Blocking and Deblocking of Diisocyanate to Synthesize Polyurethanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedure of 4-(3-Bromophenyl)-1H-Pyrazole-Blocked Toluene Diisocyanate Adducts
2.2. Synthetic Procedure for the Preparation of Polyurethane from 4-(3-Bromophenyl)-1H-Pyrazole-Blocked TDI Adducts
2.3. Spectroscopic and Thermal Analysis
3. Results
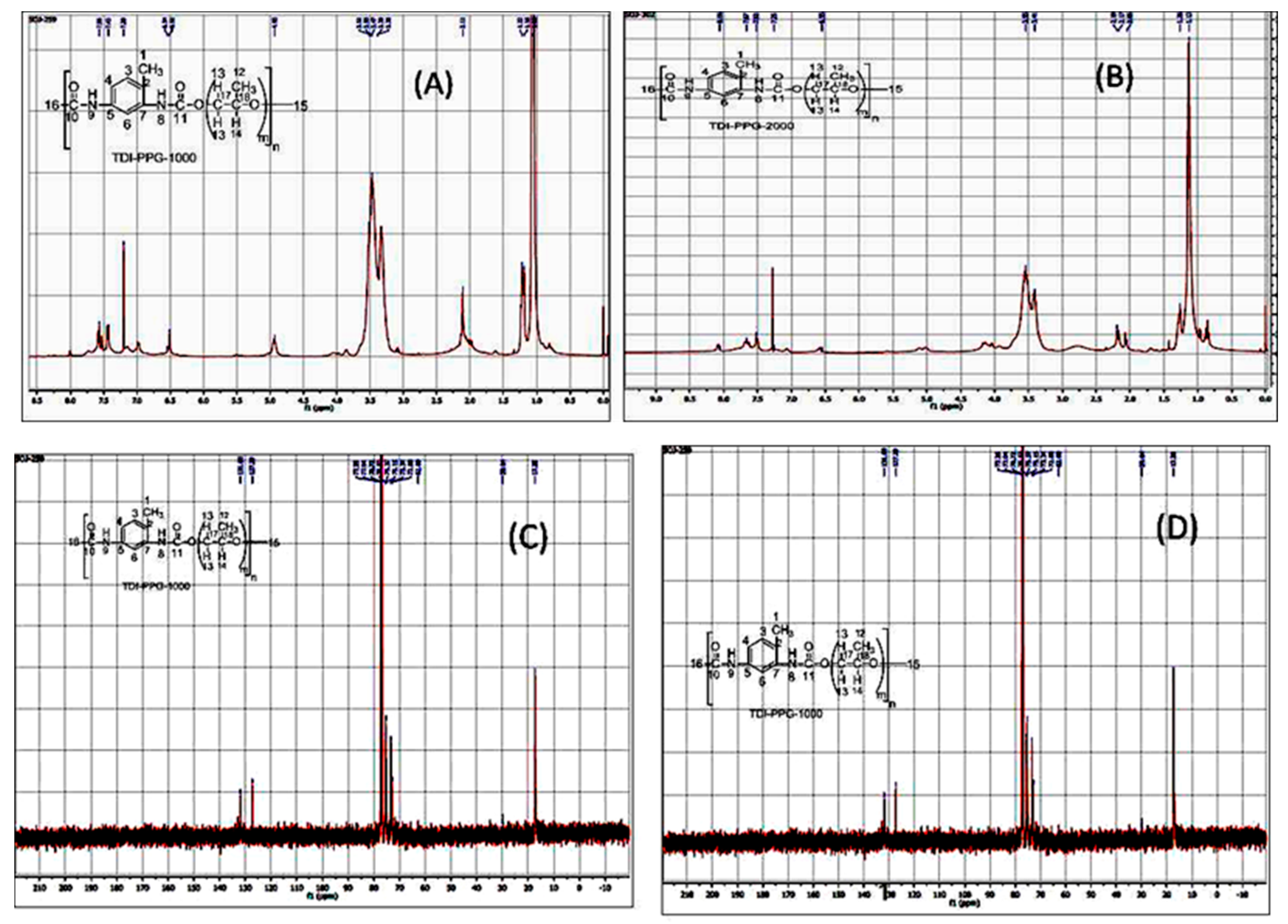
3.1. NMR Analysis
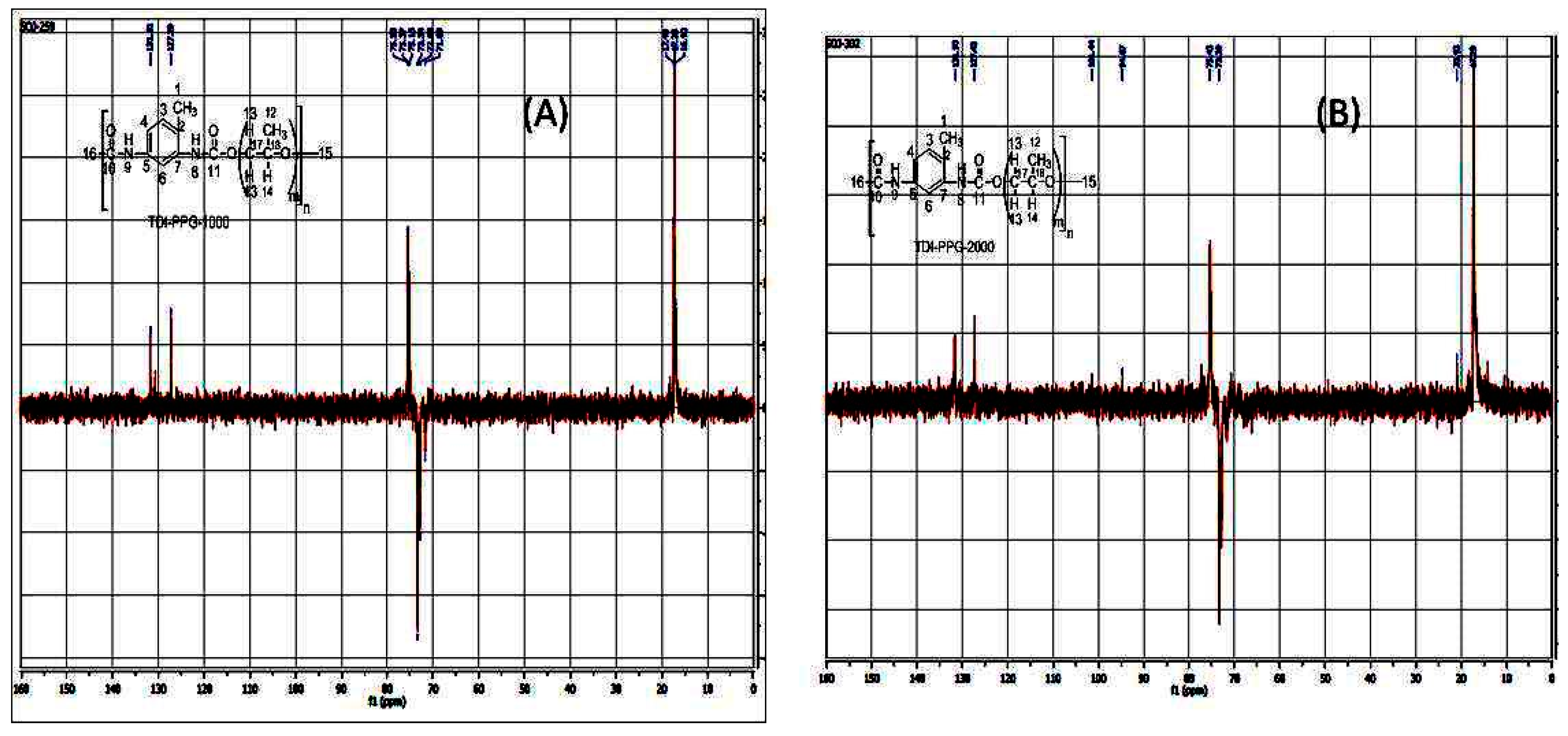
3.2. DEPT-135
3.3. FTIR Analysis
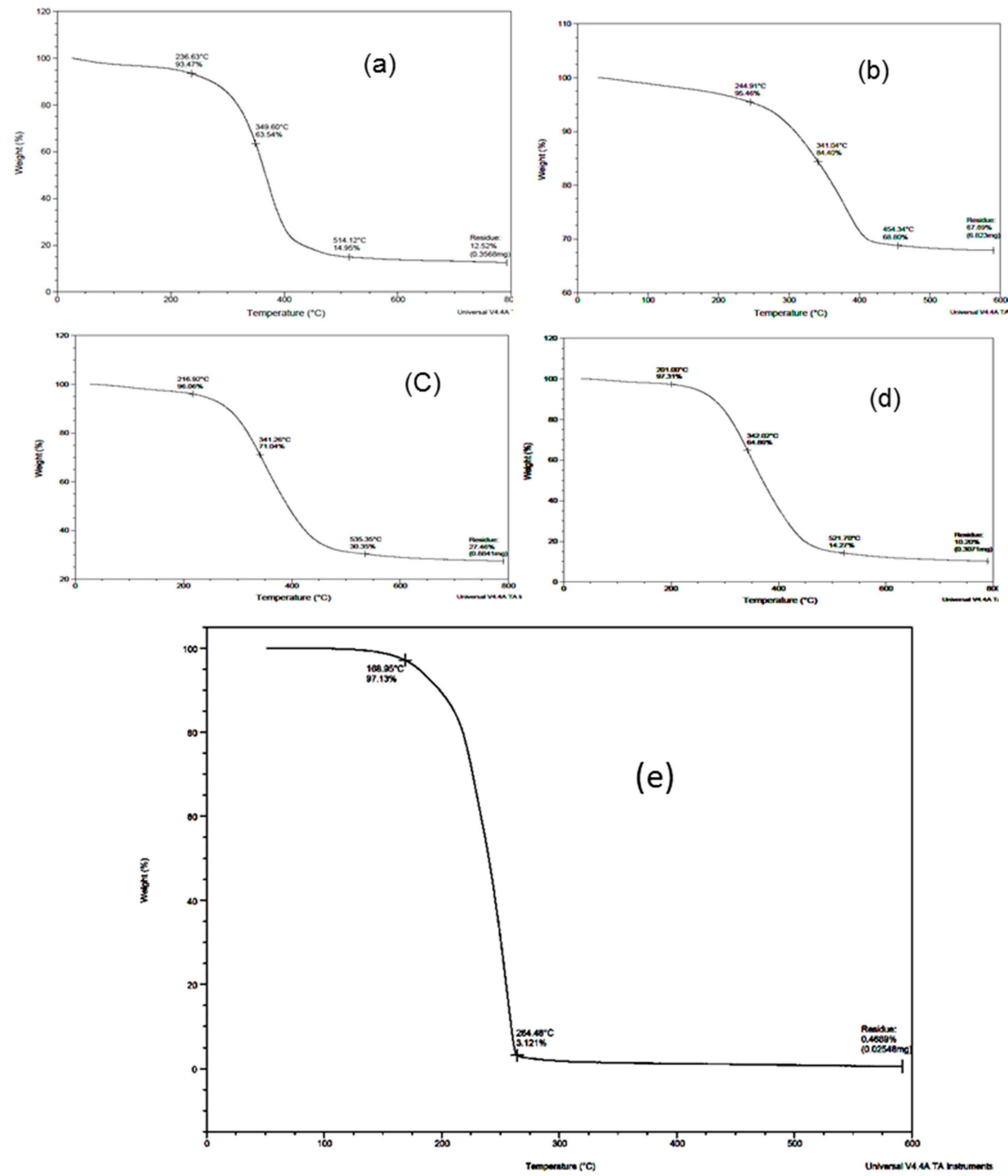
3.4. TGA Thermogram
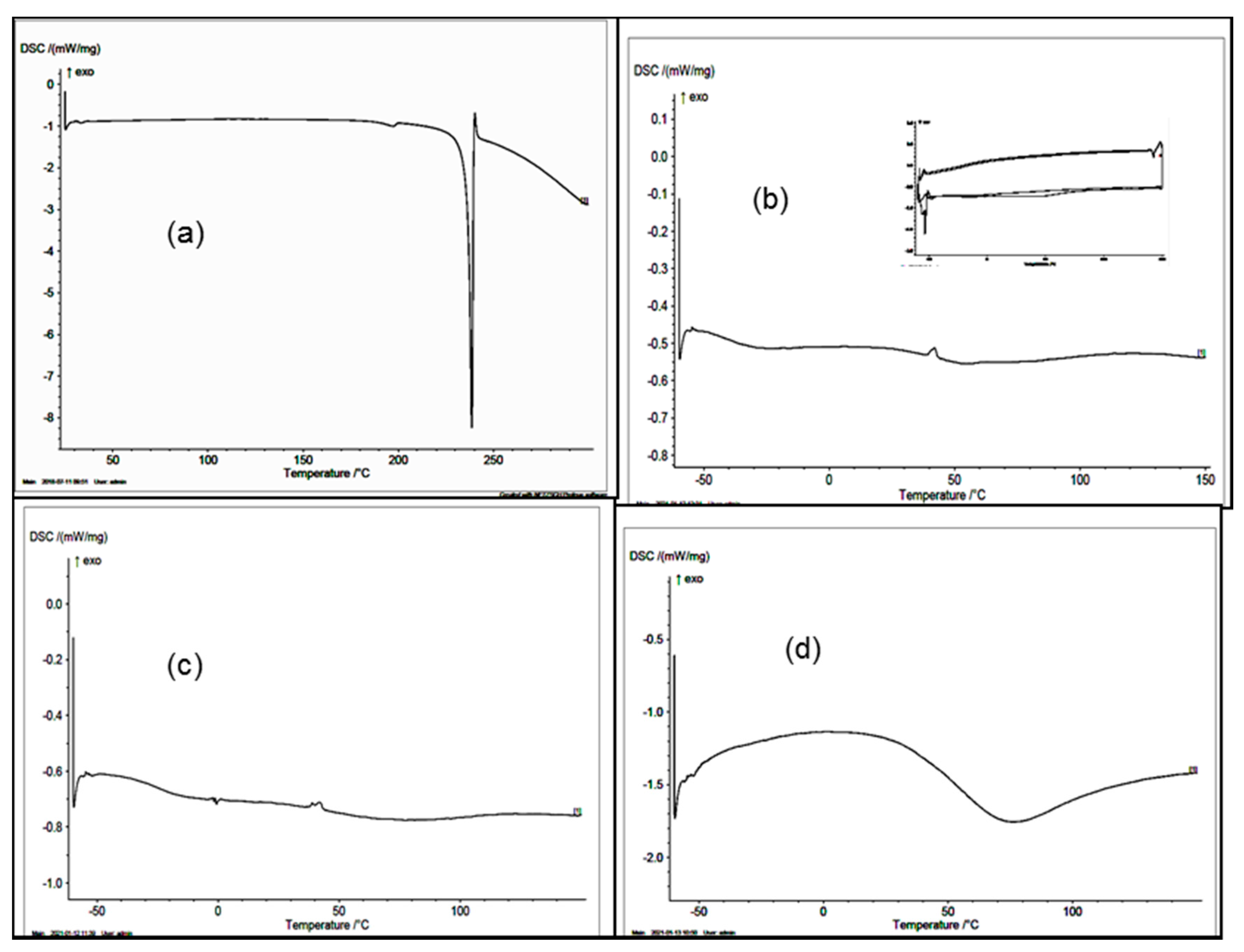
3.5. DSC Thermogram
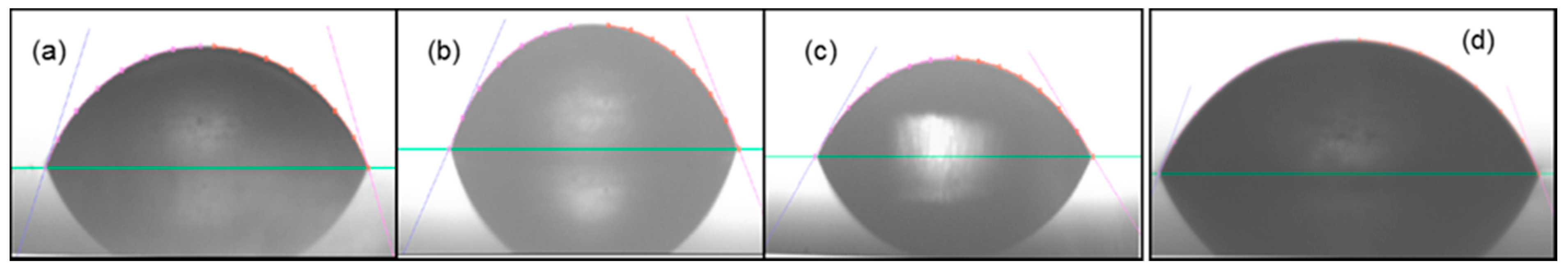
3.6. Contact Angle Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Gite, V.V.; Mahulikar, P.P.; Hundiwale, D.G. Preparation and properties of polyurethane coatings based on acrylic polyols and trimer of isophorone diisocyanate. Prog. Org. Coat. 2010, 68, 307–312. [Google Scholar] [CrossRef]
- Sang, S.L.; Li, Y.Y.; Wang, K.J.; Tang, J.L. Application of blocked isocyanate in preparation of polyurethane(urea) elastomers. J. Polym. Sci. Part A Polym. Chem. 2021, 138, 50582. [Google Scholar]
- Sathiyaraj, S.; Shanavas, A.; Kumar, K.A.; Sathiyaseelan, A.; Senthilselvan, J.; Kalaichelvan, P.T.; Nasar, A.S. The first example of bis(indolyl)methane based hyperbranched polyurethanes: Synthesis, solar cell application and anti-bacterial and anti-oxidant properties. Eur. Polym. J. 2017, 95, 216–231. [Google Scholar] [CrossRef]
- Tassel, X.; Barbry, D.; Tighzert, L. A new blocking agent of isocyanates. Eur. Polym. J. 2000, 36, 1745–1751. [Google Scholar] [CrossRef]
- Nasar, A.S.; Jaisankar, S.N.; Subramani, S.; Radhakrishnan, G. Synthesis and Properties of Imidazole-Blocked Toluene Diisocyanates. J. Macromol. Sci. Part A Pure Appl. Chem. 1997, 34, 1237–1247. [Google Scholar] [CrossRef]
- Dyer, E.; Glenn, J.F.; Lendrat, E.G. The Kinetics of the Reactions of Phenyl Isocyanate with Thiols1. J. Org. Chem. 1961, 26, 2919–2925. [Google Scholar] [CrossRef]
- Shen, T.F.; Lu, M.G.; Liang, L.Y. Preparation and Properties of Non-ionic Polyurethane Crosslinkers from 2-Ethoxyethanol/epsilon-caprolactam-BlockedDiisocyanate. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 611–618. [Google Scholar] [CrossRef]
- Bertoldo, M.; Cappelli, C.; Catanorchi, S.; Liuzzo, V.; Bronco, S. Understanding theAccelerating Effect of ε-Caprolactam on the Formation of Urethane Linkages. Macromolecules 2005, 38, 1385–1394. [Google Scholar] [CrossRef]
- He, Z.A.; Blank, W.J. Crosslinking with malonate blocked isocyanates and with melamine resins. J. Coat. Technol. 1999, 71, 85–90. [Google Scholar]
- Carter, J.W.; Pappas, S.P. A Novel Design Strategy for Blocked Isocyanates to Enhance Their Reactivity with Alcohols-Oxime Blocking-Agents Which Undergo Intramolecular Cyclization. J. Coat. Technol. 1992, 64, 29–36. [Google Scholar]
- Zhou, Z.; Lv, H.; Wang, X.; Ren, F.; Xu, W. Deblocking of the water-soluble isophorone diisocyanate blocked by sodium bisulfite and its application. J. Appl. Polym. Sci. 2013, 128, 597–599. [Google Scholar] [CrossRef]
- Nasar, A.S.; Subramani, S.; Radhakrishnan, G. Synthesis and properties of aromatic secondary amine-blocked isocyanates. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1815–1821. [Google Scholar] [CrossRef]
- Jana, S.; Ramar, P.; Samanta, D.; Jaisankar, S.N. Bromo-substituted blocked hexamethylene diisocyanate adduct: Synthesis, characterization and application toward polyurethane coating. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 58, 298–308. [Google Scholar] [CrossRef]
- Mulebach, A. Pyrazoles—A novel class of blocking agents for isocyanates. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 753–765. [Google Scholar] [CrossRef]
- Shen, T.F.; Zhou, D.W.; Liang, L.Y.; Zheng, J.; Lan, Y.X.; Lu, M.G. Synthesis and Characterization of Reactive Blocked-Isocyanate Coupling Agents from Methyl Ethyl Ketoxime, Ethyl Cellosolve/epsilon-Caprolactam Blocked Aromatic and Aliphatic Diisocyanates. J. Appl. Polym. Sci. 2011, 122, 748–757. [Google Scholar] [CrossRef]
- Nasar, A.S.; Shrinivas, V.; Shanmugam, T.; Raghavan, A. Synthesis and deblocking of cardanol-and anacardate-blocked toluene diisocyanates. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4047–4055. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Peng, P.P.; Wu, Q.Y.; Zhang, J.A.; Wu, M.Y.; Liu, J.Y.; Yang, J.J. Preparation and antibacterial properties of poly (hexamethylene guanidine hydrochloride) modified ionic waterborne polyurethane. Prog. Org. Coat. 2021, 156, 106246. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Liu, W.Q.; Liang, L.Y.; Wang, S.; Shi, H.Y.; Xie, Y.K.; Yang, M.P.; Pi, K. The effect of functional graphene oxide nanoparticles on corrosion resistance of waterborne polyurethane. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124565. [Google Scholar] [CrossRef]
- Ahmad, I.; Zaidi, J.H.; Hussain, R.; Munir, A. Synthesis, characterization and thermal dissociation of 2-butoxyethanol-blocked diisocyanates and their use in the synthesis of isocyanate-terminated prepolymers. Polym. Int. 2007, 56, 1521–1529. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Ranjbar, Z.; Montazeri, S.; Nayini, M.M.R.; Jannesari, A. Synthesis and characterization of diethylene glycol monobutyl ether-Blocked diisocyanate crosslinkers. Prog. Org. Coat. 2010, 69, 426–431. [Google Scholar] [CrossRef]
- Shen, T.F.; Sun, Y.J.; Sun, C.F.; Lu, M.G. Preparation and Characterization of Polyurethane Bioadhesive from Hydroxyl-terminated Polylactide and Imidazole-blocked Isocyanate. Polym. Korea 2013, 37, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Rolph, M.S.; Markowska, A.L.J.; Warriner, C.N.; O’Reilly, R.K. Blocked isocyanates: From analytical and experimental considerations to non-polyurethane applications. Polym. Chem. 2016, 7, 7351–7364. [Google Scholar] [CrossRef] [Green Version]
- Prakash, D.; Jaisankar, S.N. Thermoplastic poly(urethane-thiourethane) triblock copolymers with SWCNTs composite. Diamond Relat. Mater. 2019, 93, 34–41. [Google Scholar] [CrossRef]
- Murali, A.; Gurusamy-Thangavelu, S.A.; Jaisankar, S.N.; Mandal, A.B. Enhancement of the physicochemical properties of polyurethane-perovskite nanocomposites via addition of nickel titanate nanoparticles. RSC Adv. 2015, 5, 102488–102494. [Google Scholar] [CrossRef]
- Meera, K.M.S.; Sankar, R.M.; Paul, J.; Jaisankar, S.N.; Mandal, A.B. The influence of applied silica nanoparticles on a bio-renewable castor oil based polyurethanenanocomposite and its physicochemical properties. Phys. Chem. Chem. Phys. 2014, 16, 9276–9288. [Google Scholar] [CrossRef]
- Sankar, R.M.; Meera, K.S.; Mandal, A.B.; Jaisankar, S.N. Thermoplastic polyurethane/single-walled carbon nanotube composites with low electrical resistance surfaces. High Perform. Polym. 2013, 25, 135–146. [Google Scholar] [CrossRef]
- Mustapha, S.; Andou, Y. Enhancing Mechanical Properties of Polyurethane with Cellulose Acetate as Chain Extender. Fibers Polym. 2021. [Google Scholar] [CrossRef]
- Gu, L.; Wu, Q.Y. Recyclable bio-based crosslinked polyurethanes with self-healing ability. J. Appl. Polym. Sci. 2018, 135, 46272. [Google Scholar] [CrossRef]
- Xie, F.W.; Zhang, T.L.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar]
- Mukherjee, M.; Gurusamy-Thangavelu, S.A.; Chelike, D.K.; Alagumalai, A.; Das, B.N.; Jaisankar, S.N.; Mandal, A.B. Biodegradable polyurethane foam as shoe insole to reduce footwear waste: Optimization by morphological physicochemical and mechanical properties. Appl. Surf. Sci. 2020, 499, 143966. [Google Scholar] [CrossRef]




| S. No. | TDI Gram × 10−4 | Blocking Agent (Gram) | Adduct (Gram) | Solvent | Reflux Time (Hour) | Catalyst DBTDL (wt.%) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1. | 3.9 | 0.10 | 0.13 | CHCl3 | 3.0 | 0.85 | 91 |
| 2. | 3.9 | 0.10 | 0.12 | CHCl3 | 3.0 | 0.65 | 85 |
| 3. | 3.9 | 0.10 | 0.12 | CHCl3 | 3.0 | 0.50 | 88 |
| 4. | 3.9 | 0.10 | 0.11 | CHCl3 | 3.0 | 1.0 | 81 |
| S. No. | Wt. of Blocked Adduct (Gram) | Polyether | Wt of Polyether (Gram × 10−4) | Polyurethane | Wt of Polyurethane Gram | Yield (%) | Temp. (°C) | Gelation Time (h) |
|---|---|---|---|---|---|---|---|---|
| 1. | 0.10 | PEG-400 | 6.44 | TDI-PEG-400 | 0.061 | 95 | 240 | 3 |
| 2. | 0.19 | PPG-1000 | 3.22 | TDI-PPG-1000 | 0.175 | 34 | 240 | 4 |
| 3. | 0.1 | PPG-2000 | 3.22 | TDI-PPG-2000 | 0.13 | 32 | 240 | 4 |
| 4. | 0.1 | PPG-3000 | 4.83 | TDI-PPG-3000 | 0.18 | 32 | 240 | 4 |
| Compound | 1H NMR (in ppm) | 13C NMR (in ppm) |
|---|---|---|
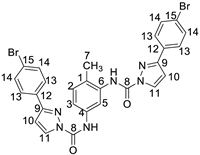 | 2.43 (CH3-7), 6.69 (2 H-10), 7.51 (H-2, H-3), 7.51 (4 H-13), 7.71(4 H-14), 8.0 (H-5), 8.21 and 8.31 (2 H-11), 9.10 (2 N-H). | 16.93 (CH3-7), 108 (C-5), 127.18 (4 C-13), 130 (4 C-14), 152 (2 C-8). |
| Compound | 1H NMR (in ppm) | 13C NMR (in ppm) |
|---|---|---|
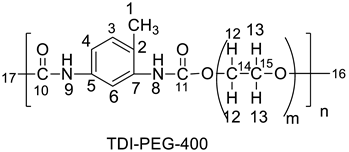 | 1.54, 1.19 (H-16), 2.11 (H-17), 2.24 (H-1), 3.65 (2H-13), 4.22 (2H-12), 6.97 (N-H-8), 7.42 (H-3), 7.57 (H-4), 7.69 (H-6), 8.00 (N-H-9). | 17.37 (C-1), 31.25 (C-17), 61.49 (C-16), 64.32 (C-14), 67.3 (C-15), 115 (C-6), 122 (C-4), 127 (C-2), 130.7–131.7 (C-7), 136–137 (C-5), 149 (C-10), 153 (C-11). |
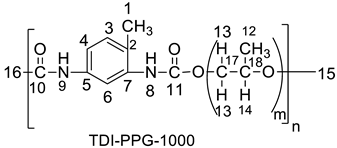 | 1.10 (H-15), 1.24 (H-12), 2.14 (H-1), 3.34 (H-14), 3.47 (H-13), 6.51 (H-9), 7.2 (H-3), 7.48 (H-4), 7.56 (H-6), 8.01 (H-8) | 17.28 (C-15, C-1), 29.64 (C-16), 72.8 (C-15), 73 (C-17), 75 (C-18), 127 (C-4, C-6), 131.8 (C-7, C-5). |
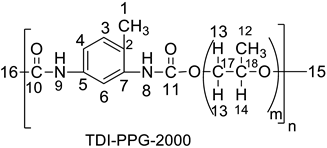 | 1.15 (H-15), 1.23 (H-12), 2.06, 2.26 (C-1), 3.41 (2H-13), 3.55 (H-14), 7.51 (H-3, H-4), 7.65 (H-6), 8.09 (H-8, H-9). | 17.2 (C-15), 22.62 (C-1), 28.29 (C-12), 45 (C-16), 72.75 (C-17), 75 (C-18), 127 (C-4, C-6), 131 (C-7,C-5), 170 (C-10), 196 (C-11). |
 | 1.06 (H-16), 1.19 (H-12), 3.33 and 3.45 (2H-13), 4.12 (H-14), 7.45 (H-3, H-4), 7.54 (H-6), 8.01 (N-H-9). | 17.3 (C-1), 17.4 (C-12), 22 (C-16), 31 (C-17), 73, 127.28 (C-5, C-7), 131 (C-3,C-4, C-6). |
| Compound | DEPT-135 (in ppm) |
|---|---|
 | 17.11 (up)[CH3-1], 61.28 (down)[CH2-14], 63.5 (down) [CH2-13], 77.72 (down) [CH2-16], 126.9 (up) [CH-3], 130.13 (up) [CH-4], 131.7 (up) [CH-6]. |
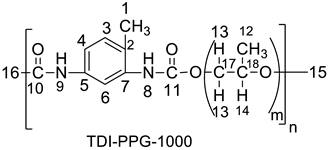 | 17.12 (up) [CH3-1], 17.4 (down) [CH3-12], 71.69 (down) [CH2-15], 72.88 (down) [CH2-17], 73.61 (down) [CH2-16], 127.29 (up) [CH-3], 130 (up) [CH-4], 131 (up) [CH-6]. |
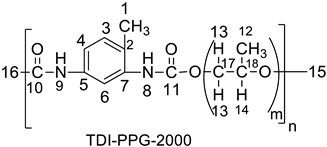 | 17.79 (up) [CH3-12], 20.9 (up) [CH3 -1], 73.39 (down) [CH2-17], 75.43 (down) [CH2-15], 77.30 (up) [CH-7],127 (up) [CH-3, CH-4], 131.5 (up) [CH-6]. |
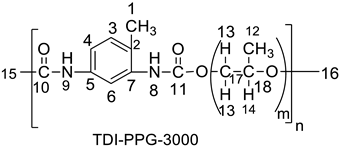 | 17.45 (up) [CH3-1, CH3-12], 73.33 (down [CH2-17], 74.95 (down) [CH2-16], 127.19 (up), [CH-3, CH-4], 131 (up) [CH-6]. |
| Compound | −NH Stretch (cm−1) | −NCO Stretch (cm−1) | −C=O Stretch (cm−1) | CH Stretch (cm−1) | C–N Stretch (cm−1) | C–O− Stretch (cm−1) | C−H Bending (cm−1) |
|---|---|---|---|---|---|---|---|
| Blocked TDI | 3355 | - | 1730 | 3143 | 1230 | 1000 | 752 |
| TDI-PEG-400 | 3306 | - | 1730 | 2880 | 1230 | 1081 | 781 |
| TDI-PPG-1000 | 3306 | - | 1730 | 2868–2992 | 1230 | 1093 | 756 |
| TDI-PPG-2000 | 3318 | - | 1730 | 2980–2868 | 1230 | 1081 | 769 |
| TDI-PPG-3000 | 3318 | - | 1730 | 2868–2980 | 1230 | 1093 | 769 |
| Compound | Starting Point | Ending Point |
|---|---|---|
| Blocked TDI | 168.95 °C (97.13%) | 264.48 °C (3.121%) |
| PU-TDI-PEG-400 | 236.63 °C (93.47%) | 514.12 °C (14.95%) |
| PU-TDI-PPG-1000 | 244.9 °C (95.46%) | 454.34 °C (68.80%) |
| PU-TDI-PPG-2000 | 216.92 °C (96.06%) | 535.35 °C (30.35%) |
| PU-TDI-PPG-3000 | 201.00 °C (97.31%) | 521.70 °C (14.27%) |
| Compound | Contact Angle |
|---|---|
| PU-TDI-PPG-1000 | 66.3° |
| PU-TDI-PPG-2000 | 56.7° |
| PU-TDI-PPG-3000 | 43.0° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jana, S.; Samanta, D.; Fahad, M.M.; Jaisankar, S.N.; Kim, H. Blocking and Deblocking of Diisocyanate to Synthesize Polyurethanes. Polymers 2021, 13, 2875. https://doi.org/10.3390/polym13172875
Jana S, Samanta D, Fahad MM, Jaisankar SN, Kim H. Blocking and Deblocking of Diisocyanate to Synthesize Polyurethanes. Polymers. 2021; 13(17):2875. https://doi.org/10.3390/polym13172875
Chicago/Turabian StyleJana, Sourita, Debasis Samanta, Mir Muhammad Fahad, Sellamuthu N. Jaisankar, and Hongdoo Kim. 2021. "Blocking and Deblocking of Diisocyanate to Synthesize Polyurethanes" Polymers 13, no. 17: 2875. https://doi.org/10.3390/polym13172875






