Nanocelluloses Reinforced Bio-Waterborne Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of the WBPU
2.3. Synthesis of Nanocelluloses
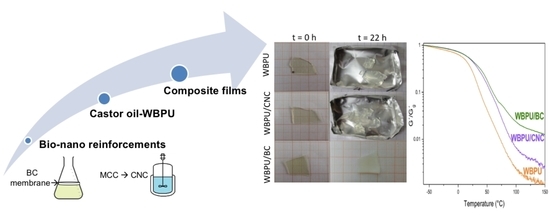
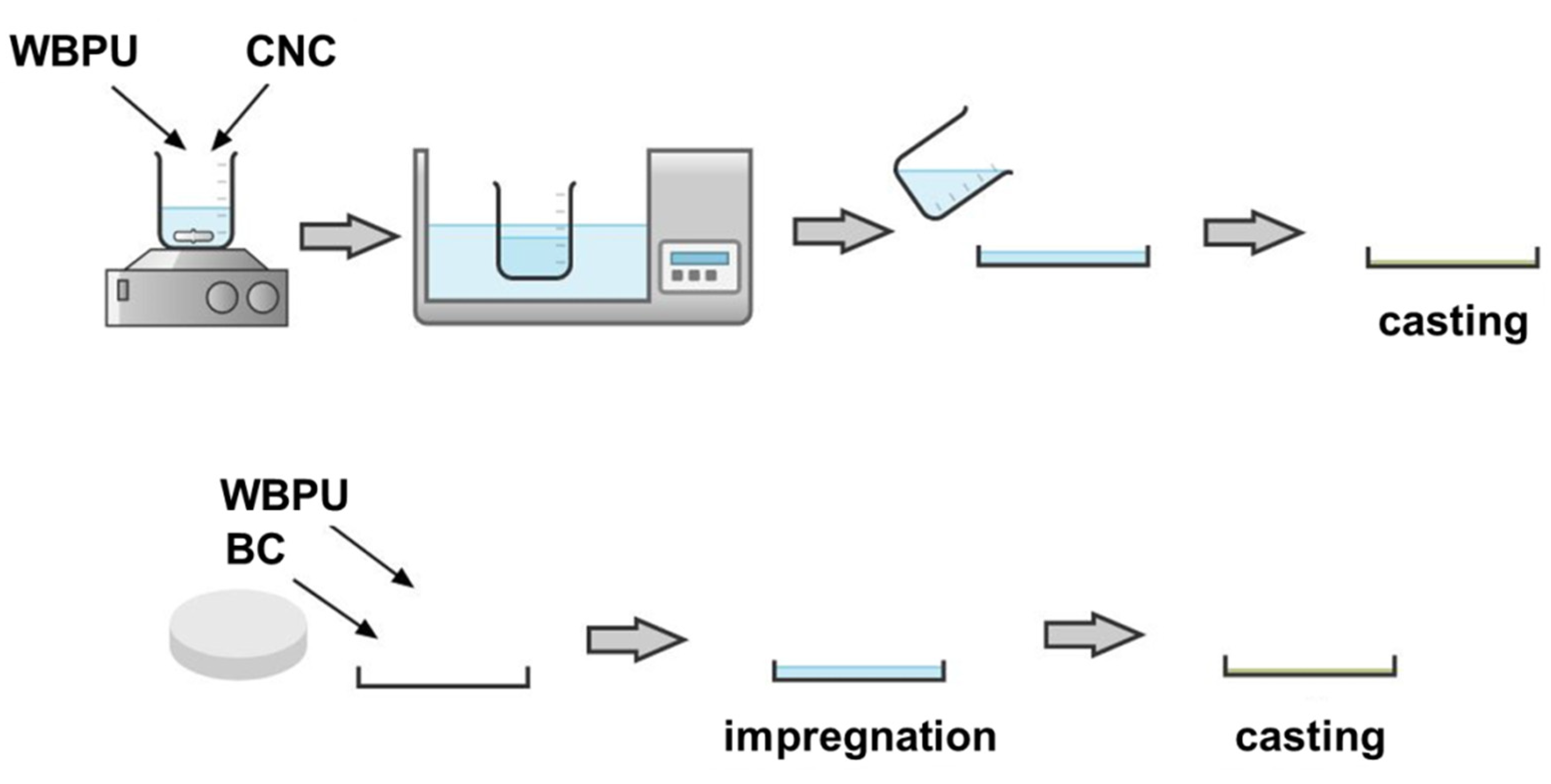
2.4. Composites Preparation
2.5. Characterization Methods
3. Results and Discussion
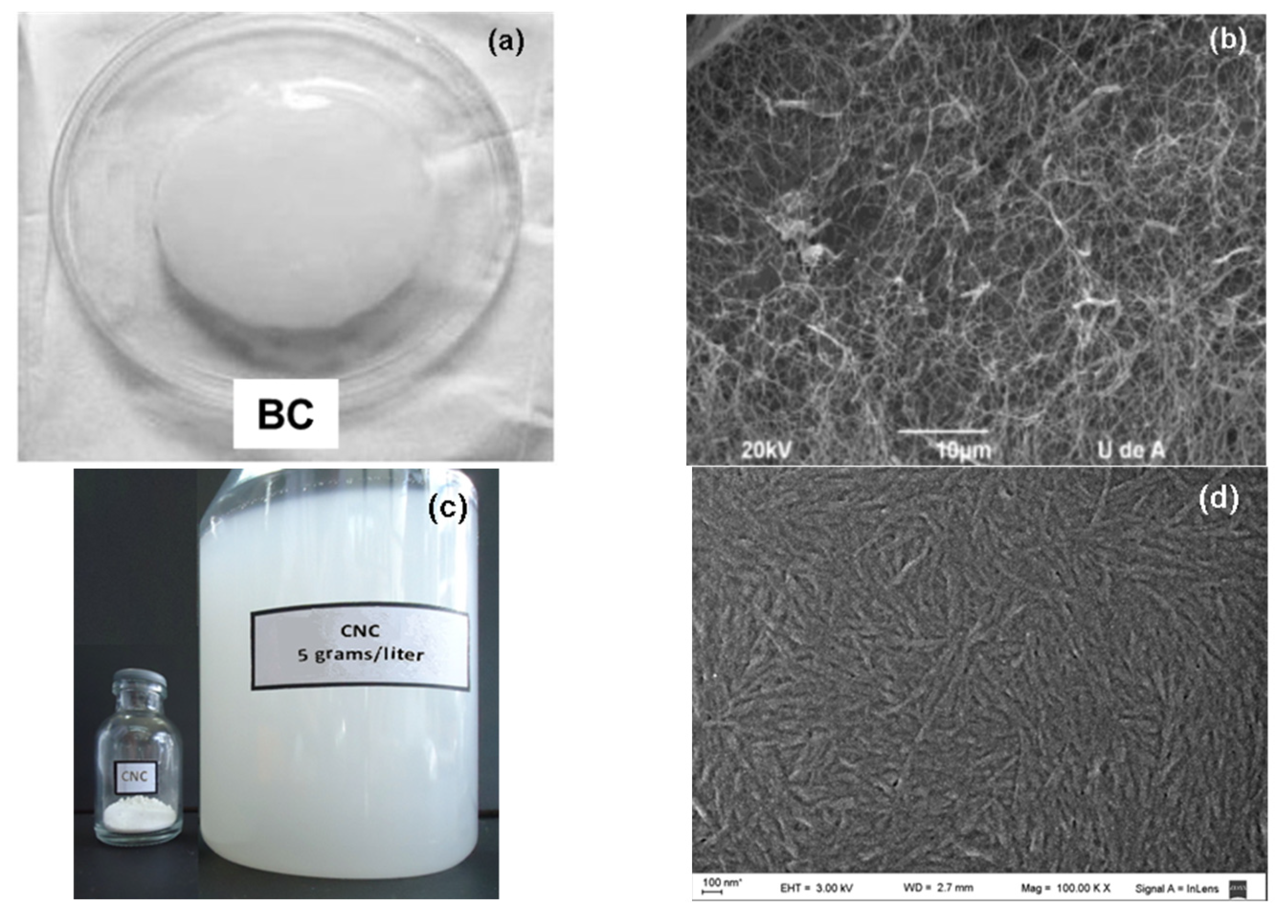
3.1. Characterization of Nanocelluloses
3.1.1. Microscopic Structure of the Nanocelluloses
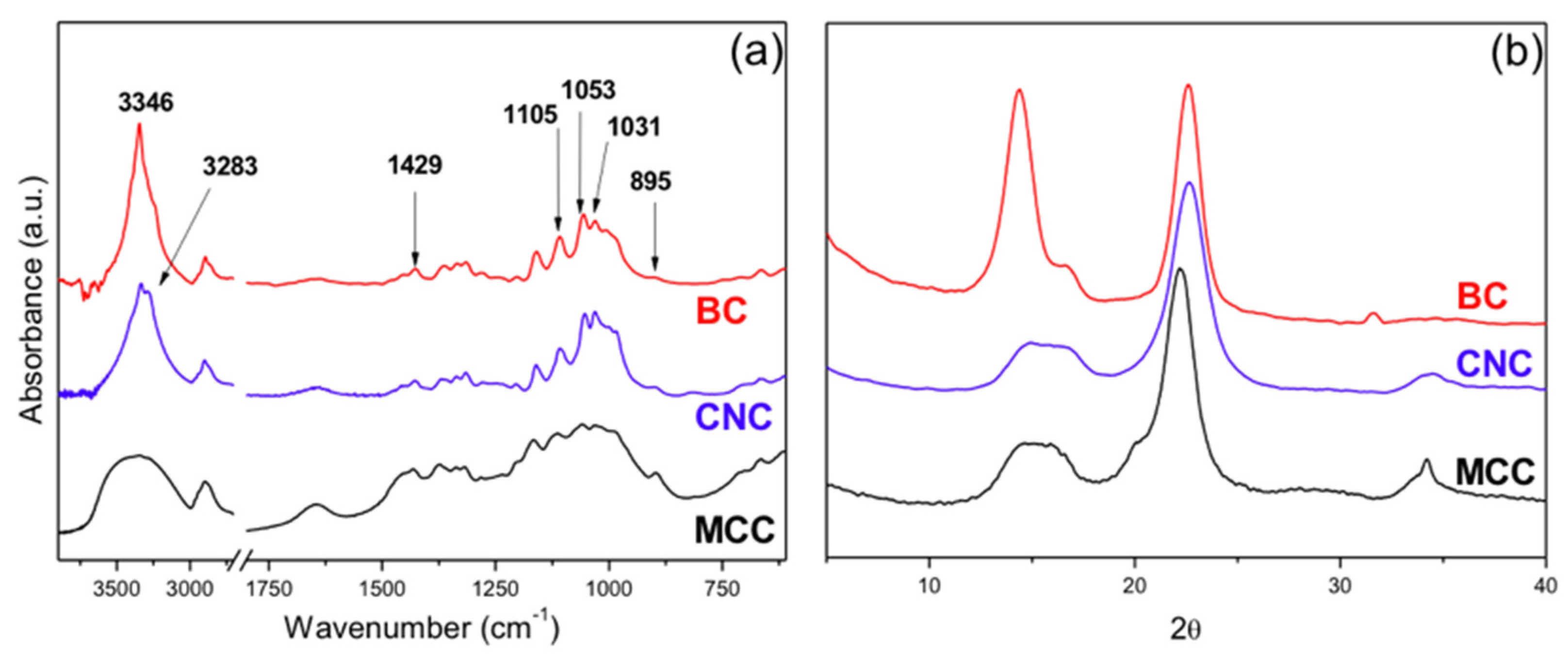
3.1.2. FTIR Characterization
3.1.3. X-ray Diffraction Characterization
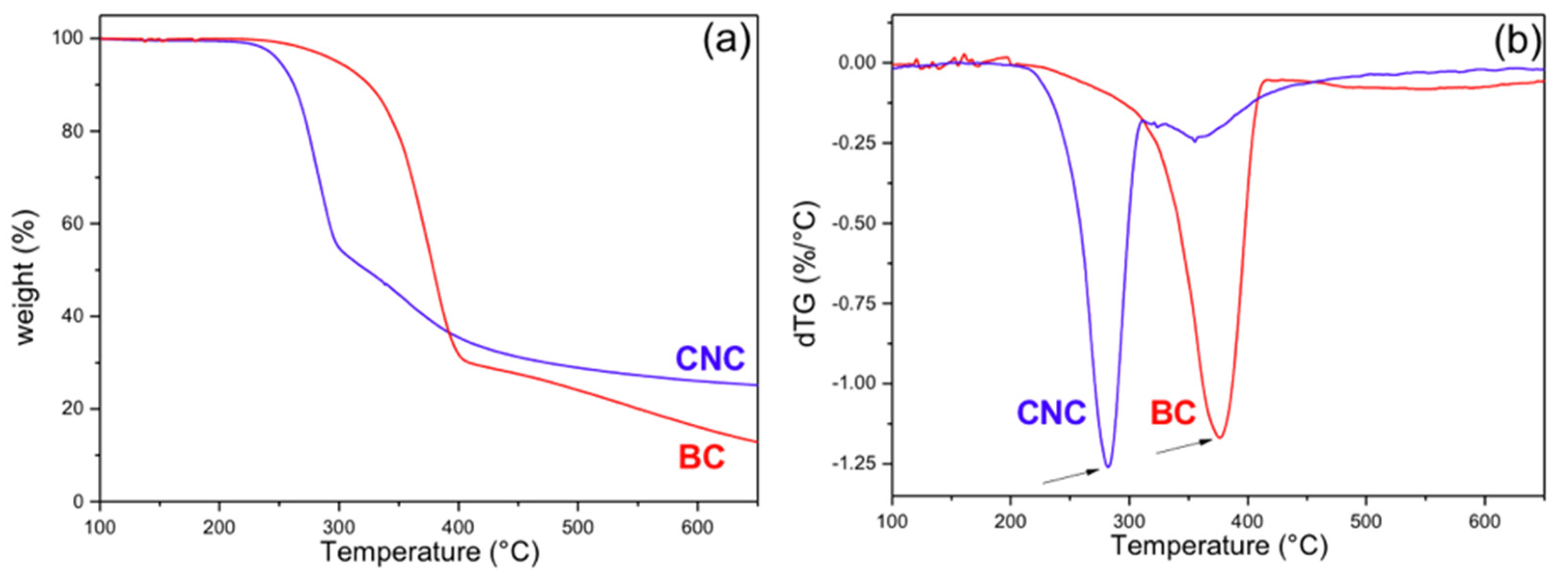
3.1.4. Thermal Degradation (Thermogravimetric Analysis (TGA))
3.2. Characterization of the Composite Films
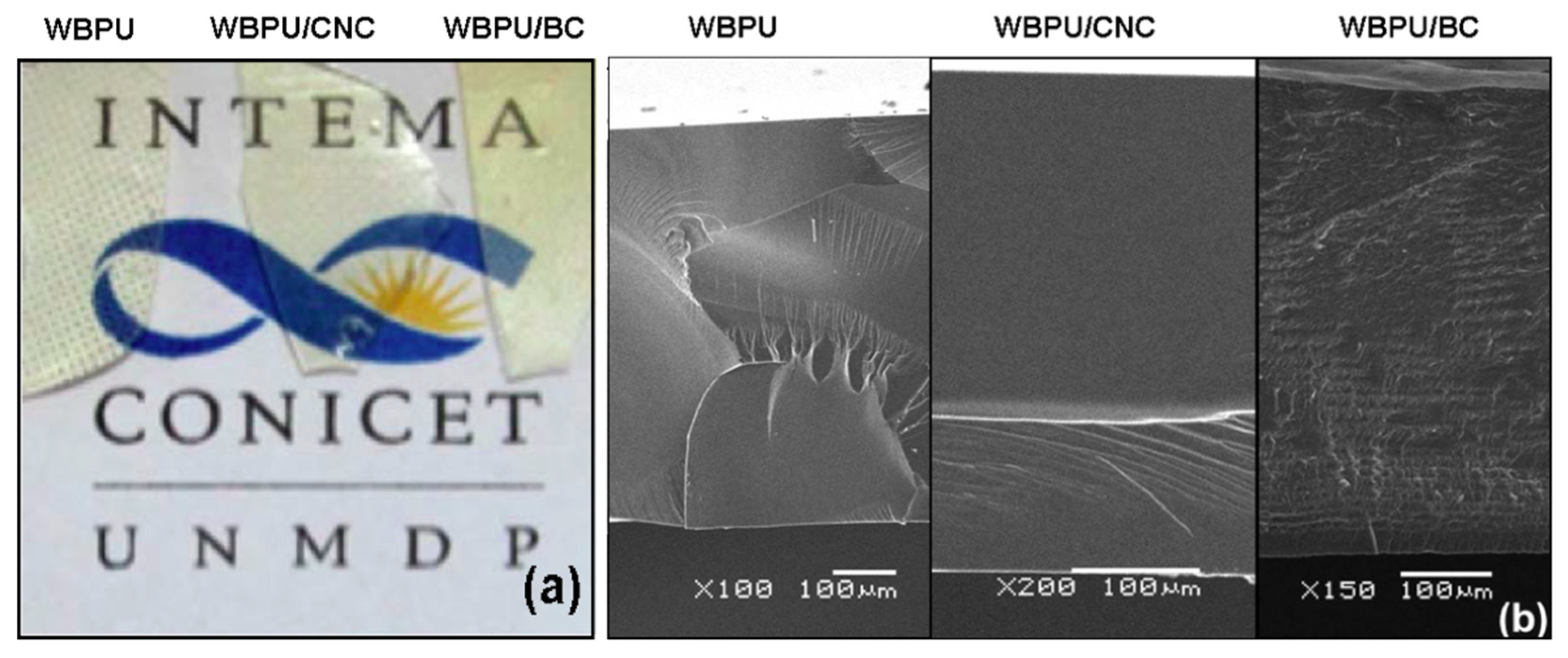
3.2.1. Optical Aspect and SEM Topology
3.2.2. FTIR and DRX Analysis of Composites
3.2.3. Thermal Characterization of the Films (DSC, DMA, and TGA)
3.2.4. Static Contact Angle and Water Absorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balart, R.; Montanes, N.; Dominici, F.; Boronat, T.; Torres-Giner, S. Environmentally friendly polymers and polymer composites. Materials 2020, 13, 4892. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Ha, S.K.; Verma, K.; Tiwari, S.K. Recent progress in selected bio-nanomaterials and their engineering applications: An overview. J. Sci. Adv. Mater. Devices 2018, 3, 263–288. [Google Scholar] [CrossRef]
- Cao, X.; Dong, H.; Li, C.M. New Nanocomposite Materials Reinforced with Flax Cellulose Nanocrystals in Waterborne Polyurethane. Biomacromolecules 2007, 8, 899–904. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Aranguren, M.I. Recent Developments in Plant Oil Based Functional Materials. Polym. Int. 2016, 65, 28–38. [Google Scholar] [CrossRef]
- Mucci, V.L.; Hormaiztegui, M.E.V.; Aranguren, M.I. Plant oil-based waterborne polyurethanes: A brief review. J. Renew. Mater. 2020, 8, 579–601. [Google Scholar] [CrossRef]
- Zafar, F.; Ghosal, A.; Sharmin, E.; Chaturvedi, R.; Nishat, N. A review on cleaner production of polymeric and nanocomposite coatings based on waterborne polyurethane dispersions from seed oils. Prog. Org. Coat. 2019, 131, 259–275. [Google Scholar] [CrossRef]
- Hormaiztegui, M.E.V.; Daga, B.; Aranguren, M.I.; Mucci, V.L. Bio-based waterborne polyurethanes reinforced with cellulose nanocrystals as coating films. Prog. Org. Coat. 2020, 144, 105649. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Xia, Y.; Kessler, M.R. Rheological behavior of environmentally friendly castor oil-based waterborne polyurethane dispersions. Macromolecules 2013, 46, 4606–4616. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Basri, M.; Jusoh, E.R.; Mamat, S. Colloidal stability and rheology of jatropha oil-based waterborne polyurethane (JPU) dispersion. Prog. Org. Coat. 2018, 125, 348–357. [Google Scholar] [CrossRef]
- Liang, H.; Liu, L.; Lu, J.; Chen, M.; Zhang, C. Castor oil-based cationic waterborne polyurethane dispersions: Storage stability, thermo-physical properties and antibacterial properties. Ind. Crops Prod. 2018, 117, 169–178. [Google Scholar] [CrossRef]
- Moreno, M.; Goikoetxea, M.; Barandiaran, M.J. Fatty Acid-Based Waterborne Coatings. In Biobased and Environmental Benign Coatings; Tiwari, A., Galanis, A., Soucek, M.D., Eds.; Scrivener Publishing LLC-Wiley: Beverly, MA, USA, 2016; pp. 161–182. [Google Scholar]
- Madbouly, S.A.; Otaigbe, J.U. Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films. Prog. Polym. Sci. 2009, 34, 1283–1332. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Preparation and properties of aqueous castor oil-based polyurethane-silica nanocomposite dispersions through a sol-gel process. Macromol. Rapid Commun. 2011, 32, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, D.H.; Raghu, A.V.; Reddy, K.R.; Lee, H.I.; Yoon, K.S.; Jeong, H.M.; Kim, B.K. Properties of graphene/waterborne polyurethane nanocomposites cast from colloidal dispersion mixtures. J. Macromol. Sci. Part B Phys. 2012, 51, 197–207. [Google Scholar] [CrossRef]
- Fu, C.; Hu, X.; Yang, Z.; Shen, L.; Zheng, Z. Preparation and properties of waterborne bio-based polyurethane/siloxane cross-linked films by an in situ sol-gel process. Prog. Org. Coat. 2015, 84, 18–27. [Google Scholar] [CrossRef]
- Liao, L.; Li, X.; Wang, Y.; Fu, H.; Li, Y. Effects of surface structure and morphology of nanoclays on the properties of jatropha curcas oil-based waterborne polyurethane/clay nanocomposites. Ind. Eng. Chem. Res. 2016, 55, 11689–11699. [Google Scholar] [CrossRef]
- Gao, Z.; Peng, J.; Zhong, T.; Sun, J.; Wang, X.; Yue, C. Biocompatible elastomer of waterborne polyurethane based on castor oil and polyethylene glycol with cellulose nanocrystals. Carbohydr. Polym. 2012, 87, 2068–2075. [Google Scholar] [CrossRef]
- Mondragon, G.; Santamaria-Echart, A.; Hormaiztegui, M.E.V.; Arbelaiz, A.; Peña-Rodriguez, C.; Mucci, V.; Corcuera, M.; Aranguren, M.I.; Eceiza, A. Nanocomposites of Waterborne Polyurethane Reinforced with Cellulose Nanocrystals from Sisal Fibres. J. Polym. Environ. 2018, 26, 1869–1880. [Google Scholar] [CrossRef]
- Hormaiztegui, M.E.V.; Mucci, V.L.; Aranguren, M.I. Composite films obtained from a waterborne biopolyurethane. Incorporation of tartaric acid and nanocellulose. Ind. Crops Prod. 2019, 142, 111879. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Stojiljković, D.T.; Nikolić, N.N.; Todorović, B.; Radosavljević-Stevanović, N.V. Effect of the silica nanofiller on the properties of castor oil-based waterborne polyurethane hybrid dispersions based on recycled PET waste. Polym. Bull. 2019, 76, 1217–1238. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Y.; Li, X.; Chen, W. Synthesis of vegetable oil-based waterborne polyurethane/silver-halloysite antibacterial nanocomposites. Compos. Sci. Technol. 2016, 126, 86–93. [Google Scholar] [CrossRef]
- Panda, S.S.; Panda, B.P.; Mohanty, S.; Nayak, S.K. Synthesis and properties of castor oil-based waterborne polyurethane cloisite 30B nanocomposite coatings. J. Coatings Technol. Res. 2017, 14, 377–394. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent advances in Nanocellulose-based different Biomaterials: Types, Properties, and Emerging Applications. J. Mater. Res. Technol. 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Buffa, J.M.; Casado, U.; Mucci, V.; Aranguren, M.I. Cellulose nanocrystals in aqueous suspensions: Rheology of lyotropic chiral liquid crystals. Cellulose 2019, 26, 2317–2332. [Google Scholar] [CrossRef]
- Casado, U.; Mucci, V.L.; Aranguren, M.I. Cellulose nanocrystals suspensions: Liquid crystal anisotropy, rheology and films iridescence. Carbohydr. Polym. 2021, 261, 117848. [Google Scholar] [CrossRef]
- Ludueña, L.; Fasce, D.; Alvarez, V.A.; Stefani, P.M. Nanocellulose from rice husk following alkaline treatment to remove silica. BioResources 2011, 6, 1440–1453. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Qin, Y. Alginate fibers: An overwiew of the production processes and applications in wound management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Auad, M.L.; Richardson, T.; Hicks, M.; Mosiewicki, M.A.; Aranguren, M.I.; Marcovich, N.E. Shape memory segmented polyurethanes: Dependence of behavior on nanocellulose addition and testing conditions. Polym. Int. 2012, 61, 321–327. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallegos, A.M.A.; Carrera, S.H.; Parra, R.; Keshavarz, T.; Iqbal, H.M.N. Bacterial cellulose: A sustainable source to develop value-added products—A review. BioResources 2016, 11, 5641–5655. [Google Scholar] [CrossRef]
- Vasconcellos, V.M.; Farinas, C.S. The effect of the drying process on the properties of bacterial cellulose films from Gluconacetobacter hansenii. Chem. Eng. Trans. 2018, 64, 145–150. [Google Scholar]
- Feng, Z.; Li, M.; Jin, X.; Zheng, Y.; Liu, J.; Zhao, L.; Wang, Y.; Li, H.; Zuo, D. Design and characterization of plasticized bacterial cellulose/waterborne polyurethane composite with antibacterial function for nasal stenting. Regen. Biomater. 2020, 7, 597–608. [Google Scholar] [CrossRef]
- Nimeskern, L.; Martínez Ávila, H.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ramírez, C.; Castro, C.; Zuluaga, R.; Gañán, P. Physical Characterization of Bacterial Cellulose Produced by Komagataeibacter medellinensis Using Food Supply Chain Waste and Agricultural By-Products as Alternative Low-Cost Feedstocks. J. Polym. Environ. 2018, 26, 830–837. [Google Scholar] [CrossRef]
- Castro, C.; Cleenwerck, I.; Zuluaga, R.; Caro, G.; Putaux, J.-L.; Rojas, O.J.; Gañán, P. Production of Bacterial Cellulose: Use of a New Strain of Microorganism. In Handbook of Green Materials; Oksman, K., Mathew, A.P., Rojas, O., Sain, M., Eds.; World Scientific: Singapore, 2014; pp. 105–122. [Google Scholar]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Advances in waterborne polyurethane and polyurethane-urea dispersions and their eco-friendly derivatives: A review. Polymers 2021, 13, 409. [Google Scholar] [CrossRef]
- Urbina, L.; Alonso-Varona, A.; Saralegi, A.; Palomares, T.; Eceiza, A.; Corcuera, M.Á.; Retegi, A. Hybrid and biocompatible cellulose/polyurethane nanocomposites with water-activated shape memory properties. Carbohydr. Polym. 2019, 216, 86–96. [Google Scholar] [CrossRef]
- Hormaiztegui, M.E.V.; Aranguren, M.I.; Mucci, V.L. Synthesis and characterization of a waterborne polyurethane made from castor oil and tartaric acid. Eur. Polym. J. 2018, 102, 151–160. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Castro, C.; Cleenwerck, I.; Trcek, J.; Zuluaga, R.; de Vos, P.; Caro, G.; Aguirre, R.; Putaux, J.L.; Gañán, P. Gluconacetobacter medellinensis sp. nov., cellulose- and non-cellulose-producing acetic acid bacteria isolated from vinegar. Int. J. Syst. Evol. Microbiol. 2013, 63, 1119–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffa, J.M.; Grela, M.A.; Aranguren, M.I.; Mucci, V.L. EPR spectroscopy applied to the study of the TEMPO mediated oxidation of nanocellulose. Carbohydr. Polym. 2016, 136, 744–749. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Álvarez, C.; Putaux, J.L.; Caro, G.; Rojas, O.J.; Mondragon, I.; Gañán, P. Bacterial cellulose produced by a new acid-resistant strain of Gluconacetobacter genus. Carbohydr. Polym. 2012, 89, 1033–1037. [Google Scholar] [CrossRef]
- Yamanaka, S.; Sugiyama, J. Structural modification of bacterial cellulose. Cellulose 2000, 7, 213–225. [Google Scholar] [CrossRef]
- Liang, C.Y.; Marchessault, R.H. Infrared spectra of crystalline polysaccharides. II. Native celluloses in the region from 640 to 1700 cm−1. J. Polym. Sci. 1959, 39, 269–278. [Google Scholar] [CrossRef]
- Duchemin, B.; Le Corre, D.; Leray, N.; Dufresne, A.; Staiger, M.P. All-cellulose composites based on microfibrillated cellulose and filter paper via a NaOH-urea solvent system. Cellulose 2015, 23, 593–609. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Application of Spectroscopic Analysis in Cellulose Chemistry. In Comprehensive Cellulose Chemistry, Comprehensive Cellulose Chemistry: Fundamentals and Analytical Methods; Klemm, D., Philipp, B., Heinze, T., Heinze, U., Wagenknecht, W., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1998; pp. 181–195. [Google Scholar]
- Lu, J.; Askeland, P.; Drzal, L.T. Surface modification of microfibrillated cellulose for epoxy composite applications. Polymer 2008, 49, 1285–1296. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Borsoi, C.; Zimmernnam, M.V.G.; Zattera, A.J.; Santana, R.M.C.; Ferreira, C.A. Thermal degradation behavior of cellulose nanofibers and nanowhiskers. J. Therm. Anal. Calorim. 2016, 126, 1867–1878. [Google Scholar] [CrossRef]
- Morán, J. Extracción De Celulosa Y Obtención De Nanocelulosa a Partir De Fibra Sisal—Caracterización. Genesis 2008, 16–17. [Google Scholar]
- Marcovich, N.E.; Reboredo, M.M.; Aranguren, M.I. FTIR spectroscopy applied to woodflour. Compos. Interfaces 1996, 4, 119–132. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Marcovich, N.E.; Aranguren, M.I. Characterization of fiber surface treatments in natural fiber composites by infrared and Raman spectroscopy. In Interface Engineering Nature Fibre Composites Maximum Perform—Part 1. Processing and Surface Treatments to Compose the Interface in Natural Fibre Composites; Zafeiropoulos, N.E., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 117–145. [Google Scholar]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform—Materials Analysis; Salih, S.M., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 45–68. [Google Scholar]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural Characteristics and Thermal Properties of Native Cellulose. In Cellulose—Fundamental Aspects; van de Ven, T., Godbout, L., Eds.; IntechOpen: Rijeka, Croatia, 2013; pp. 45–68. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons Ltd.: Middlesex, UK, 2004. [Google Scholar]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous Cellulose—Structure and Characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Vasconcellos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Souza Filho, M.d.S.M.; Rosa, M.d.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Fernandes, S.N.; De Oliveira, A.G.; Fernandes, J.G.; Grey, P.; Pontes, R.V.; Pereira, L.; Martins, R.; Godinho, M.H.; Fortunato, E. Nanocrystalline cellulose applied simultaneously as the gate dielectric and the substrate in flexible field effect transistors. Nanotechnology 2014, 25, 094008. [Google Scholar] [CrossRef] [Green Version]
- Börjesson, M.; Sahlin, K.; Bernin, D.; Westman, G. Increased thermal stability of nanocellulose composites by functionalization of the sulfate groups on cellulose nanocrystals with azetidinium ions. J. Appl. Polym. Sci. 2018, 135, 45963. [Google Scholar] [CrossRef]
- Kafle, K.; Greeson, K.; Lee, C.; Kim, S.H. Cellulose polymorphs and physical properties of cotton fabrics processed with commercial textile mills for mercerization and liquid ammonia treatments. Text. Res. J. 2014, 84, 1692–1699. [Google Scholar] [CrossRef]
- Lee, C.M.; Mittal, A.; Barnette, A.L.; Kafle, K.; Park, Y.B.; Shin, H.; Johnson, D.K.; Park, S.; Kim, S.H. Cellulose polymorphism study with sum-frequency-generation (SFG) vibration spectroscopy: Identification of exocyclic CH2OH conformation and chain orientation. Cellulose 2013, 20, 991–1000. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef]
- Kulkarni, P.K.; Anil Dixit, S.; Singh, U.B. Evaluation of bacterial cellulose produced form Acetobacter xylinum as pharmaceutical excipient. Am. J. Drug Discov. Dev. 2012, 2, 72–86. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the interpretation of X-ray diffraction powder patterns in terms of the nanostructure of cellulose I fibres. Macromol. Chem. Phys. 2005, 206, 1568–1575. [Google Scholar] [CrossRef]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Biodegradation and thermal stability of bacterial cellulose as biomaterial: The relevance in biomedical applications. Polym. Degrad. Stab. 2020, 179, 109232. [Google Scholar] [CrossRef]
- George, J.; Sajeevkumar, V.A.; Kumar, R.; Ramana, K.V.; Sabapathy, S.N.; Bawa, A.S. Enhancement of Thermal Stability Associated with the Chemical Treatment of Bacterial (Gluconacetobacter xylinus) Cellulose. J. Appl. Polym. Sci. 2008, 108, 1845–1851. [Google Scholar] [CrossRef]
- Lichtenstein, K.; Lavoine, N. Toward a deeper understanding of the thermal degradation mechanism of nanocellulose. Polym. Degrad. Stab. 2017, 146, 53–60. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer. 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Castro, C.; Vesterinen, A.; Zuluaga, R.; Caro, G.; Filpponen, I.; Rojas, O.J.; Kortaberria, G.; Gañán, P. In situ production of nanocomposites of poly(vinyl alcohol) and cellulose nanofibrils from Gluconacetobacter bacteria: Effect of chemical crosslinking. Cellulose 2014, 21, 1745–1756. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Soybean-oil-based waterborne polyurethane dispersions: Effects of polyol functionality and hard segment content on properties. Biomacromolecules 2008, 9, 3332–3340. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Y.; Ou, C.; Xia, Z.; Zhong, L. Synthesis and characterization of waterborne polyurethanes with alkoxy silane groups in the side chains for potential application in waterborne ink. Prog. Org. Coat. 2016, 92, 85–94. [Google Scholar] [CrossRef]
- Hormaiztegui, M.E.V.; Mucci, V.L.; Santamaria-Echart, A.; Corcuera, M.Á.; Eceiza, A.; Aranguren, M.I. Waterborne polyurethane nanocomposites based on vegetable oil and microfibrillated cellulose. J. Appl. Polym. Sci. 2016, 133, 44207. [Google Scholar] [CrossRef]
- Buffa, J.M.; Mondragon, G.; Corcuera, M.A.; Eceiza, A.; Mucci, V.; Aranguren, M.I. Physical and mechanical properties of a vegetable oil based nanocomposite. Eur. Polym. J. 2018, 98, 116–124. [Google Scholar] [CrossRef]
- Pascault, J.-P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers, 1st ed.; CRC Press: New York, NY, USA, 2002. [Google Scholar]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Retegi, A.; Arbelaiz, A.; Corcuera, M.A.; Eceiza, A. Development of waterborne polyurethane-ureas added with plant extracts: Study of different incorporation routes and their influence on particle size, thermal, mechanical and antibacterial properties. Prog. Org. Coat. 2018, 117, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Xu, Q.; Hu, C.P. Preparation and characterization of waterborne polyurethaneurea composed of dimer fatty acid polyester polyol. J. Nanomater. 2006, 2006, 014906. [Google Scholar] [CrossRef]
- Alonso-Lerma, B.; Larraza, I.; Barandiaran, L.; Ugarte, L.; Saralegi, A.; Corcuera, M.A.; Perez-Jimenez, R.; Eceiza, A. Enzymatically produced cellulose nanocrystals as reinforcement for waterborne polyurethane and its applications. Carbohydr. Polym. 2021, 254, 117478. [Google Scholar] [CrossRef]
- Romanzini, D.; Lavoratti, A.; Ornaghi, H.L.; Amico, S.C.; Zattera, A.J. Influence of fiber content on the mechanical and dynamic mechanical properties of glass/ramie polymer composites. Mater. Des. 2013, 47, 9–15. [Google Scholar] [CrossRef]
- Vincent, J.F.V. From cellulose to cell. J. Exp. Biol. 1999, 202, 3263–3268. [Google Scholar] [CrossRef]
- Mariano, M.; Kissi, N.E.; Dufresne, A. Cellulose Nanocrystals and Related Nanocomposites: Review of some Properties and Challenges. J. Polym. Sci. Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Ugarte, L.; Arbelaiz, A.; Gabilondo, N.; Corcuera, M.A.; Eceiza, A. Two different incorporation routes of cellulose nanocrystals in waterborne polyurethane nanocomposites. Eur. Polym. J. 2016, 76, 99–109. [Google Scholar] [CrossRef]
- Amri, M.R.; Guan, C.T.; Osman Al-Edrus, S.S.; Md Yasin, F.; Mohamad, S.F. Effect of Cellulose Nanofibrils on the Properties of Jatropha Oil-Based Waterborne Polyurethane Nanocomposite Film. Polymers 2021, 13, 1460. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Effects of cellulose nanocrystals and cellulose nanofibers on the structure and properties of polyhydroxybutyrate nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef] [Green Version]
- Naomi, R.; Idrus, R.B.H.; Fauzi, M.B. Plant-vs. Bacterial-derived cellulose for wound healing: A review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hormaiztegui, M.E.V.; Marin, D.; Gañán, P.; Stefani, P.M.; Mucci, V.; Aranguren, M.I. Nanocelluloses Reinforced Bio-Waterborne Polyurethane. Polymers 2021, 13, 2853. https://doi.org/10.3390/polym13172853
Hormaiztegui MEV, Marin D, Gañán P, Stefani PM, Mucci V, Aranguren MI. Nanocelluloses Reinforced Bio-Waterborne Polyurethane. Polymers. 2021; 13(17):2853. https://doi.org/10.3390/polym13172853
Chicago/Turabian StyleHormaiztegui, M. E. Victoria, Diana Marin, Piedad Gañán, Pablo Marcelo Stefani, Verónica Mucci, and Mirta I. Aranguren. 2021. "Nanocelluloses Reinforced Bio-Waterborne Polyurethane" Polymers 13, no. 17: 2853. https://doi.org/10.3390/polym13172853
APA StyleHormaiztegui, M. E. V., Marin, D., Gañán, P., Stefani, P. M., Mucci, V., & Aranguren, M. I. (2021). Nanocelluloses Reinforced Bio-Waterborne Polyurethane. Polymers, 13(17), 2853. https://doi.org/10.3390/polym13172853