DPD Study on the Interfacial Properties of PEO/PEO-PPO-PEO/PPO Ternary Blends: Effects of Pluronic Structure and Concentration
Abstract
:1. Introduction
2. Method
2.1. Model
2.2. Simulation Details
3. Results and Discussion
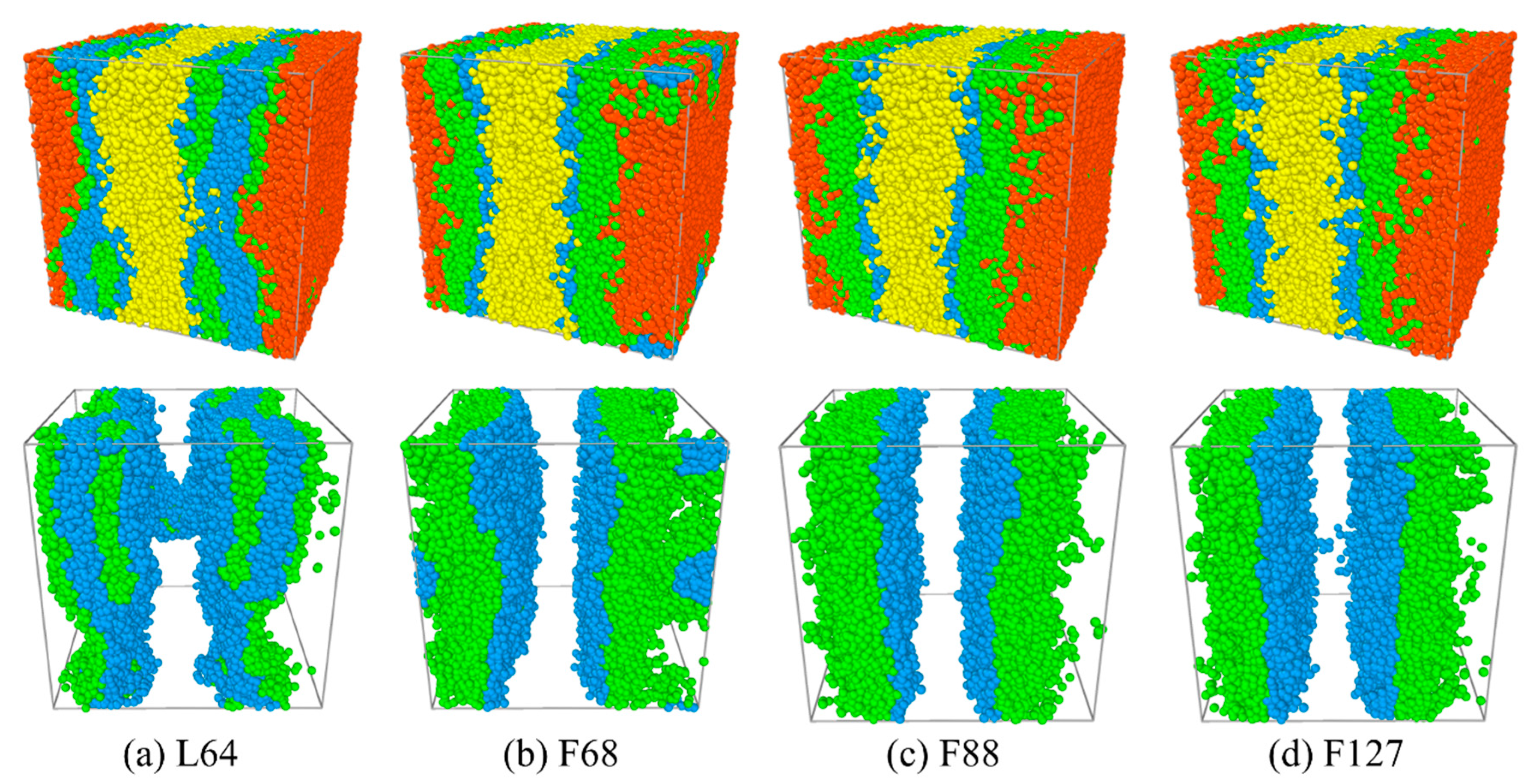
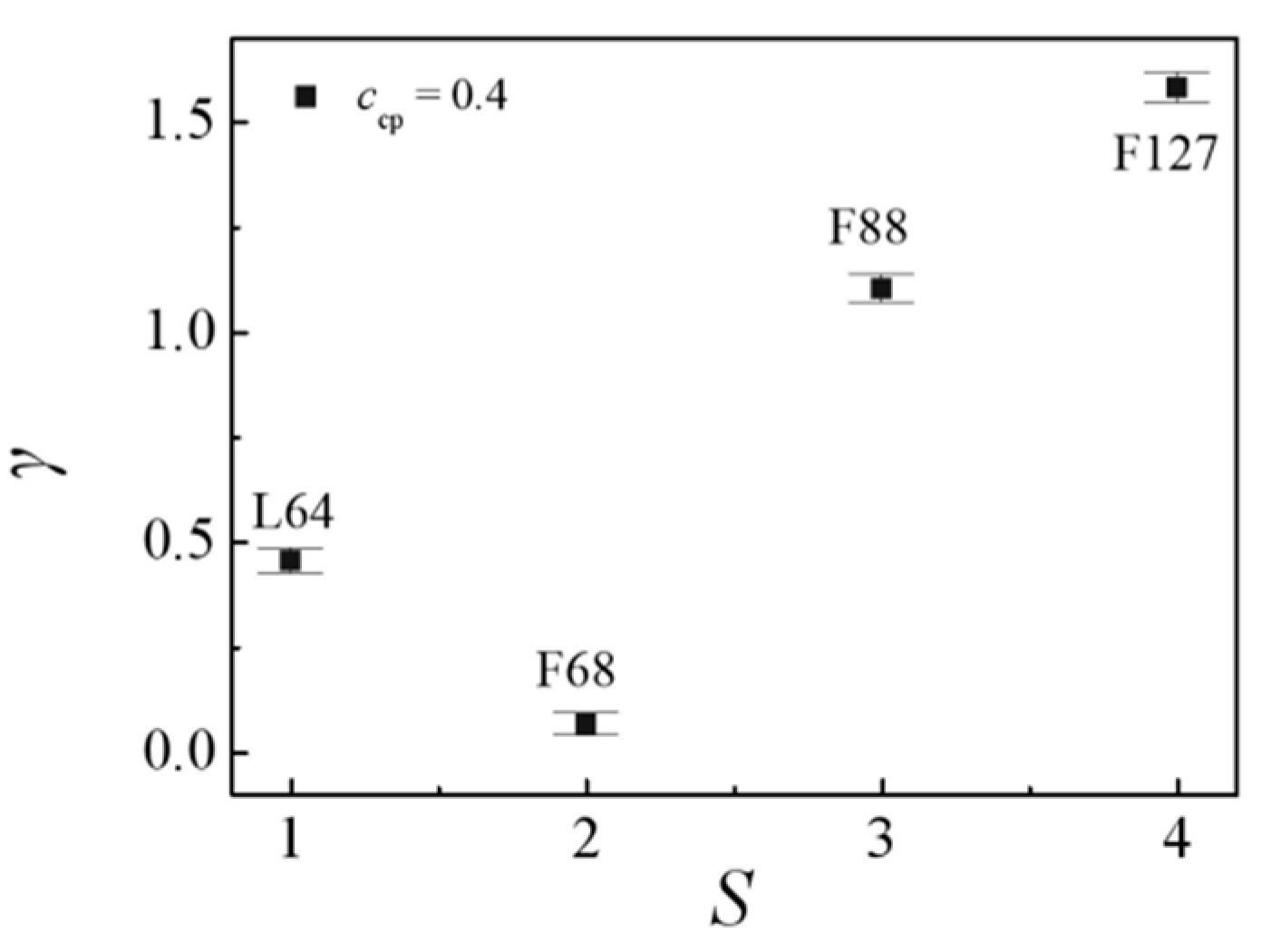
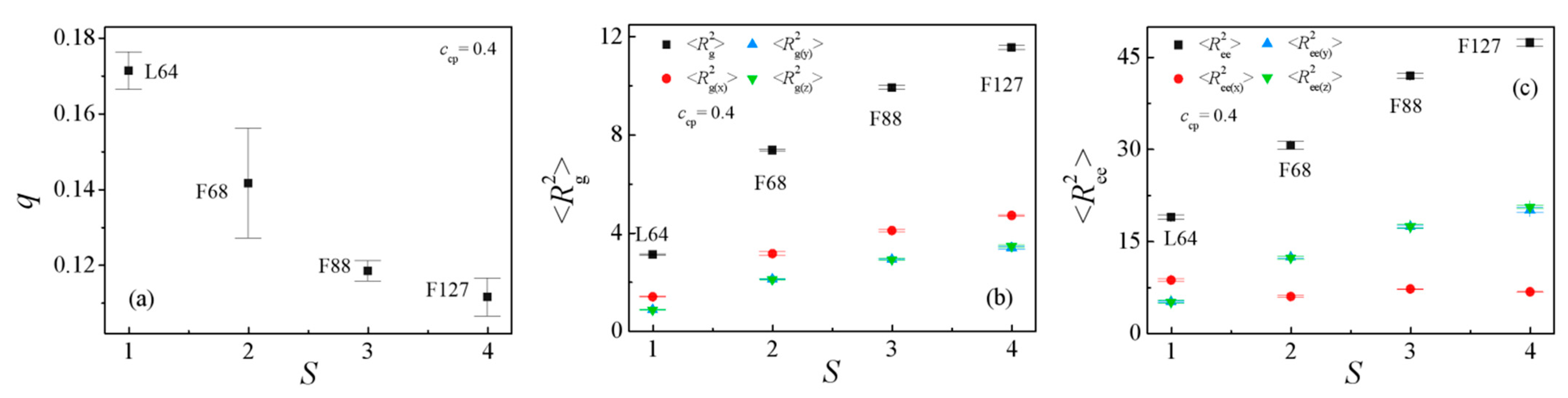
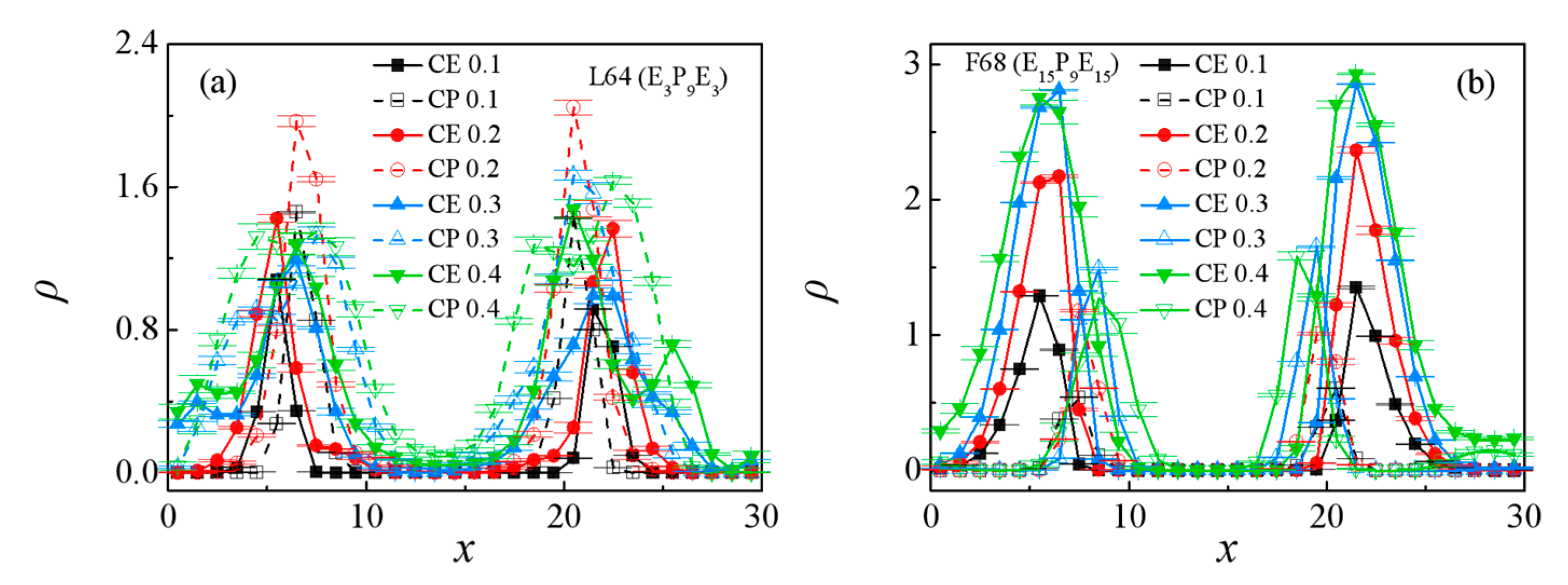
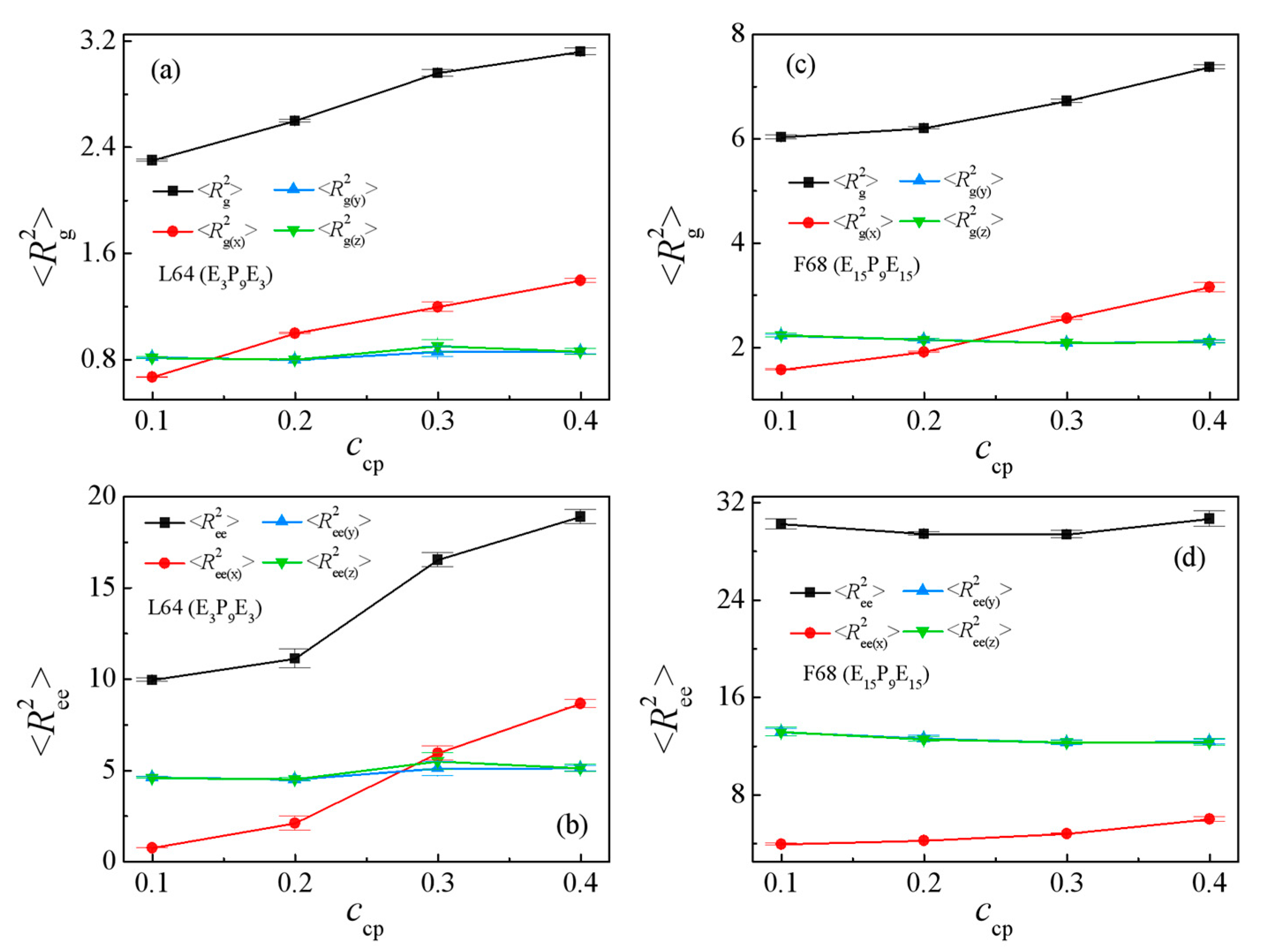
3.1. Effect of the Pluronic Copolymer Structure
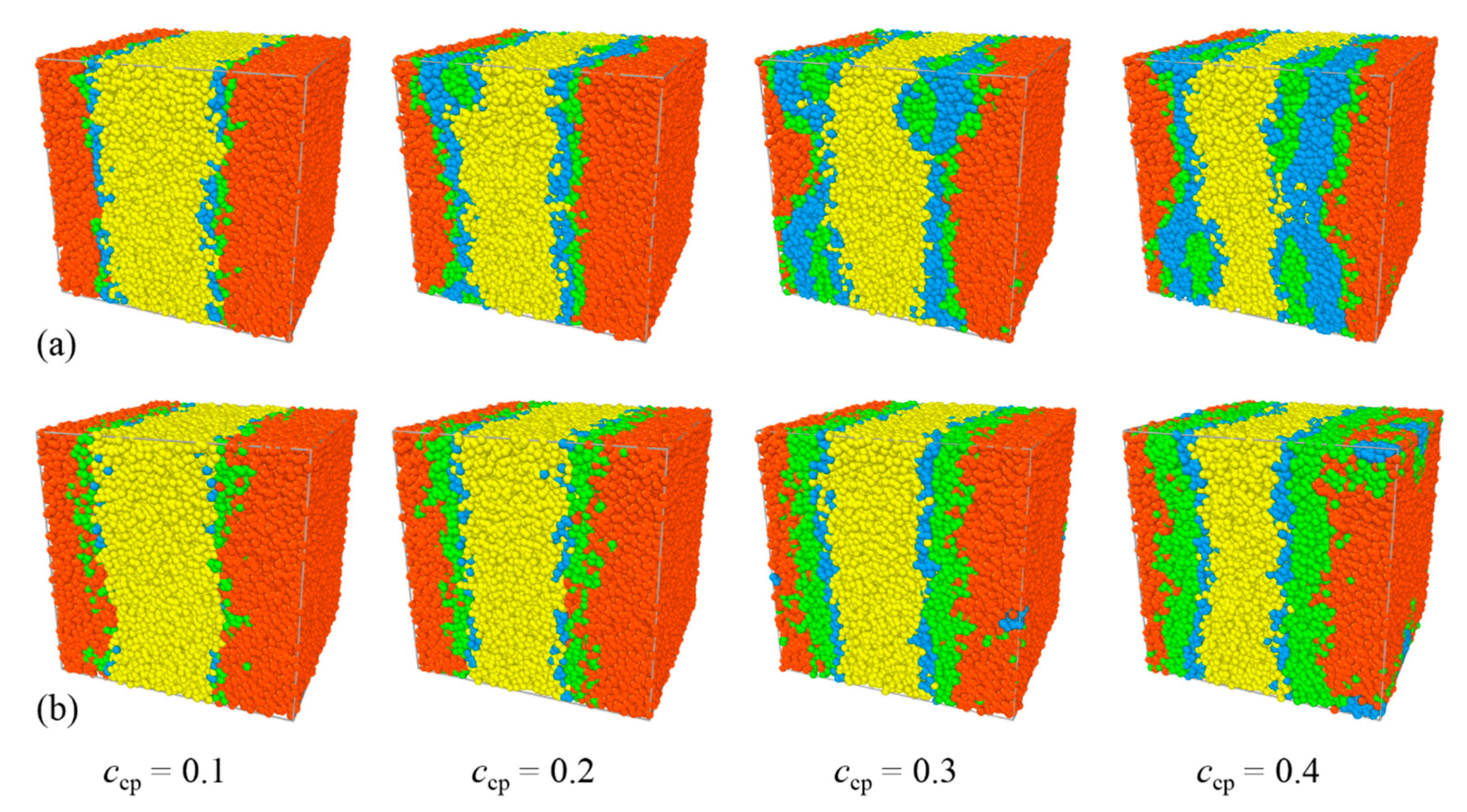
3.2. Effect of Pluronic Copolymer Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pérez-Mosqueda, L.M.; Maldonado-Valderrama, J.; Ramírez, P.; Cabrerizo-Vílchez, M.; Muñoz, J. Interfacial characterization of Pluronic PE9400 at biocompatible (air-water and limonene-water) interfaces. Colloids Surf. B Biointerfaces 2013, 111, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Fainerman, V.B.; Lucassen-Reynders, E.H.; Miller, R. Description of the adsorption behavior of proteins at water/fluid interfaces in the framework of a two-dimensional solution model. Adv. Colloid Interface Sci. 2003, 106, 237–259. [Google Scholar] [CrossRef]
- Berthier, D.L.; Schmidt, I.; Fieber, W.; Schatz, C.; Furrer, A.; Wong, K.; Lecommandoux, S. Controlled Release of Volatile Fragrance Molecules from PEO-b-PPO-b-PEO Block Copolymer Micelles in Ethanol-Water Mixtures. Langmuir 2010, 26, 7953–7961. [Google Scholar] [CrossRef] [PubMed]
- Exerowa, D.; Gotchev, G.; Kolarov, T.; Kristov, K.; Levecke, B.; Tadros, T. Comparison of oil-in-water emulsion films produced using ABA or ABn copolymers. Colloid Surf. A 2009, 335, 50–54. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Zhang, Y.; Zhou, Y.; Chen, X.; Huang, Y.; Liu, Y. Novel mixed polymeric micelles for enhancing delivery of anticancer drug and overcoming multidrug resistance in tumor cell lines simultaneously. Pharm. Res. 2010, 27, 1498–1511. [Google Scholar] [CrossRef]
- Lorena, T.; Rita, M.; Sonia, T.; Roberta, C.; Nevio, P. Effect of formulations variables on the in vitro percutaneous permeation of Sodium Diclofenac from new vesicular systems obtained from Pluronic triblock copolymers. Colloids Surf. B Biointerfaces 2010, 79, 227–234. [Google Scholar]
- Kabanov, A.V.; Lemieux, P.; Vinogradov, S.; Alakhov, V. Pluronic block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002, 54, 223–233. [Google Scholar] [CrossRef]
- Torcello-Gómez, A.; Maldonado-Valderrama, J.; Martín-Rodríguez, A.; Mcclements, D.J. Physicochemical properties and digestibility of emulsified lipids in simulated intestinal fluids: Influence of interfacial characteristics. Soft Matter 2011, 7, 6167–6177. [Google Scholar] [CrossRef]
- Wulff-Pérez, M.; Torcello-Gómez, A.; Gálvez-Ruíz, M.J.; Martín-Rodríguez, A. Stability of emulsions for parenteral feeding: Preparation and characterization of o/w nanoemulsions with natural oils and Pluronic f68 as surfactant. Food Hydrocoll. 2009, 23, 1096–1102. [Google Scholar] [CrossRef]
- Wulff-Pérez, M.; Vicente, J.D.; Martín-Rodríguez, A.; Galvez-Ruiz, A. Controlling lipolysis through steric surfactants: New insights on the controlled degradation of submicron emulsions after oral and intravenous administration. Int. J. Pharm. 2012, 423, 161–166. [Google Scholar] [CrossRef]
- Torcello-Gómez, A.; Santander-Ortega, M.J.; Peula-García, J.; Maldonado-Valderrama, J.; Gálvez-Ruiz, M.; Ortega-Vinuesa, J.L.; Martín-Rodríguez, A. Adsorption of antibody onto Pluronic F68-covered nanoparticles: Link with surface properties. Soft Matter 2011, 7, 8450–8461. [Google Scholar] [CrossRef]
- Welge, I.; Wolf, B.A. Reduction of the interfacial tension between ‘immiscible’ polymers: To which phase one should add a compatibilizer. Polymer 2001, 42, 3467–3473. [Google Scholar] [CrossRef]
- Vidhi, S.; Bhavesh, B.; Shah, D.O. Effect of molecular weight and diffusivity on the adsorption of PEO-PPO-PEO block copolymers at PTFE-water and oil-water interfaces. Colloid Polym. Sci. 2018, 296, 1333–1340. [Google Scholar]
- Sun, N.N.; Li, Y.M.; Wang, D.X.; Bao, M.T.; Tong, L.J. Mesoscopic Simulation Studies on the Self-assembly of Pluronic at Oil/Water Interface. Acta Chim. Sin. 2013, 71, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Zhao, S.; Fa Ng, S.; Ma, Y.; Duan, M. Mesoscopic Simulations of Adsorption and Association of PEO-PPO-PEO Triblock Copolymers on a Hydrophobic Surface: From Mushroom Hemisphere to Rectangle Brush. Langmuir 2016, 32, 11375–11385. [Google Scholar] [CrossRef] [PubMed]
- Ballal, D.; Srivastava, R. Modeling the interfacial properties of Poly(Ethylene oxide-Co-Propylene oxide) polymers at water-toluene interface. Fluid Phase Equilibria 2016, 427, 209–218. [Google Scholar] [CrossRef]
- Azum, N. Synergistic Behavior of Mixed Monolayer/Mixed Micelle Formation between Cationic Monomeric and Dimeric Surfactants with PEO-PPO-PEO Triblock Copolymer. Int. J. Electrochem. Sci. 2018, 13, 2090–2101. [Google Scholar] [CrossRef]
- Deguchi, T.; Nakahara, T.; Imamura, K.; Ishida, N. Direct measurement of interaction force between hydrophilic silica surfaces in triblock copolymer solutions with salt by atomic force microscopy. Adv. Powder Technol. 2020, 32, 30–36. [Google Scholar] [CrossRef]
- Guo, H.X.; Cruz, M.O. A computer simulation study of the segregation of amphiphiles in binary immiscible matrices: Short asymmetric copolymers in short homopolymers. J. Chem. Phys. 2005, 123, 174903. [Google Scholar] [CrossRef]
- Qian, H.J.; Lu, Z.Y.; Chen, L.J.; Li, Z.S.; Sun, C.C. Dissipative particle dynamics study on the interfaces in incompatible A/B homopolymer blends and with their block copolymers. J. Chem. Phys. 2005, 122, 187907. [Google Scholar] [CrossRef]
- Liu, D.M.; Duan, X.Z.; Shi, T.F.; Jiang, F.; Zhang, H.Z. Monte Carlo simulation of effects of homopolymer chain length on interfacial properties of A/AB/B ternary polymer blends. Chem. J. Chin. Univ. Chin. 2015, 36, 2532–2539. [Google Scholar]
- Liu, D.M.; Dai, L.J.; Duan, X.Z.; Shi, T.F.; Zhang, H.Z. Monte Carlo Simulation of Interfacial Properties in Homopolymer/Diblock Copolymer/Homopolymer Ternary Polymer Blends. Chem. J. Chin. Univ. Chin. 2015, 36, 1752–1758. [Google Scholar]
- Goodson, A.D.; Liu, G.L.; Rick, M.S.; Raymond, A.W.; Uddin, M.F.; Ashbaugh, H.S.; Albert, J.N.L. Nanostructure stability and swelling of ternary block copolymer/homopolymer blends: A direct comparison between dissipative particle dynamics and experiment. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 794–803. [Google Scholar] [CrossRef]
- Liu, D.M.; Gong, K.; Lin, Y.; Liu, T.; Liu, Y.; Duan, X.Z. Dissipative Particle Dynamics Study on Interfacial Properties of Symmetric Ternary Polymeric Blends. Polymers 2021, 13, 1516. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Gong, K.; Lin, Y.; Bo, H.F.; Liu, T.; Duan, X.Z. Effects of repulsion parameter and chain length of homopolymers on interfacial properties of An/Ax/2BxAx/2/Bm blends: A DPD simulation study. Polymers 2021, 14, 2333. [Google Scholar] [CrossRef] [PubMed]
- Centeno, R.C.; Bustamante-Rendón, R.A.; Hernández-Fragoso, J.S.; Arroyo-Ordoñez, I.; Pérez, E.; Alas, S.J.; Goicochea, A.G. Surfactant chain length and concentration influence on the interfacial tension of two immiscible model liquids: A coarse-grained approach. J. Mol. Model. 2017, 23, 306. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xu, J.B.; He, X.F. Effect of surfactants on the deformation of single droplet in shear flow studied by dissipative particle dynamics. Mol. Phys. 2018, 116, 1851–1861. [Google Scholar] [CrossRef]
- Liang, X.P.; Wu, J.Q.; Yang, X.G. Investigation of oil-in-water emulsion stability with relevant interfacial characteristics simulated by dissipative particle dynamics. Colloid Surf. A Physicochem. Eng. Asp. 2018, 546, 107–114. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Wang, R.; Tian, R.; Zhang, X.; Sun, Q.; Liu, L. Dissipative particle dynamics study on the temperature dependent interfacial tension in surfactant-oil-water mixtures. J. Pet. Sci. Eng. 2018, 169, 81–95. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. Effects of salt and surfactant on interfacial characteristics of water/oil systems: Molecular dynamic simulations and dissipative particle dynamics. Ind. Eng. Chem. Res. 2019, 58, 8817–8834. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, F.; Kondori, J.; Rezaei, N.; Zendehboudi, S. Meso- and molecular-scale modeling to provide new insights into interfacial and structural properties of hydrocarbon/water/surfactant systems. J. Mol. Liq. 2019, 295, 111357. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhao, L.; Lu, Z.Y. Microscopic characteristics of janus nanoparticles prepared via a grafting-from reaction at the immiscible liquid interface. Phys. Chem. Chem. Phys. 2020, 22, 5347–5354. [Google Scholar] [CrossRef] [PubMed]
- Schlijper, A.G.; Hoogerbrugge, P.J.; Manke, C.W. Computer-simulation of dilute polymer-solutions with the dissipative particle dynamics method. J. Rheol. 1995, 39, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Boker, A.; He, J.; Sill, K.; Xiang, H.; Abetz, C.; Li, X.; Wang, J.; Emrick, T.; Long, S.; et al. Self-directed self-assembly of nanoparticle/copolymer mixtures. Nature 2005, 434, 55–59. [Google Scholar] [CrossRef]
- Hong, Z.H.; Xiao, N.; Li, L.; Xie, X.N. Investigation of nanoemulsion interfacial properties: A mesoscopic simulation. J. Food Eng. 2020, 276, 109877. [Google Scholar] [CrossRef]
- Zhang, J.W.; Chen, L.; Wang, A.L.; Yan, Z.C. Dissipative particle dynamics simulation of ionic liquid-based microemulsion: Quantitative properties and emulsification mechanism. Ind. Eng. Chem. Res. 2020, 59, 763–773. [Google Scholar] [CrossRef]
- Hoogerbrugge, P.J.; Koelman, J. Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics. Europhys. Lett. 1992, 19, 155–160. [Google Scholar] [CrossRef]
- Koelman, J.; Hoogerbrugge, P.J. Dynamic simulations of hard-sphere suspensions under steady shear. Europhys. Lett. 1993, 21, 363–368. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Prhashanna, A.; Khan, S.A.; Chen, S.B. Co-Micellization Behavior in Poloxamers: Dissipative Particle Dynamics Study. J. Phys. Chem. B 2015, 119, 572–582. [Google Scholar] [CrossRef]
- Gai, J.G.; Li, H.L.; Schrauwen, C.; Hu, G.H. Dissipative particle dynamics study on the phase morphologies of the ultrahigh molecular weight polyethylene/polypropylene/poly(ethylene glycol) blends. Polymer 2009, 50, 336–346. [Google Scholar] [CrossRef]
- Luo, Z.L.; Jiang, J.W. Molecular dynamics and dissipative particle dynamics simulations for the miscibility of poly(ethylene oxide)/poly(vinyl chloride) blends. Polymer 2010, 51, 291–299. [Google Scholar] [CrossRef]
- Eslami, H.; Khanjari, N.; Muller-Plathe, F. A local Order Parameter-Based Method for Simulation of Free Energy Barriers in Crystal Nucleation. J. Chem. Theory Comput. 2017, 13, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Gharibi, A.; Muller-Plathe, F. Mechanisms of Nucleation and Solid-Solid-Phase Transitions in Triblock Janus Assemblies. J. Chem. Theory Comput. 2021, 17, 1742–1754. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, X.P.; Zeng, Q.X. Simulation studies of the interfaces of incompatible glycidyl azide polymer/hydroxyl-terminated polybutadiene blends by dissipative particle dynamics. I. The effect of block copolymers and plasticizers. J. Appl. Polym. Sci. 2012, 125, 1530–1537. [Google Scholar] [CrossRef]
- Field, M.J.; Bash, P.A.; Karplus, M. A combined quantum mechanical and molecular mechanical potential for molecular dynamics simulations. J. Comput. Chem. 1990, 11, 700–733. [Google Scholar] [CrossRef]
- Dai, K.H.; Washiyama, J.; Kramer, E.J. Segregation Study of a BAB Triblock Copolymer at the A/B Homopolymer Interface. Macromolecules 1994, 27, 4544–4553. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, Y.; Kong, M.; Yang, Q.; Li, G. Assessment of compatibilization efficiency of SEBS in the PP/PS blend. J. Appl. Polym. Sci. 2018, 135, 46244. [Google Scholar] [CrossRef]
- Shull, K.R.; Kramer, E.J.; Hadziioannou, G.; Tang, W. Segregation of block copolymers to interfaces between immiscible homopolymers. Macromolecules 1990, 23, 4780–4787. [Google Scholar] [CrossRef]
- Eslami, H.; Khani, M.; Muller-Plathe, F. Gaussian Charge Distributions for Incorporation of Electrostatic Interactions in Dissipative Particle Dynamics: Application to Self-Assembly of Surfactants. J. Chem. Theory Comput. 2019, 15, 4197–4207. [Google Scholar] [CrossRef] [PubMed]


| Name | Polymer Structure | Coarse-Grained DPD Chains |
|---|---|---|
| PEO | EO229 | E46 |
| PPO | PO42 | P13 |
| L64 | EO13PO30EO13 | E3P9E3 |
| F68 | EO76PO29EO76 | E15P9E15 |
| F88 | EO104PO39EO104 | E21P12E21 |
| F127 | EO106PO70EO106 | E21P21E21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Yang, M.; Wang, D.; Jing, X.; Lin, Y.; Feng, L.; Duan, X. DPD Study on the Interfacial Properties of PEO/PEO-PPO-PEO/PPO Ternary Blends: Effects of Pluronic Structure and Concentration. Polymers 2021, 13, 2866. https://doi.org/10.3390/polym13172866
Liu D, Yang M, Wang D, Jing X, Lin Y, Feng L, Duan X. DPD Study on the Interfacial Properties of PEO/PEO-PPO-PEO/PPO Ternary Blends: Effects of Pluronic Structure and Concentration. Polymers. 2021; 13(17):2866. https://doi.org/10.3390/polym13172866
Chicago/Turabian StyleLiu, Dongmei, Meiyuan Yang, Danping Wang, Xueying Jing, Ye Lin, Lei Feng, and Xiaozheng Duan. 2021. "DPD Study on the Interfacial Properties of PEO/PEO-PPO-PEO/PPO Ternary Blends: Effects of Pluronic Structure and Concentration" Polymers 13, no. 17: 2866. https://doi.org/10.3390/polym13172866






