Development and Characterization of Biocompatible Membranes from Natural Chitosan and Gelatin for Pervaporative Separation of Water–Isopropanol Mixture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
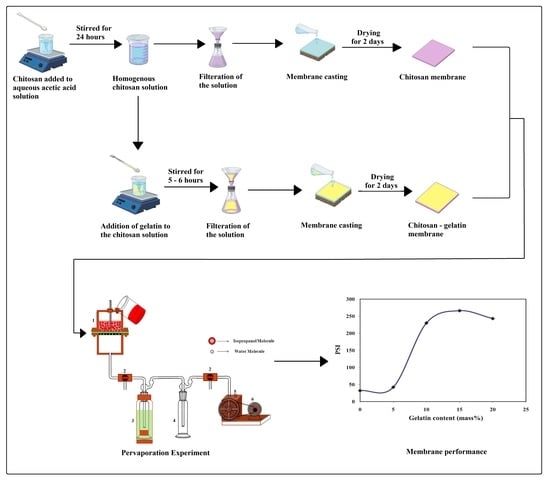
2.2. Membrane Fabrication
2.3. Membrane Characterization
2.4. Equilibrium Swelling
2.5. Pervaporation Experiments
3. Results
3.1. Membrane Characterization
3.1.1. FTIR Studies
3.1.2. Wide-Angle X-ray Diffraction Studies (WAXD)
3.1.3. Thermogravimetric Analysis (TGA)
3.1.4. Differential Scanning Calorimetry (DSC)
3.1.5. Scanning Electron Microscopy (SEM)
3.2. Effects of the Amount of Gelatin and Feed Composition on Membrane Swelling
3.3. Effects of the Amount of Gelatin and Feed Composition on Pervaporation
3.4. Effect of Gelatin on Pervaporation Separation Index (PSI)
3.5. Effect of Temperature on Membrane Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volova, T.G.; Vinnik, Y.S.; Shishatskaya, E.I.; Markelova, N.M.; Zaikov, G.E. Natural-Based Polymers for Biomedical Applications; Apple Academic Press Inc.: Waretown, NJ, USA, 2017. [Google Scholar]
- Tong, J.J.; Zhang, H.; Wang, J.; Liu, Y.; Mao, S.Y.; Xiong, B.H.; Jiang, L.S. Effects of different molecular weights of chitosan on methane production and bacterial community structure in vitro. J. Integr. Agric. 2020, 19, 1644–1655. [Google Scholar] [CrossRef]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte complexes of natural polymers and their biomedical applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes based on non-synthetic (natural) polymers for wastewater treatment. Polym. Test. 2020, 84, 106381. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Nejayote biopolyelectrolytes multifunctionality (glucurono ferulauted arabinoxylans) in the separation of hazardous metal ions from industrial wastewater. Chem. Eng. J. 2021, 423, 130210. [Google Scholar] [CrossRef]
- Ibarra-Rodrígue, D.; Lizardi-Mendoza, J.; López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Capacity of ‘nopal’ pectin as a dual coagulant-flocculant agent for heavy metals removal. Chem. Eng. J. 2017, 323, 19–28. [Google Scholar] [CrossRef]
- García, O.G.Z.; Oropeza-Guzmán, M.T.; Monal, W.M.A.; López-Maldonado, E.A. Design and mechanism of action of multifunctional BPE’s with high performance in the separation of hazardous metal ions from polluted water Part I: Chitosan-poly(N-vinylcaprolactam) and its derivatives. Chem. Eng. J. 2019, 359, 840–851. [Google Scholar] [CrossRef]
- Martínez-Quiroz, M.; López-Maldonado, E.A.; Ochoa-Terán, A.; Pina-Luis, G.E.; Oropeza-Guzmán, M.T. Modification of chitosan with carbamoyl benzoic acids for testing its coagulant-flocculant and binding capacities in removal of metallic ions typically contained in plating wastewater. Chem. Eng. J. 2018, 332, 749–756. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Badi, S.M.; Sajjan, A.M.; Banapurmath, N.R.; Kariduraganavar, M.Y.; Shettar, A.S. Preparation and characterization of B2SA grafted hybrid poly(vinyl alcohol) membranes for pervaporation separation of water–isopropanol mixtures. Chem. Data Coll. 2019, 22, 100245. [Google Scholar] [CrossRef]
- Kalahal, P.B.; Kulkarni, A.S.; Sajjan, A.M.; Khan, T.M.Y.; Badruddin, I.A.; Kamangar, S.; Banapurmath, N.R.; Ayachit, N.H.; Naik, M.L.; Marakatti, V.S. Fabrication and physicochemical study of B2SA grafted poly(vinyl Alcohol)–graphene hybrid membranes for dehydration of bioethanol by pervaporation. Membranes 2021, 11, 110. [Google Scholar] [CrossRef]
- Sugumaran, J.; Ahmad, A.L.; Zaulkiflee, N.D. Improvement of ionic conductivity of titanium dioxide incorporated PVDF-HFP/cellulose acetate electrolyte membrane. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 052025. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Sajjan, A.M.; Ashwini, M.; Banapurmath, N.R.; Ayachit, N.H.; Shirnalli, G.G. Novel fabrication of PSSAMA_Na capped silver nanoparticle embedded sodium alginate membranes for pervaporative dehydration of bioethanol. RSC Adv. 2020, 10, 22645–22655. [Google Scholar] [CrossRef]
- Song, K.M.; Hong, W.H. Dehydration of ethanol and isopropanol using tubular type cellulose acetate membrane with ceramic support in pervaporation process. J. Membr. Sci. 1997, 123, 27–33. [Google Scholar] [CrossRef]
- Dubey, V.; Saxena, C.; Singh, L.; Ramana, K.V.; Chauhan, R.S. Pervaporation of binary water–ethanol mixtures through bacterial cellulose membrane. Sep. Purif. Technol. 2002, 27, 163–171. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of chitin to defined chitosan oligomers: Current status and future prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulik, S.; Vani, B.; Chandrasekhar, S.S.; Sridhar, S. Chitosan-polytetrafluoroethylene composite membranes for separation of methanol and toluene by pervaporation. Carbohydr. Polym. 2018, 193, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Vinu, M.; Raja, D.S.; Jiang, Y.; Liu, T.; Xie, Y.; Linb, Y.; Yang, C.; Lin, C.; Alshehri, S.M.; Ahamad, T.; et al. Effects of structural crystallinity and defects in microporous Al-MOF filled chitosan mixed matrix membranes for pervaporation of water/ethanol mixtures. J. Taiwan Inst. Chem. Eng. 2017, 83, 143–151. [Google Scholar] [CrossRef]
- Chrzanowska, E.; Gierszewska, M.; Kujawa, J.; Raszkowska-Kaczor, A.; Kujawski, W. Development and characterization of polyamide-supported chitosan nanocomposite membranes for hydrophilic pervaporation. Polymers 2018, 10, 868. [Google Scholar] [CrossRef] [Green Version]
- Achari, D.; Rachipudi, P.; Naik, S.; Karuppannan, R.; Kariduraganavar, M. Polyelectrolyte complex membranes made of chitosan—PSSAMA for pervaporation separation of industrially important azeotropic mixtures. J. Ind. Eng. Chem. 2019, 78, 383–395. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and characterization of polyelectrolyte complex membranes for the pervaporation separation of water–isopropanol mixtures using sodium alginate and gelatin. Polym. Bull. 2018, 75, 851–875. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and characterization of GTMAC grafted chitosan membranes for the dehydration of low water content isopropanol by pervaporation. J. Ind. Eng. Chem. 2015, 25, 151–161. [Google Scholar] [CrossRef]
- Fan, L.; Du, Y.; Huang, R.; Wang, Q.; Wang, X.; Zhang, L. Preparation and characterization of alginate/gelatin blend fibers. J. Appl. Polym. Sci. 2005, 96, 1625–1629. [Google Scholar] [CrossRef]
- Poverenov, E.; Rutenberg, R.; Danino, S.; Horev, B.; Rodov, V. Gelatin-chitosan composite films and edible coatings to enhance the quality of food products: Layer-by-layer vs. blended formulations. Food Bioproc. Tech. 2014, 7, 3319–3327. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Nguyen, V.B.; Hsieh, M.F. Curcumin-loaded chitosan/gelatin composite sponge for wound healing application. Int. J. Polym. Sci. 2013, 2013, 106570. [Google Scholar] [CrossRef] [Green Version]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef] [PubMed]
- Rachipudi, P.S.; Kittur, A.A.; Sajjan, A.M.; Kariduraganavar, M.Y. Synthesis and characterization of hybrid membranes using chitosan and 2-(3,4-epoxycyclohexyl) ethyltrimethoxysilane for pervaporation dehydration of isopropanol. J. Membr. Sci. 2013, 441, 83–92. [Google Scholar] [CrossRef]
- Ioelovich, M. Crystallinity and Hydrophility of Chitin and Chitosan. Res. Rev. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Sajjan, A.M.; Banapurmath, N.R.; Shivayyanavar, N.M.; Kulkarni, A.S.; Shettar, A.S. Development and characterization of silicon carbide incorporated graphene amine-based polymer nanocomposites for structural applications. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012073. [Google Scholar] [CrossRef]
- Corazzari, I.; Nistico, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Parvez, S.; Rahman, M.M.; Khan, M.A.; Khan, M.A.H.; Islam, J.M.M.; Ahmed, M.; Rahman, M.F.; Ahmed, B. Preparation and characterization of artificial skin using chitosan and gelatin composites for potential biomedical application. Polym. Bull. 2012, 69, 715–731. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Tiwari, A. Carboxymethyl chitosan and its applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular interactions in gelatin/chitosan composite films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, A.M.; Naik, M.L.; Kulkarni, A.S.; Rudgi, U.; Ashwini, M.; Shirnalli, G.G.; Sharanappa, A.; Kalahal, P.B. Preparation and characterization of PVA-Ge/PEG-400 biodegradable plastic blend films for packaging applications. Chem. Data Collect. 2020, 26, 100338. [Google Scholar] [CrossRef]












| Mass% of Water | J × 10−2 (kg/m2 h) | αsep. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M-0 | M-5 | M-10 | M-15 | M-20 | M-0 | M-5 | M-10 | M-15 | M-20 | |
| 10 | 3.733 | 3.617 | 3.584 | 4.201 | 4.402 | 848.1 | 1160 | 6420 | 6330 | 5512 |
| 15 | 6.428 | 6.350 | 6.138 | 6.355 | 6.605 | 730.3 | 879.8 | 2018 | 2002 | 1604 |
| 20 | 9.701 | 9.076 | 8.776 | 9.012 | 10.21 | 201.1 | 412.7 | 621.0 | 618.0 | 405.5 |
| 25 | 14.50 | 14.01 | 12.09 | 12.95 | 14.59 | 151.0 | 174.5 | 197.0 | 195.6 | 134.6 |
| Mass% of Water | Pi/l(GPU) * | ||||
|---|---|---|---|---|---|
| M-0 | M-5 | M-10 | M-15 | M-20 | |
| 10 | 829 | 803 | 796 | 933 | 978 |
| 15 | 1428 | 1411 | 1364 | 1412 | 1467 |
| 20 | 2156 | 2017 | 1950 | 2002 | 2269 |
| 25 | 3222 | 3113 | 2687 | 2877 | 3242 |
| Mass% of Water | J w × 10−2 (kg/m2 h) | J IPA × 10−2 (kg/m2 h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M-0 | M-5 | M-10 | M-15 | M-20 | M-0 | M-5 | M-10 | M-15 | M-20 | |
| 10 | 3.694 | 3.589 | 3.579 | 4.194 | 4.395 | 0.039 | 0.028 | 0.005 | 0.007 | 0.008 |
| 15 | 6.378 | 6.309 | 6.121 | 6.337 | 6.580 | 0.049 | 0.041 | 0.017 | 0.018 | 0.024 |
| 20 | 9.512 | 8.989 | 8.720 | 8.954 | 10.11 | 0.189 | 0.087 | 0.056 | 0.058 | 0.100 |
| 25 | 14.14 | 13.77 | 11.91 | 12.77 | 14.27 | 0.362 | 0.236 | 0.181 | 0.183 | 0.318 |
| Temperature (°C) | J × 10−2 (kg/m2 h) | αsep. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M-0 | M-5 | M-10 | M-15 | M-20 | M-0 | M-5 | M-10 | M-15 | M-20 | |
| 30 | 3.733 | 3.617 | 3.584 | 4.201 | 4.402 | 848.1 | 1160 | 6420 | 6330 | 5512 |
| 40 | 5.241 | 5.180 | 5.174 | 5.364 | 6.129 | 523.5 | 722.7 | 1791 | 1397 | 922.7 |
| 50 | 6.640 | 6.321 | 6.299 | 7.299 | 9.982 | 452.5 | 652.7 | 1159 | 928.5 | 723.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.S.; Sajjan, A.M.; Khan, T.M.Y.; Badruddin, I.A.; Kamangar, S.; Banapurmath, N.R.; Ayachit, N.H.; Ashwini, M.; Sharanappa, A. Development and Characterization of Biocompatible Membranes from Natural Chitosan and Gelatin for Pervaporative Separation of Water–Isopropanol Mixture. Polymers 2021, 13, 2868. https://doi.org/10.3390/polym13172868
Kulkarni AS, Sajjan AM, Khan TMY, Badruddin IA, Kamangar S, Banapurmath NR, Ayachit NH, Ashwini M, Sharanappa A. Development and Characterization of Biocompatible Membranes from Natural Chitosan and Gelatin for Pervaporative Separation of Water–Isopropanol Mixture. Polymers. 2021; 13(17):2868. https://doi.org/10.3390/polym13172868
Chicago/Turabian StyleKulkarni, Akshay S., Ashok M. Sajjan, T. M. Yunus Khan, Irfan Anjum Badruddin, Sarfaraz Kamangar, Nagaraj R. Banapurmath, Narasimha H. Ayachit, M. Ashwini, and A. Sharanappa. 2021. "Development and Characterization of Biocompatible Membranes from Natural Chitosan and Gelatin for Pervaporative Separation of Water–Isopropanol Mixture" Polymers 13, no. 17: 2868. https://doi.org/10.3390/polym13172868
APA StyleKulkarni, A. S., Sajjan, A. M., Khan, T. M. Y., Badruddin, I. A., Kamangar, S., Banapurmath, N. R., Ayachit, N. H., Ashwini, M., & Sharanappa, A. (2021). Development and Characterization of Biocompatible Membranes from Natural Chitosan and Gelatin for Pervaporative Separation of Water–Isopropanol Mixture. Polymers, 13(17), 2868. https://doi.org/10.3390/polym13172868