Design and Fabrication of a Fast Response Resistive-Type Humidity Sensor Using Polypyrrole (Ppy) Polymer Thin Film Structures
Abstract
:1. Introduction
1.1. Literature Review
1.2. Measuring Parameters for Humidity Sensors
1.3. Classification of Humidity Sensors Based on Their Fabrication Materials
1.3.1. Ceramic Humidity Sensor (Semiconductor)
1.3.2. Organic/Inorganic Hybrid Composites Type Humidity Sensors
1.3.3. Polymer-Based Humidity Sensors
Capacitive-Type Humidity Sensor
Resistive-Type Humidity Sensors
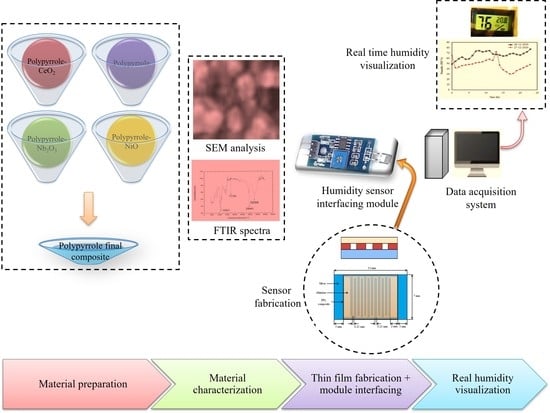
2. Material and Methods
2.1. Analytical Reagent Grade Pyrrole Material
2.1.1. Synthesis of Polypyrrole
2.1.2. Synthesis of Polypyrrole-NiO Composite
2.1.3. Synthesis of Polypyrrole-Nb2O5 Composite
2.1.4. Synthesis of Polypyrrole-CeO2 Composite
2.1.5. Preparation of Pellets
2.2. Characterization Techniques
2.2.1. Fourier Transform Infrared Radiation (FTIR) Spectra Analysis
2.2.2. Differential Thermal Analysis (DTA)/Thermo-Gravimetric Analysis (TGA)
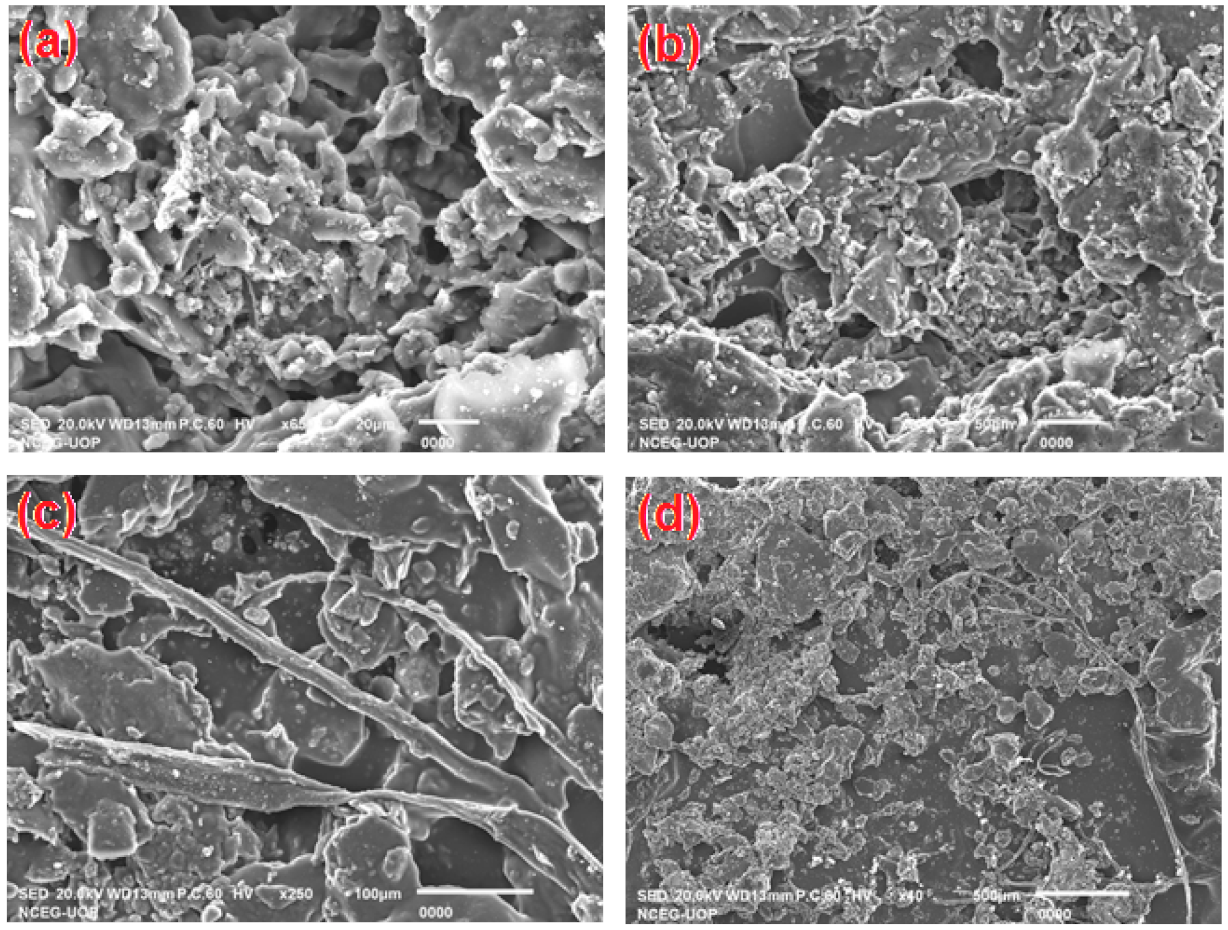
2.2.3. Scanning Electron Microscopy (SEM)
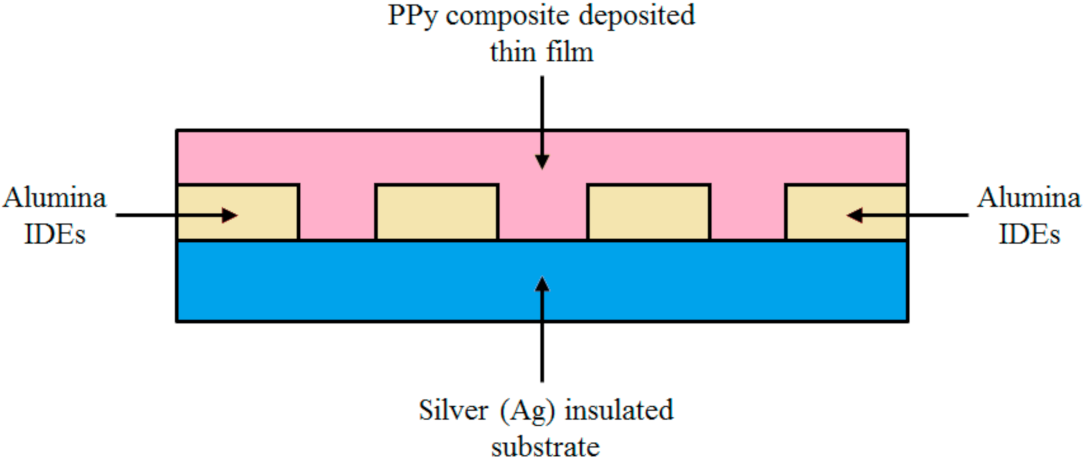
2.3. Fabrication of the Humidity Sensor of PVA Polypyrrole
3. Results and Discussion
3.1. Stability and Repeatability
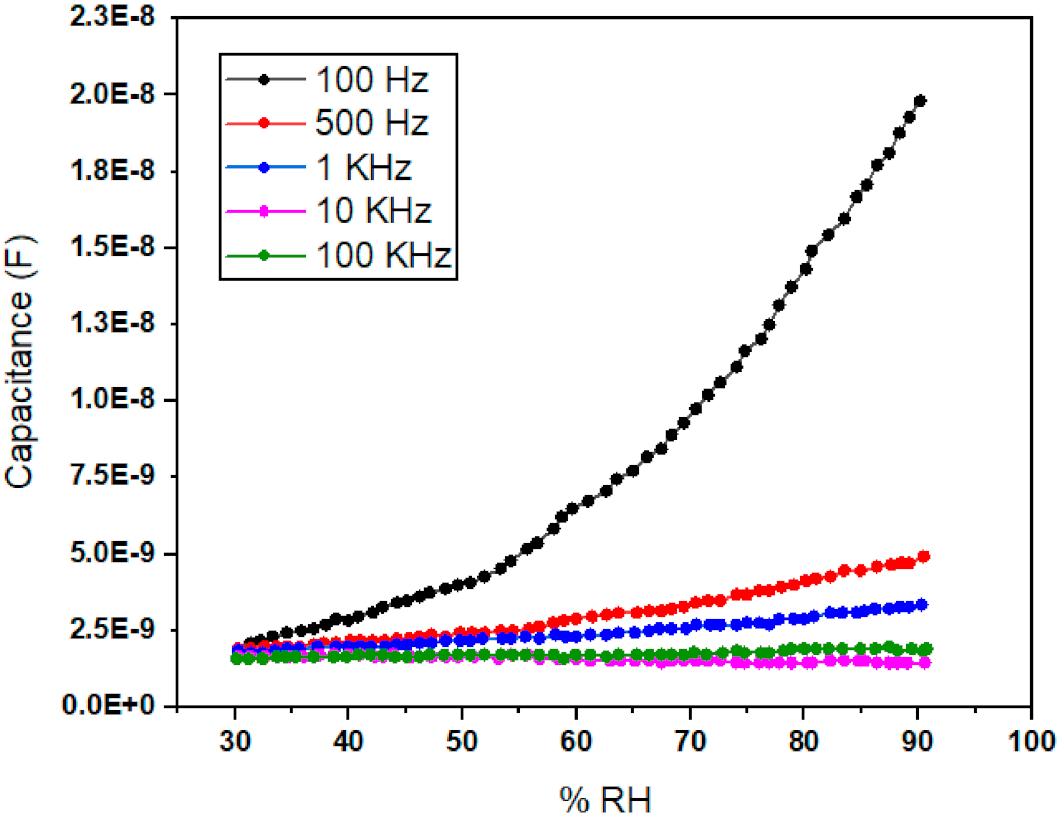
3.2. Capacitance verses Humidity
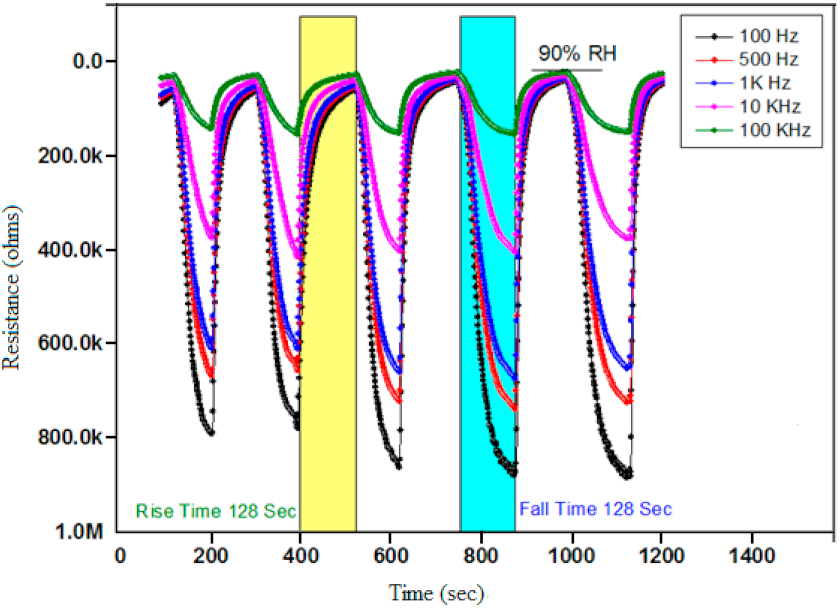
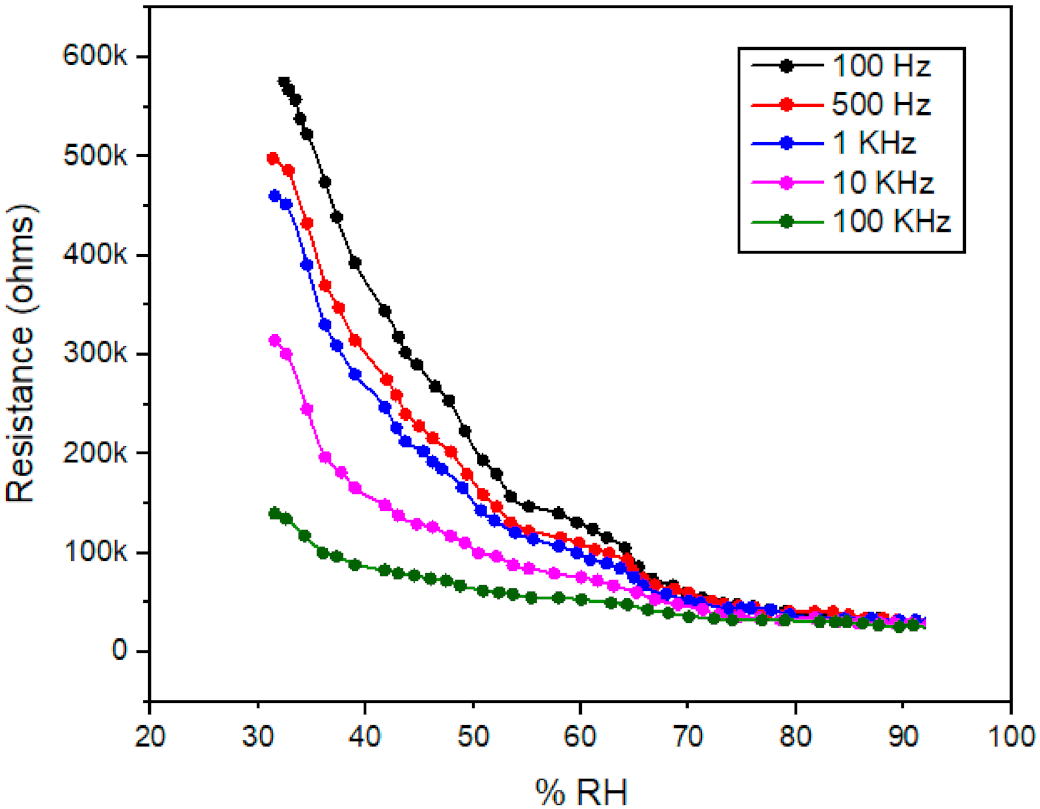
3.3. Resistance verses Humidity
3.4. Hysteresis of Ppy Thin Film Sensor
3.5. Real-Time Humidity Response of the Sensor
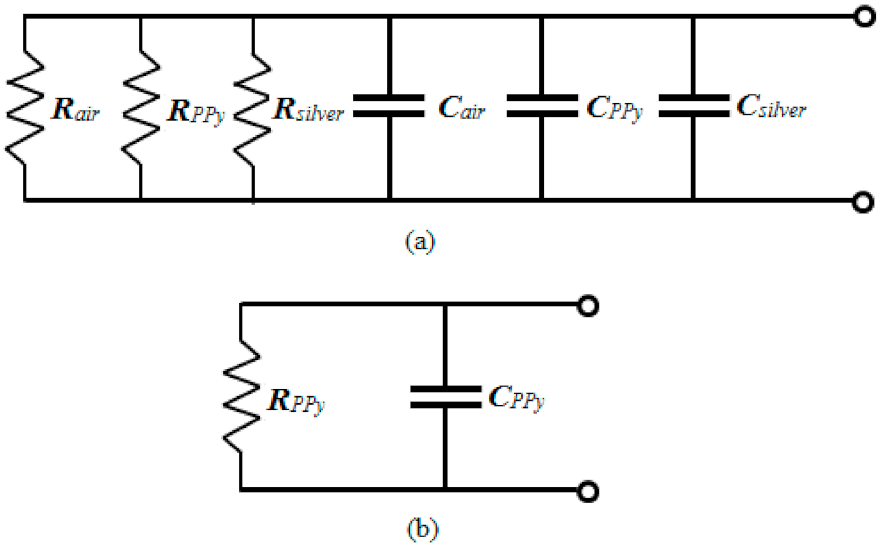
3.6. Equivalent Circuit Diagrams of Ppy Composite Sensor
4. Conclusions and Future Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diaz, A.F.; Kanazawa, K.K.; Gardini, G.P. Electrochemical polymerization of pyrrole. J. Chem. Soc. Chem. Commun. 1979, 14, 635–636. [Google Scholar] [CrossRef]
- Cheng, G.; Ding, J.; Zhang, Z.; Ling, Z.; Pu, H. Study on the preparation and multiproperties of the polypyrrole films doped with different ions. Surf. Interface Anal. 2012, 44, 844–850. [Google Scholar] [CrossRef]
- Harraz, F.A.; Salim, M.S.; Sakka, T.; Ogata, Y.H. Hybrid nanostructure of polypyrrole and porous silicon prepared by galvanostatic technique. Electrochim. Acta 2008, 53, 3734–3740. [Google Scholar] [CrossRef]
- Yoshino, K.; Hayashi, S.; Sugimoto, R.-I. Preparation and properties of conducting heterocyclic polymer films by chemical method. Jpn. J. Appl. Phys. 1984, 23, 247–252. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Rendu, P.L.; Tran, V.H.; Parkhutik, V.; Fenollosa Esteve, R. Electrical and optical properties of conducting polymer/porous silicon structures. J. Porous Mater. 2000, 7, 393–396. [Google Scholar] [CrossRef]
- Harraz, F.A. Electrochemical polymerization of pyrrole into nanostructured p-type porous silicon. J. Electrochem. Soc. 2006, 153, C349–C356. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Dispersed conducting polymer nanocomposites with glucose oxidase and gold nanoparticles for the design of enzymatic glucose biosensors. Polymers 2021, 13, 2173. [Google Scholar] [CrossRef] [PubMed]
- Samukaite-Bubniene, U.; Valiūnienė, A.; Bucinskas, V.; Genys, P.; Ratautaite, V.; Ramanaviciene, A.; Aksun, E.; Tereshchenko, A.; Zeybek, B.; Ramanavicius, A. Towards supercapacitors: Cyclic voltammetry and fast fourier transform electrochemical impedance spectroscopy based evaluation of polypyrrole electrochemically deposited on the pencil graphite electrode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125750. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge transfer and biocompatibility aspects in conducting polymer-based enzymatic biosensors and biofuel cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Emir, G.; Dilgin, Y.; Ramanaviciene, A.; Ramanavicius, A. Amperometric nonenzymatic glucose biosensor based on graphite rod electrode modified by Ni-nanoparticle/polypyrrole composite. Microchem. J. 2021, 161, 105751. [Google Scholar] [CrossRef]
- Xiaoqiang, L.; Qian, S.; Yan, K.; Yanan, Z.; Zengyuan, P.; Yang, J.; Mengjuan, L.; Chronakis, I.S. Self-powered humidity sensor based on polypyrrole modified melamine aerogel. Mater. Lett. 2020, 277, 128281. [Google Scholar] [CrossRef]
- Mazhar, S.; Qarni, A.A.; Haq, Y.Q.; Haq, Z.U.; Murtaza, I.; Ahmad, N.; Jabeen, N.; Amin, S. Electrospun PVA/TiC nanofibers for high performance capacitive humidity sensing. Microchem. J. 2020, 157, 104974. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Qi, D.; Zhao, C. High sensitive and fast response humidity sensor based on polymer composite nanofibers for breath monitoring and non-contact sensing. Sens. Actuators B Chem. 2020, 330, 129239. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, H.; Lin, X.; Liu, S.; Fei, T.; Zhang, T. Design strategy for ultrafast-response humidity sensors based on gel polymer electrolytes and application for detecting respiration. Sens. Actuators B Chem. 2020, 304, 127270. [Google Scholar] [CrossRef]
- Kastner, W.; Neugschwandtner, G.; Soucek, S.; Newman, H.M. Communication systems for building automation and control. Proc. IEEE 2005, 93, 1178–1203. [Google Scholar] [CrossRef]
- Wise, D.L. Electrical and Optical Polymer Systems: Fundamentals: Methods, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Dian, J.; Konečný, M.; Broncová, G.; Kronďák, M.; Matolínová, I. Electrochemical fabrication and characterization of porous silicon/polypyrrole composites and chemical sensing of organic vapors. Int. J. Electrochem. Sci. 2013, 8, 1559–1572. [Google Scholar]
- Funt, L.; Tanner, J. Electrochem, Synth, Poly, Tech, Electro-Org, Synth; Weinger, N.L., Ed.; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Pokhrel, S.; Jeyaraj, B.; Nagaraja, K.S. Humidity-sensing properties of ZnCr2O4–ZnO composites. Mater. Lett. 2003, 57, 3543–3548. [Google Scholar] [CrossRef]
- Chen, Y.S.; Li, Y.; Yang, M.J. Humidity sensitive properties of NaPSS/MWNTs nanocomposites. J. Mater. Sci. 2005, 40, 5037–5039. [Google Scholar] [CrossRef]
- Arrizabalaga, O.; Velasco, J.; Zubia, J.; De Ocáriz, I.S.; Villatoro, J. Miniature interferometric humidity sensor based on an off-center polymer cap onto optical fiber facet. Sens. Actuators B Chem. 2019, 297, 126700. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, T.; Guan, X.; Dai, J.; Liu, S.; Zhao, H.; Fei, T. Capacitive humidity sensors based on mesoporous silica and poly(3,4 ethylenedioxythiophene) composites. J. Colloid Interface Sci. 2020, 565, 592–600. [Google Scholar] [CrossRef]
- Karunarathne, T.S.E.F.; Wijesinghe, W.P.S.L.; Rathuwadu, N.P.W.; Karalasingam, A.; Manoharan, N.; Sameera, S.A.L.; Sandaruwan, C.; Amaratunga, G.A.; De Silva, S.G.M. Fabrication and characterization of partially conjugated poly (vinyl alcohol) based resistive humidity sensor. Sens. Actuator A Phys. 2020, 314, 112263. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lee, S.; Kim, B.; Kim, J.-H.; So, J.-H.; Koo, H.-J. Impedance study on humidity dependent conductivity of polymer composites with conductive nanofillers. Compos. B Eng. 2020, 202, 108412. [Google Scholar] [CrossRef]
- Lang, C.; Liu, Y.; Cao, K.; Li, Y.; Qu, S. Ultra-compact, fast-responsive and highly-sensitive humidity sensor based on a polymer micro-rod on the end-face of fiber core. Sens. Actuators B Chem. 2019, 290, 23–27. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimizu, Y. Humidity sensors: Principles and applications. Sens. Actuators 1986, 10, 379–398. [Google Scholar] [CrossRef]
- Fagan, J.G.; Amarakoon, R.W. Reliability and reproducibility of ceramic sensors. III: Humidity sensors. Am. Ceram. Soc. Bull. 1993, 72, 119–130. [Google Scholar]
- Goldberg, H.D.; Brown, R.B.; Liu, D.P.; Meyerhoff, M.E. Screen printing: A technology for the batch fabrication of integrated chemical-sensor arrays. Sens. Actuators B Chem. 1994, 21, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Kulwicki, B.M. Humidity sensors. J. Am. Ceram. 1991, 74, 697–708. [Google Scholar] [CrossRef]
- Dean, R.N.; Rane, A.K.; Baginski, M.E.; Richard, J.; Hartzog, Z.; Elton, D.J. A capacitive fringing field sensor design for moisture measurement based on printed circuit board technology. IEEE Trans. Instrum. Meas. 2011, 61, 1105–1112. [Google Scholar] [CrossRef]
- Lin, W.D.; Chang, H.M.; Wu, R.J. Applied novel sensing material graphene/polypyrrole for humidity sensor. Sens. Actuators B Chem. 2013, 181, 326–331. [Google Scholar] [CrossRef]
- Xu, C.N.; Miyazaki, K.; Watanabe, T. Humidity sensors using manganese oxides. Sens. Actuators B Chem. 1998, 46, 87–96. [Google Scholar] [CrossRef]
- Pelino, M.; Colella, C.; Cantalini, C.; Faccio, M.; Ferri, G.; D’Amico, A. Microstructure and electrical properties of an α-hematite ceramic humidity sensor. Sens. Actuators B Chem. 1992, 7, 464–469. [Google Scholar] [CrossRef]
- Klym, H.; Hadzaman, I.; Shpotyuk, O.; Brunner, M. P3.6-multifunctional T/RH-sensitive thick-film structures for environmental sensors. In Proceedings of the SENSOR+TEST Conferences 2011 Proceedings on SENSOR, Nürnberg, Germany, 6–9 September 2011; pp. 744–748. [Google Scholar]
- Traversa, E.; Bearzotti, A. A novel humidity-detection mechanism for ZnO dense pellets. Sens. Actuators B Chem. 1995, 23, 181–186. [Google Scholar] [CrossRef]
- Gusmano, G.; Montesperelli, G.; Nunziante, P.; Traversa, E. Study of the conduction mechanism of MgAl2O4 at different environmental humidities. Electrochim. Acta 1993, 38, 2617–2621. [Google Scholar] [CrossRef]
- Smetana, W.; Unger, M. Design and characterization of a humidity sensor realized in LTCC-technology. Microsyst. Technol. 2008, 14, 979–987. [Google Scholar] [CrossRef]
- Karimov, K.S.; Cheong, K.Y.; Saleem, M.; Murtaza, I.; Farooq, M.; Noor, A.F.M. Ag/PEPC/NiPc/ZnO/Ag thin film capacitive and resistive humidity sensors. J. Semicond. 2010, 31, 054002. [Google Scholar] [CrossRef]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Connolly, E.J.; O’Halloran, G.M.; Pham, H.T.M.; Sarro, P.M.; French, P.J. Comparison of porous silicon, porous polysilicon and porous silicon carbide as materials for humidity sensing applications. Sens. Actuator A Phys. 2002, 99, 25–30. [Google Scholar] [CrossRef]
- Salehi, A.; Kalantari, D.J.; Goshtasbi, A. Rapid response of Au/porous-GaAs humidity sensor at room temperature. In Proceedings of the 2006 IEEE Conference on Optoelectronic and Microelectronic Materials and Devices, Perth, WA, Australia, 6–8 December 2006; pp. 125–128. [Google Scholar] [CrossRef]
- Yang, B.; Aksak, B.; Lin, Q.; Sitti, M. Compliant and low-cost humidity nanosensors using nanoporous polymer membranes. Sens. Actuators B Chem. 2006, 114, 254–262. [Google Scholar] [CrossRef]
- Shah, J.; Kotnala, R.K.; Singh, B.; Kishan, H. Microstructure-dependent humidity sensitivity of porous MgFe2O4–CeO2 ceramic. Sens. Actuators B Chem. 2007, 128, 306–311. [Google Scholar] [CrossRef]
- Santha, H.; Packirisamy, M.; Stiharu, I.; Li, X.; Rinaldi, G. A polyimide based resistive humidity sensor. Sens. Rev. 2005, 25, 271–276. [Google Scholar]
- Cho, N.B.; Lim, T.H.; Jeon, Y.M.; Gong, M.S. Inkjet printing of polymeric resistance humidity sensor using UV-curable electrolyte inks. Macromol. Res. 2008, 16, 149–154. [Google Scholar] [CrossRef]
- Dwiputra, M.A.; Fadhila, F.; Imawan, C.; Fauzia, V. The enhanced performance of capacitive-type humidity sensors based on ZnO nanorods/WS2 nanosheets heterostructure. Sens. Actuators B Chem. 2020, 310, 127810. [Google Scholar] [CrossRef]
- Jachowicz, R.S.; Senturia, S.D. A thin-film capacitance humidity sensor. Sens. Actuators 1981, 2, 171–186. [Google Scholar] [CrossRef]
- Pal, B.N.; Chakravorty, D. Humidity sensing by composites of glass ceramics containing silver nanoparticles and their conduction mechanism. Sens. Actuators B Chem. 2006, 114, 1043–1051. [Google Scholar] [CrossRef]
- Jeseentharani, V.; Reginamary, L.; Jeyaraj, B.; Dayalan, A.; Nagaraja, K.S. Nanocrystalline spinel NixCu0.8-xZn0.2Fe2O4: A novel material for humidity sensing. J. Mater. Sci. 2012, 47, 3529–3534. [Google Scholar] [CrossRef]
- Krutovertsev, S.A.; Tarasova, A.E.; Krutovertseva, L.S.; Chuprin, M.V.; Ivanova, O.M.; Sazhinev, Y.S. Technology and characteristics of microhumidity sensors. In Proceedings of the 2011 IEEE 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 621–624. [Google Scholar] [CrossRef]
- Jain, M.K.; Bhatnagar, M.C.; Sharma, G.L. Effect of Li+ doping on ZrO2–TiO2 humidity sensor. Sens. Actuators B Chem. 1999, 55, 180–185. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korvink, J.G.; Chandran, L.; Boltshauser, T.; Baltes, H. Accurate 3D capacitance evaluation in integrated capacitive humidity sensors. Sens. Mater. 1993, 4, 323–336. [Google Scholar]
- Nellikkal, M.A.N.; Ahmad, Z.; Shakoor, R.A. Organic thin-film capacitive and resistive humidity sensors: A focus review. Adv. Mater. Interfaces 2018, 5, 1800969. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Fadzil Ain, M.; Ahmad, Z.A. Fabrication of resistance type humidity sensor based on CaCu3Ti4O12 thick film. Measurement 2016, 94, 902–908. [Google Scholar] [CrossRef]
- Su, P.-G.; Wang, C.-S. Novel flexible resistive-type humidity sensor. Sens. Actuators B Chem. 2007, 123, 1071–1076. [Google Scholar] [CrossRef]
- Najjar, R.; Nematdoust, S. A resistive-type humidity sensor based on polypyrrole and ZnO nanoparticles: Hybrid polymers vis-a-vis nanocomposites. RSC Adv. 2016, 6, 112129–112139. [Google Scholar] [CrossRef]
- Horowitz, H.H.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Narayanan, R.; Laine, R.M. Synthesis and characterization of precursors for group II metal aluminates. Appl. Organomet. Chem. 1998, 11, 919–927. [Google Scholar] [CrossRef]
- Tahira, M.; Sayyad, M.H.; Clark, J.; Wahab, F.; Aziz, F.; Shahid, M.; Munawar, M.A.; Chaudry, J.A. Humidity, light and temperature dependent characteristics of Au/N-BuHHPDI/Au surface type multifunctional sensor. Sens. Actuators B Chem. 2014, 192, 565–571. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.; Hasnain, S.; Khan, N.A.; Bano, S.; Zuhra, F.; Ali, M.; Khan, M.; Abbas, N.; Ali, A. Design and Fabrication of a Fast Response Resistive-Type Humidity Sensor Using Polypyrrole (Ppy) Polymer Thin Film Structures. Polymers 2021, 13, 3019. https://doi.org/10.3390/polym13183019
Hussain M, Hasnain S, Khan NA, Bano S, Zuhra F, Ali M, Khan M, Abbas N, Ali A. Design and Fabrication of a Fast Response Resistive-Type Humidity Sensor Using Polypyrrole (Ppy) Polymer Thin Film Structures. Polymers. 2021; 13(18):3019. https://doi.org/10.3390/polym13183019
Chicago/Turabian StyleHussain, Mushahid, Saqib Hasnain, Nadir Ali Khan, Shehar Bano, Fazeelat Zuhra, Muhammad Ali, Munawar Khan, Naseem Abbas, and Ahsan Ali. 2021. "Design and Fabrication of a Fast Response Resistive-Type Humidity Sensor Using Polypyrrole (Ppy) Polymer Thin Film Structures" Polymers 13, no. 18: 3019. https://doi.org/10.3390/polym13183019
APA StyleHussain, M., Hasnain, S., Khan, N. A., Bano, S., Zuhra, F., Ali, M., Khan, M., Abbas, N., & Ali, A. (2021). Design and Fabrication of a Fast Response Resistive-Type Humidity Sensor Using Polypyrrole (Ppy) Polymer Thin Film Structures. Polymers, 13(18), 3019. https://doi.org/10.3390/polym13183019