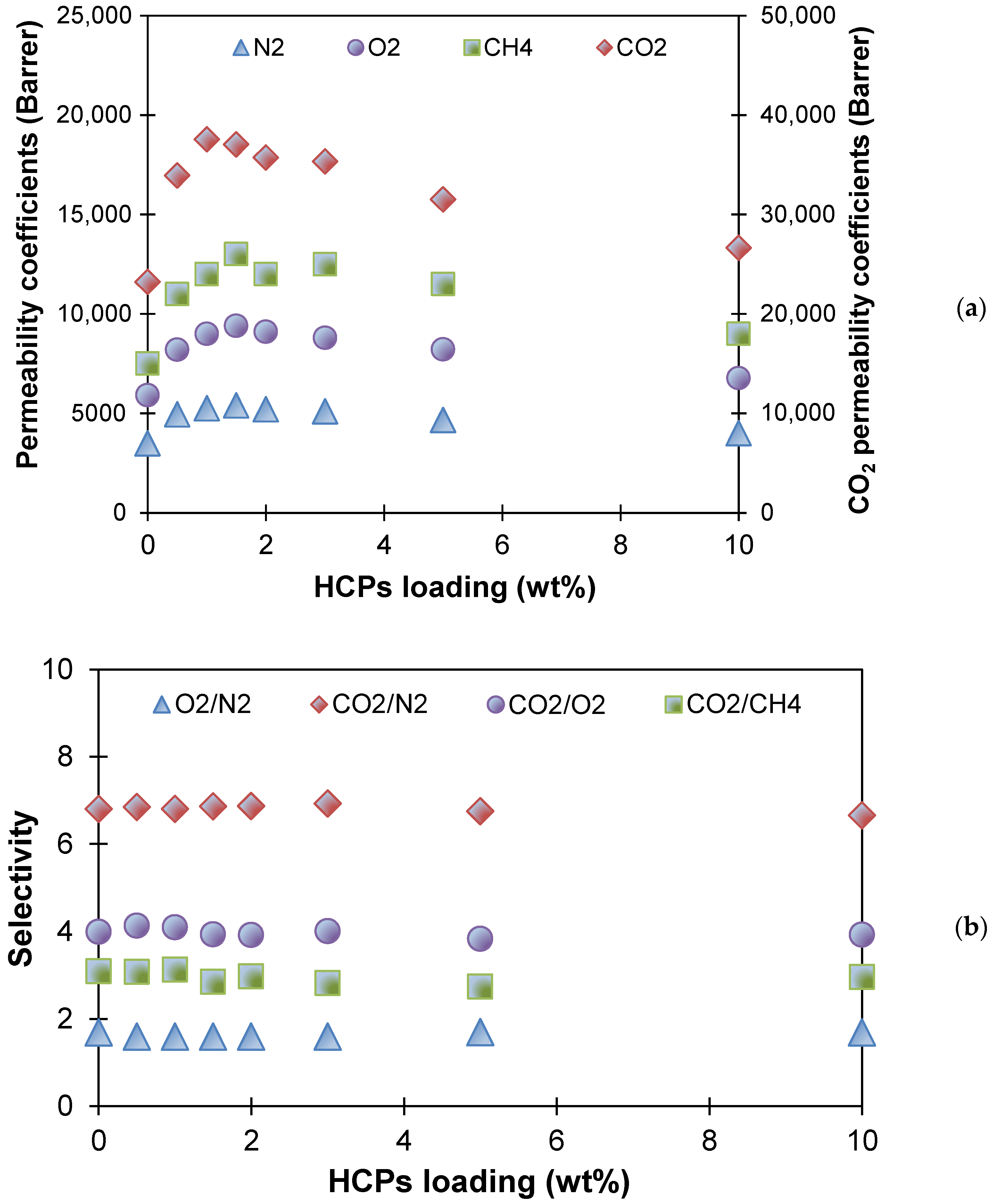
3.1. PTMSP Membranes Filled with HCPS
3.2. PTMSP Purification from Polymerization Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buonomenna, M.G. Membrane Processes for a Sustainable Industrial Growth. RSC Adv. 2013, 3, 5694–5740. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-Efficient Polymeric Gas Separation Membranes for a Sustainable Future: A Review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Pulyalina, A.; Rostovtseva, V.; Faykov, I.; Tataurov, M.; Dubovenko, R.; Shugurov, S. Development of Novel Polyamide-Imide/DES Composites and Their Application for Pervaporation and Gas Separation. Molecules 2021, 26, 990. [Google Scholar] [CrossRef] [PubMed]
- Volkov, V.; Borisov, I.; Golubev, G.; Vasilevsky, V.; Matveev, D.; Bondarenko, G.; Volkov, A. Sorption-assisted Thermopervaporation Method for Organics Recovery from ABE Fermentation Broth. J. Chem. Technol. Biotechnol. 2020, 95, 40–51. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymeric Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Volkov, A.V.; Korneeva, G.A.; Tereshchenko, G.F. Organic Solvent Nanofiltration: Prospects and Application. Russ. Chem. Rev. 2008, 77, 983. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Nagai, K.; Masuda, T.; Nakagawa, T.; Freeman, B.D.; Pinnau, I. Poly [1-(Trimethylsilyl)-1-Propyne] and Related Polymers: Synthesis, Properties and Functions. Prog. Polym. Sci. 2001, 26, 721–798. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Bermesheva, E.V.; Andreyanov, F.A.; Borisov, I.L.; Zarezin, D.P.; Bakhtin, D.S.; Gavrilova, N.N.; Ilyasov, I.R.; Nechaev, M.S.; Asachenko, A.F. Modifications of Addition Poly (5-Vinyl-2-Norbornene) and Gas-Transport Properties of the Obtained Polymers. React. Funct. Polym. 2020, 149, 104513. [Google Scholar] [CrossRef]
- Karpov, G.O.; Borisov, I.L.; Volkov, A.V.; Finkelshtein, E.S.; Bermeshev, M.V. Synthesis and Gas Transport Properties of Addition Polynorbornene with Perfluorophenyl Side Groups. Polymers 2020, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M. Polymers of Intrinsic Microporosity (PIMs): Organic Materials for Membrane Separations, Heterogeneous Catalysis and Hydrogen Storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Yampolskii, Y.; Starannikova, L.; Belov, N.; Bermeshev, M.; Gringolts, M.; Finkelshtein, E. Solubility Controlled Permeation of Hydrocarbons: New Membrane Materials and Results. J. Membr. Sci. 2014, 453, 532–545. [Google Scholar] [CrossRef]
- Talluri, V.; Patakova, P.; Moucha, T.; Vopicka, O. Transient and Steady Pervaporation of 1-Butanol–Water Mixtures through a Poly [1-(Trimethylsilyl)-1-Propyne](Ptmsp) Membrane. Polymers 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisov, I.L.; Golubev, G.S.; Patrusheva, E.V.; Sinekoy, S.P. Intensification of acetone-butanol-ethanol fermentation via products recovery: Thermopervaporation assisted by phase separation. Chem. Eng. Trans. 2018, 64, 43–48. [Google Scholar]
- Nagai, K.; Nakagawa, T. Effects of Aging on the Gas Permeability and Solubility in Poly (1-Trimethylsilyl-1-Propyne) Membranes Synthesized with Various Catalysts. J. Membr. Sci. 1995, 105, 261–272. [Google Scholar] [CrossRef]
- Smith, S.J.; Hou, R.; Konstas, K.; Akram, A.; Lau, C.H.; Hill, M.R. Control of Physical Aging in Super-Glassy Polymer Mixed Matrix Membranes. Acc. Chem. Res. 2020, 53, 1381–1388. [Google Scholar] [CrossRef]
- Tiwari, R.R.; Smith, Z.P.; Lin, H.; Freeman, B.D.; Paul, D.R. Gas Permeation in Thin Films of “High Free-Volume” Glassy Perfluoropolymers: Part I. Physical Aging. Polymer 2014, 55, 5788–5800. [Google Scholar] [CrossRef]
- Yampol’skii, Y.P.; Shishatskii, S.M.; Shantorovich, V.P.; Antipov, E.M.; Kuzmin, N.N.; Rykov, S.V.; Khodjaeva, V.L.; Plate, N.A. Transport Characteristics and Other Physicochemical Properties of Aged Poly (1-(Trimethylsilyl)-1-; Propyne). J. Appl. Polym. Sci. 1993, 48, 1935–1944. [Google Scholar] [CrossRef]
- Yushkin, A.; Grekhov, A.; Matson, S.; Bermeshev, M.; Khotimsky, V.; Finkelstein, E.; Budd, P.M.; Volkov, V.; Vlugt, T.J.; Volkov, A. Study of Glassy Polymers Fractional Accessible Volume (FAV) by Extended Method of Hydrostatic Weighing: Effect of Porous Structure on Liquid Transport. React. Funct. Polym. 2015, 86, 269–281. [Google Scholar] [CrossRef]
- Dorkenoo, K.D.; Pfromm, P.H. Accelerated Physical Aging of Thin Poly [1-(Trimethylsilyl)-1-Propyne] Films. Macromolecules 2000, 33, 3747–3751. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Sokolova, M.P.; Chen, B.; Plisko, T.V.; Markelov, D.A.; Ermakov, S.S. Impact of fullerene loading on the structure and transport properties of polysulfone mixed-matrix membranes. J. Mater. Sci. 2016, 51, 7652–7659. [Google Scholar] [CrossRef]
- Apel, P.Y.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of Membrane Science Development. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Pulyalina, A.; Rostovtseva, V.; Polotskaya, G.; Vinogradova, L.; Zoolshoev, Z.; Simonova, M.; Hairullin, A.; Toikka, A.; Pientka, Z. Hybrid Macromolecular Stars Incorporated Poly(Phenylene Oxide) Membranes: Organization, Physical, and Gas Separation Properties. Polymer 2019, 172, 355–364. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed Matrix Membranes Using Carbon Molecular Sieves: I. Preparation and Experimental Results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Smith, S.J.; Konstas, K.; Lau, C.H.; Gozukara, Y.M.; Easton, C.D.; Mulder, R.J.; Ladewig, B.P.; Hill, M.R. Post-Synthetic Annealing: Linker Self-Exchange in UiO-66 and Its Effect on Polymer–Metal Organic Framework Interaction. Cryst. Growth Des. 2017, 17, 4384–4392. [Google Scholar] [CrossRef]
- Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Surkova, V.A.; Liamin, V.P.; Markelov, D.A.; Komolkin, A.V.; Poloneeva, D.Y.; Laptenkova, A.V.; Selyutin, A.A.; et al. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66 (NH2)-EDTA for highly efficient methanol isolation. Sep. Purif. Technol. 2021, 263, 118370. [Google Scholar] [CrossRef]
- Dai, Z.; Løining, V.; Deng, J.; Ansaloni, L.; Deng, L. Poly (1-Trimethylsilyl-1-Propyne)-Based Hybrid Membranes: Effects of Various Nanofillers and Feed Gas Humidity on CO2 Permeation. Membranes 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhaimi, N.H.; Yeong, Y.F.; Ch’ng, C.W.M.; Jusoh, N. Tailoring CO2/CH4 Separation Performance of Mixed Matrix Membranes by Using ZIF-8 Particles Functionalized with Different Amine Groups. Polymers 2019, 11, 2042. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.; Sprouster, D.J. Ending Aging in Super Glassy Polymer Membranes. Angew. Chem. Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.C.; Mudie, S.; Kennedy, D.F.; Howard, S.C.; Hill, A.J.; Hill, M.R. Gas-separation Membranes Loaded with Porous Aromatic Frameworks That Improve with Age. Angew. Chem. 2015, 127, 2707–2711. [Google Scholar] [CrossRef]
- Volkov, A.V.; Bakhtin, D.S.; Kulikov, L.A.; Terenina, M.V.; Golubev, G.S.; Bondarenko, G.N.; Legkov, S.A.; Shandryuk, G.A.; Volkov, V.V.; Khotimskiy, V.S. Stabilization of Gas Transport Properties of PTMSP with Porous Aromatic Framework: Effect of Annealing. J. Membr. Sci. 2016, 517, 80–90. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Legkov, S.A.; Khotimskiy, V.S.; Levin, I.S.; Borisov, I.L.; Maksimov, A.L.; Volkov, V.V.; Karakhanov, E.A.; Volkov, A.V. Aging of Thin-Film Composite Membranes Based on PTMSP Loaded with Porous Aromatic Frameworks. J. Membr. Sci. 2018, 554, 211–220. [Google Scholar] [CrossRef]
- Bakhtin, D.; Bazhenov, S.; Polevaya, V.; Grushevenko, E.; Makaev, S.; Karpacheva, G.; Volkov, V.; Volkov, A. Aging of Thin-Film Composite Membranes Based on Crosslinked PTMSP/PEI Loaded with Highly Porous Carbon Nanoparticles of Infrared Pyrolyzed Polyacrylonitrile. Membranes 2020, 10, 419. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Mitra, T.; Adams, D.J.; Cooper, A.I.; Budd, P.M. Ultrahigh-Permeance PIM-1 Based Thin Film Nanocomposite Membranes on PAN Supports for CO2 Separation. J. Membr. Sci. 2018, 564, 878–886. [Google Scholar] [CrossRef]
- Lau, C.H.; Mulet, X.; Konstas, K.; Doherty, C.M.; Sani, M.-A.; Separovic, F.; Hill, M.R.; Wood, C.D. Hypercrosslinked Additives for Ageless Gas-Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 1998–2001. [Google Scholar] [CrossRef]
- Mitra, T.; Bhavsar, R.S.; Adams, D.J.; Budd, P.M.; Cooper, A.I. PIM-1 Mixed Matrix Membranes for Gas Separations Using Cost-Effective Hypercrosslinked Nanoparticle Fillers. Chem. Commun. 2016, 52, 5581–5584. [Google Scholar] [CrossRef] [Green Version]
- Hou, R.; O’Loughlin, R.; Ackroyd, J.; Liu, Q.; Doherty, C.M.; Wang, H.; Hill, M.R.; Smith, S.J. Greatly Enhanced Gas Selectivity in Mixed-Matrix Membranes through Size-Controlled Hyper-Cross-Linked Polymer Additives. Ind. Eng. Chem. Res. 2020, 59, 13773–13782. [Google Scholar] [CrossRef]
- Golubev, G.S.; Borisov, I.L.; Litvinova, E.G.; Khotimsky, V.S.; Bakhtin, D.S.; Pastukhov, A.V.; Davankov, V.A.; Volkov, V.V. A Novel Hybrid Material Based on Polytrimethylsilylpropyne and Hypercrosslinked Polystyrene for Membrane Gas Separation and Thermopervaporation. Pet. Chem. 2017, 57, 498–510. [Google Scholar] [CrossRef]
- Olivieri, L.; Ligi, S.; De Angelis, M.G.; Cucca, G.; Pettinau, A. Effect of Graphene and Graphene Oxide Nanoplatelets on the Gas Permselectivity and Aging Behavior of Poly (Trimethylsilyl Propyne)(PTMSP). Ind. Eng. Chem. Res. 2015, 54, 11199–11211. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Wang, Z.X.; Jiang, X.; Li, T.; Lau, C.H.; Guo, Z.; Ma, J.; Shao, L. Towards Sustainable Ultrafast Molecular-Separation Membranes: From Conventional Polymers to Emerging Materials. Prog. Mater. Sci. 2018, 92, 258–283. [Google Scholar] [CrossRef] [Green Version]
- Davankov, V.A.; Tsyurupa, M.P. Structure and Properties of Hypercrosslinked Polystyrene—the First Representative of a New Class of Polymer Networks. React. Polym. 1990, 13, 27–42. [Google Scholar] [CrossRef]
- Wood, C.D.; Tan, B.; Trewin, A.; Su, F.; Rosseinsky, M.J.; Bradshaw, D.; Sun, Y.; Zhou, L.; Cooper, A.I. Microporous Organic Polymers for Methane Storage. Adv. Mater. 2008, 20, 1916–1921. [Google Scholar] [CrossRef]
- Woodward, R.T.; Stevens, L.A.; Dawson, R.; Vijayaraghavan, M.; Hasell, T.; Silverwood, I.P.; Ewing, A.V.; Ratvijitvech, T.; Exley, J.D.; Chong, S.Y. Swellable, Water-and Acid-Tolerant Polymer Sponges for Chemoselective Carbon Dioxide Capture. J. Am. Chem. Soc. 2014, 136, 9028–9035. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Porous Structure of Hypercrosslinked Polystyrene: State-of-the-Art Mini-Review. React. Funct. Polym. 2006, 66, 768–779. [Google Scholar] [CrossRef]
- Castaldo, R.; Gentile, G.; Avella, M.; Carfagna, C.; Ambrogi, V. Microporous Hyper-Crosslinked Polystyrenes and Nanocomposites with High Adsorption Properties: A Review. Polymers 2017, 9, 651. [Google Scholar] [CrossRef] [Green Version]
- Urban, J.; Svec, F.; Fréchet, J.M. Hypercrosslinking: New Approach to Porous Polymer Monolithic Capillary Columns with Large Surface Area for the Highly Efficient Separation of Small Molecules. J. Chromatogr. A 2010, 1217, 8212–8221. [Google Scholar] [CrossRef] [Green Version]
- Polymeric Adsorbent Resins for Industrial Applications. Available online: https://www.purolite.com/dam/jcr:f016decb-bf8a-4b06-b5f7-1673fad6b255/Industrial-Adsorbents-Brochure-.pdf (accessed on 10 May 2021).
- Gerzeliev, I.M.; Temnikova, V.A.; Saitov, Z.A.; Asylbaev, D.F.; Baskhanova, M.N. Synthesis of a Catalyst for Isobutane/Butylenes Alkylation Promising for Industrial Application. Pet. Chem. 2020, 60, 1170–1175. [Google Scholar] [CrossRef]
- Shishatskii, A.M.; Yampol’skii, Y.P.; Peinemann, K.-V. Effects of Film Thickness on Density and Gas Permeation Parameters of Glassy Polymers. J. Membr. Sci. 1996, 112, 275–285. [Google Scholar] [CrossRef]
- Claes, S.; Vandezande, P.; Mullens, S.; De Sitter, K.; Peeters, R.; Van Bael, M.K. Preparation and Benchmarking of Thin Film Supported PTMSP-Silica Pervaporation Membranes. J. Membr. Sci. 2012, 389, 265–271. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, E.; Higashimura, T.; Takada, K. Poly [1-(Trimethylsilyl)-1-Propyne]: A New High Polymer Synthesized with Transition-Metal Catalysts and Characterized by Extremely High Gas Permeability. J. Am. Chem. Soc. 1983, 105, 7473–7474. [Google Scholar] [CrossRef]
- Poly(Trimethylsilyl)Propyne Properties. Available online: https://www.gelest.com/product/SSP-070/ (accessed on 10 May 2021).
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H. Enhanced Selectivity in Mixed Matrix Membranes for CO2 Capture through Efficient Dispersion of Amine-Functionalized MOF Nanoparticles. Nat. Energy 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Tasaka, S.; Inagaki, N.; Igawa, M. Effect of Annealing on Structure and Permeability of Poly [(l-trimethylsilyl)-l-propyne]. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 691–694. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Physical Aging of Thin Glassy Polymer Films Monitored by Gas Permeability. Polymer 2004, 45, 8377–8393. [Google Scholar] [CrossRef]
- Morlière, N.; Vallières, C.; Perrin, L.; Roizard, D. Impact of Thermal Ageing on Sorption and Diffusion Properties of PTMSP. J. Membr. Sci. 2006, 270, 123–131. [Google Scholar] [CrossRef]
- Li, B.; Su, F.; Luo, H.-K.; Liang, L.; Tan, B. Hypercrosslinked Microporous Polymer Networks for Effective Removal of Toxic Metal Ions from Water. Microporous Mesoporous Mater. 2011, 138, 207–214. [Google Scholar] [CrossRef]
- Khotimsky, V.S.; Tchirkova, M.V.; Litvinova, E.G.; Rebrov, A.I.; Bondarenko, G.N. Poly [1-(Trimethylgermyl)-1-propyne] and Poly [1-(Trimethylsilyl)-1-propyne] with Various Geometries: Their Synthesis and Properties. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2133–2155. [Google Scholar] [CrossRef]
- Matson, S.M.; Kossov, A.A.; Makrushin, V.P.; Levin, I.S.; Zhilyaeva, N.A.; Litvinova, E.G.; Khotimskiy, V.S. Synthesis, Structure and Properties of Poly (1-Trimethylsilyl-1-Propyne) Obtained with NbBr 5-and TaBr 5-Based Catalytic Systems. Polym. Sci. Ser. C 2019, 61, 76–85. [Google Scholar] [CrossRef]
- Nagai, K.; Freeman, B.D.; Hill, A.J. Effect of Physical Aging of Poly (1-trimethylsilyl-1-propyne) Films Synthesized with TaCl5 and NbCl5 on Gas Permeability, Fractional Free Volume, and Positron Annihilation Lifetime Spectroscopy Parameters. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1222–1239. [Google Scholar] [CrossRef]

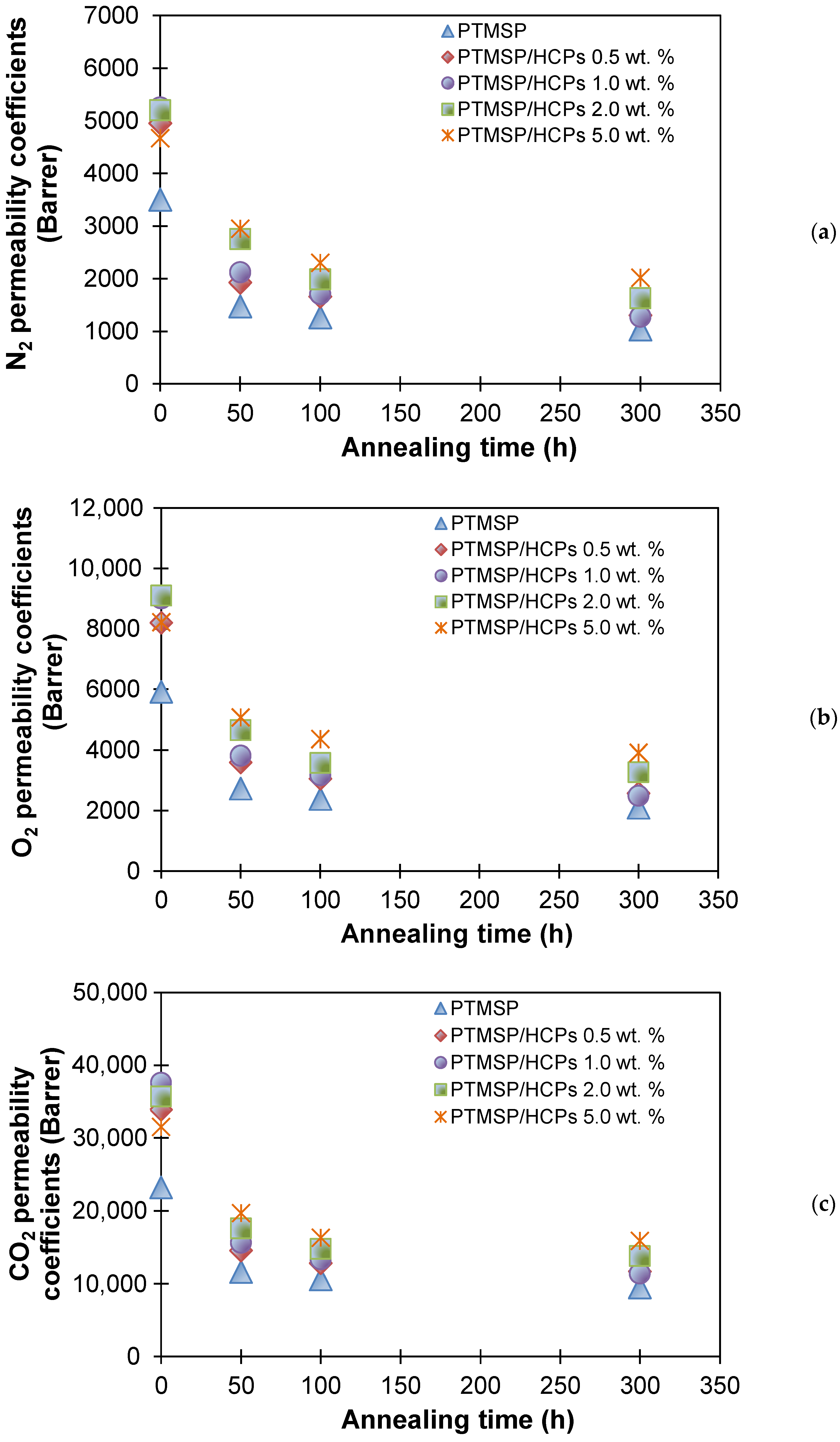
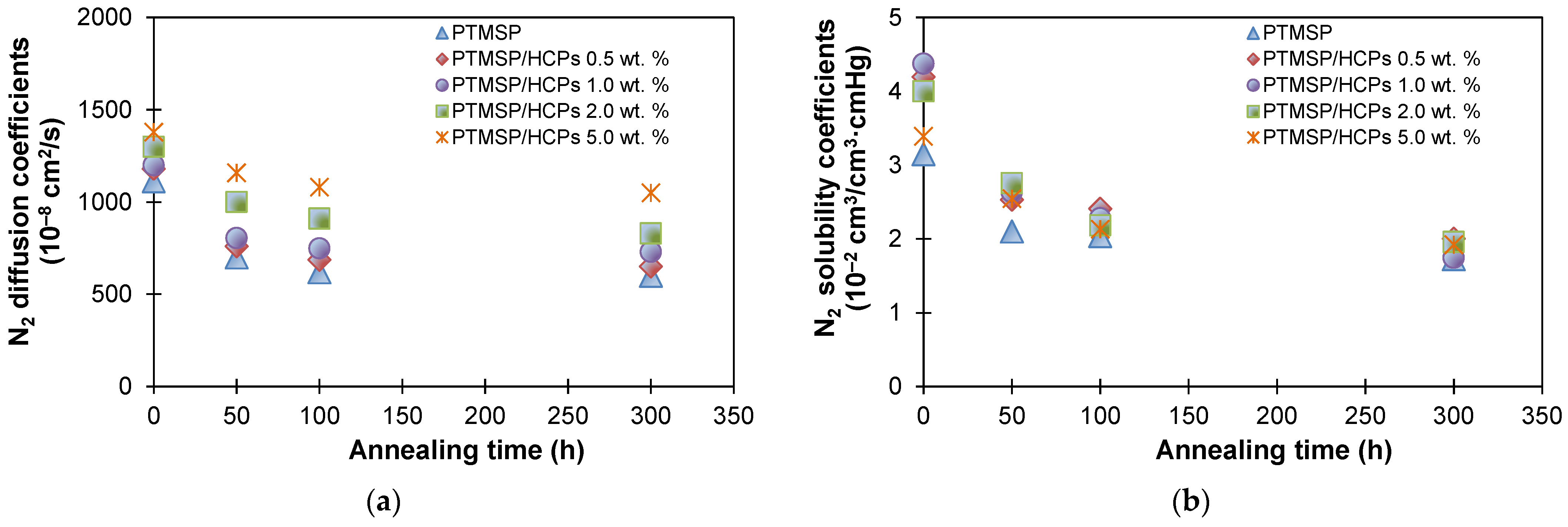
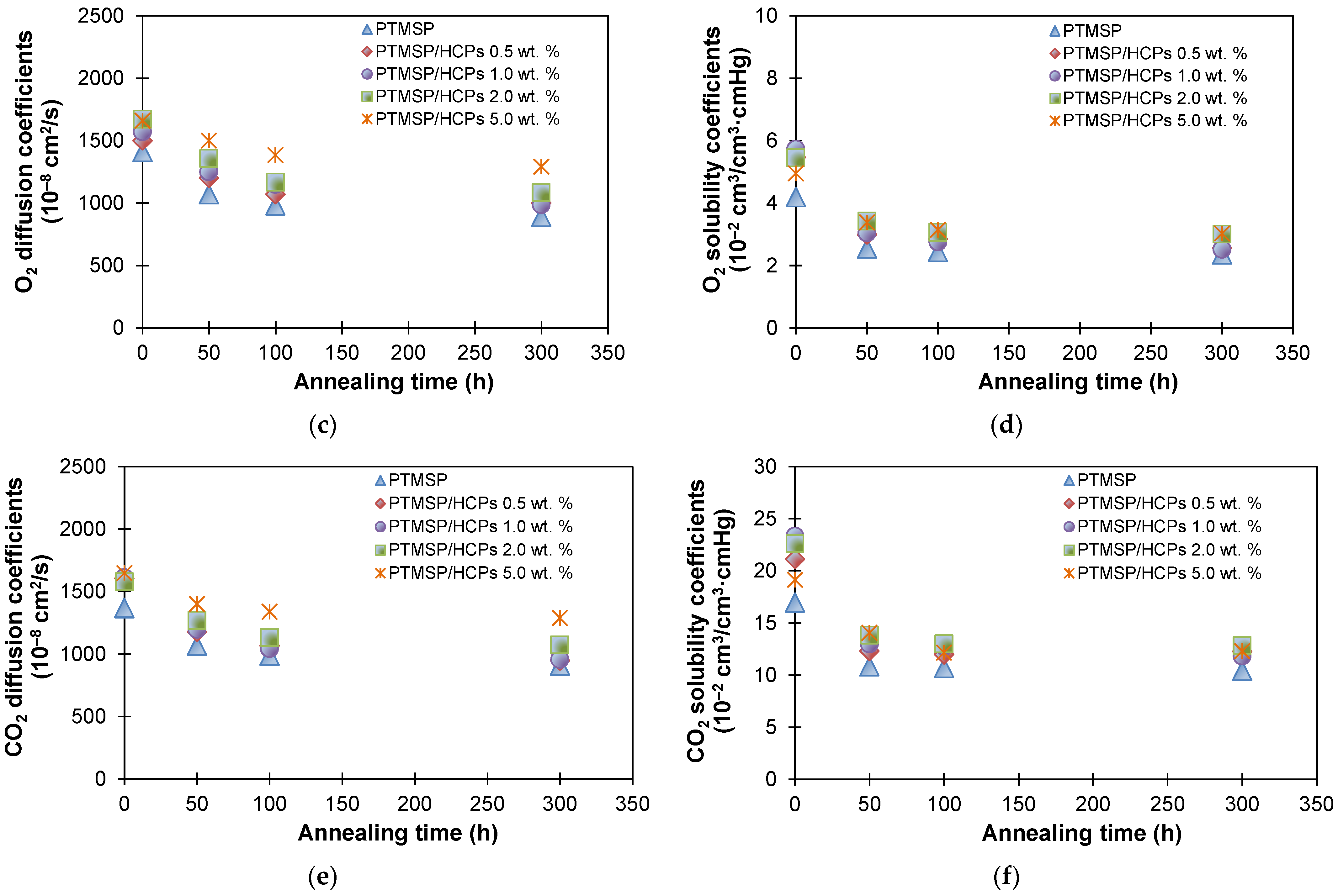
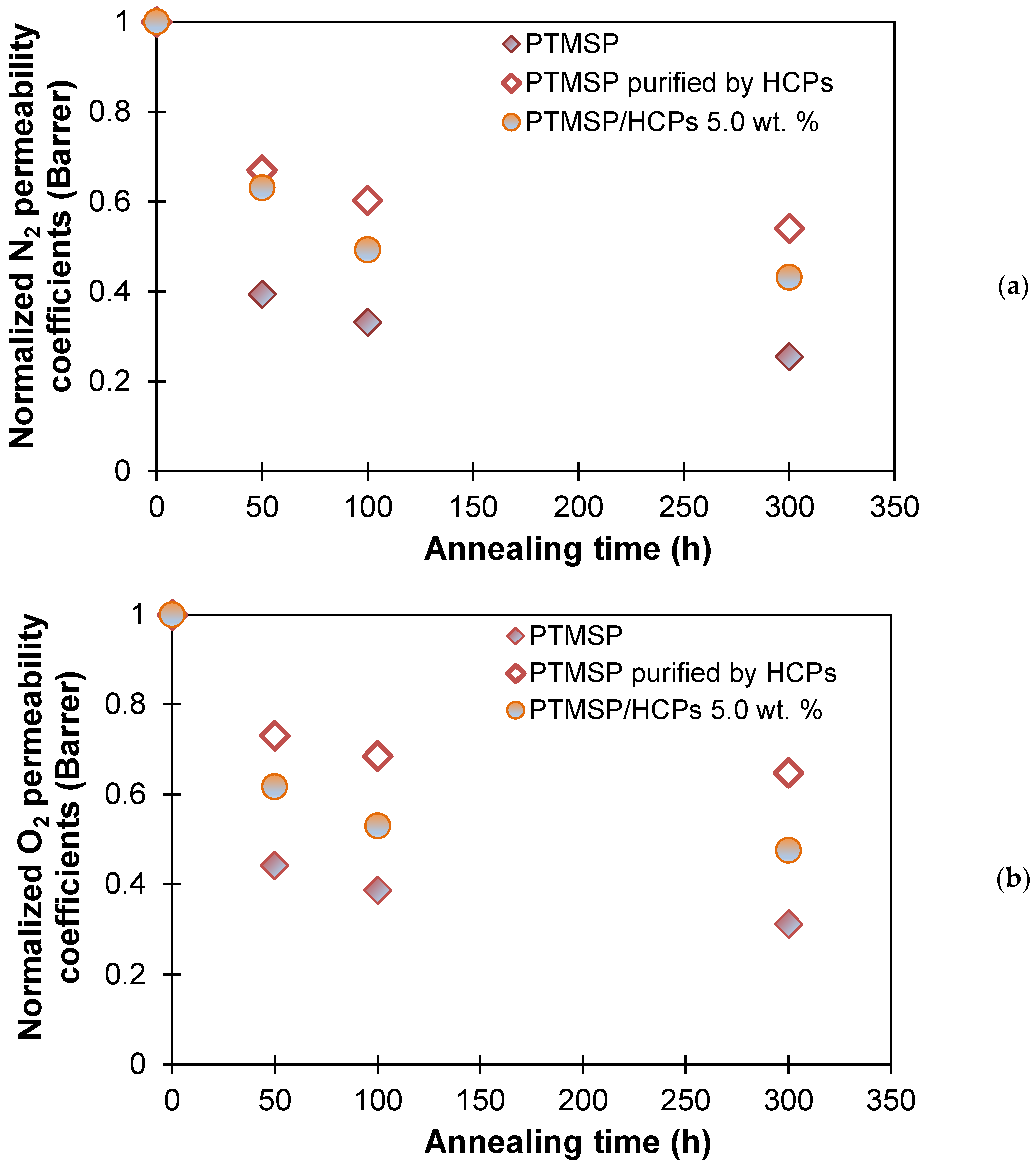

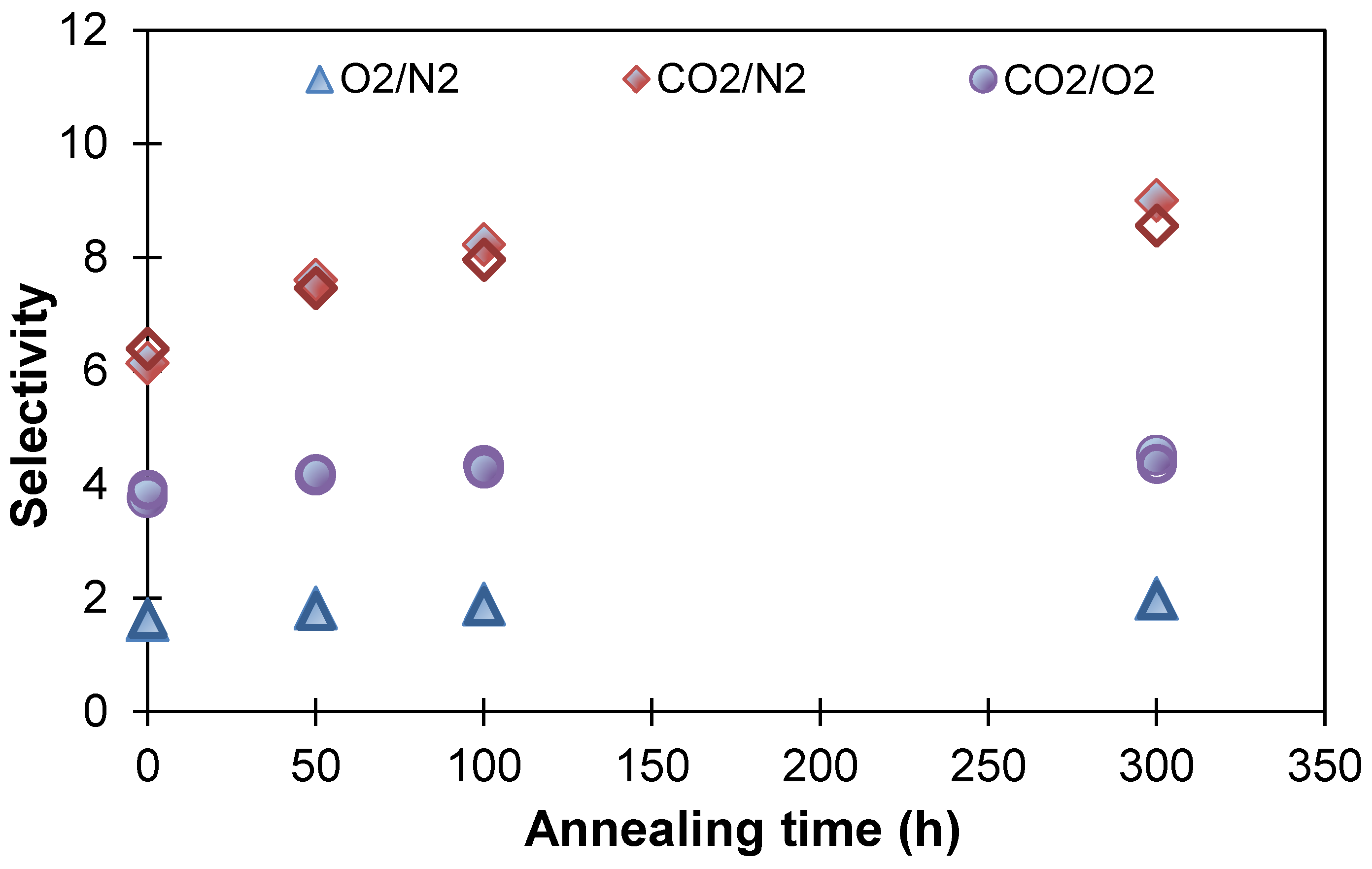
| Membranes | Decrease in Gas Permeability Coefficient, % | Selectivity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| O2/N2 | CO2/N2 | CO2/O2 | |||||||
| N2 | O2 | CO2 | Before | After | Before | After | Before | After | |
| PTMSP | 71 | 65 | 60 | 1.7 | 2.0 | 6.7 | 9.2 | 4.0 | 4.5 |
| PTMSP/HCPS 0.5 wt % | 73 | 68 | 65 | 1.7 | 2.0 | 6.8 | 9.1 | 4.1 | 4.5 |
| PTMSP/HCPS 1.0 wt % | 75 | 72 | 69 | 1.6 | 2.0 | 6.9 | 8.9 | 4.1 | 4.5 |
| PTMSP/HCPS 2.0 wt % | 69 | 64 | 61 | 1.6 | 2.0 | 6.8 | 8.7 | 4.0 | 4.3 |
| PTMSP/HCPS 5.0 wt % | 57 | 52 | 50 | 1.6 | 1.9 | 6.8 | 8.2 | 3.9 | 4.2 |
| Element | Content in the Initial PTMSP Membrane, 31 µm, wt% | Content in Membrane after Purification of PTMSP Solution by HCPS, 34 µm, wt% |
|---|---|---|
| Si | 25.2 | 25.3 |
| Ta | 0.86 | 0.06 |
| Cl | 0.07 | 0.02 |
| Al | 0.006 | 0.001 |
| Pd | 0.53 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubev, G.; Bakhtin, D.; Makaev, S.; Borisov, I.; Volkov, A. Hybrid Microporous Polymeric Materials with Outstanding Permeability and Increased Gas Transport Stability: PTMSP Aging Prevention by Sorption of the Polymerization Catalyst on HCPS. Polymers 2021, 13, 1922. https://doi.org/10.3390/polym13121922
Golubev G, Bakhtin D, Makaev S, Borisov I, Volkov A. Hybrid Microporous Polymeric Materials with Outstanding Permeability and Increased Gas Transport Stability: PTMSP Aging Prevention by Sorption of the Polymerization Catalyst on HCPS. Polymers. 2021; 13(12):1922. https://doi.org/10.3390/polym13121922
Chicago/Turabian StyleGolubev, Georgy, Danila Bakhtin, Sergey Makaev, Ilya Borisov, and Alexey Volkov. 2021. "Hybrid Microporous Polymeric Materials with Outstanding Permeability and Increased Gas Transport Stability: PTMSP Aging Prevention by Sorption of the Polymerization Catalyst on HCPS" Polymers 13, no. 12: 1922. https://doi.org/10.3390/polym13121922
APA StyleGolubev, G., Bakhtin, D., Makaev, S., Borisov, I., & Volkov, A. (2021). Hybrid Microporous Polymeric Materials with Outstanding Permeability and Increased Gas Transport Stability: PTMSP Aging Prevention by Sorption of the Polymerization Catalyst on HCPS. Polymers, 13(12), 1922. https://doi.org/10.3390/polym13121922








