Conductometric Immunosensor for Escherichia coli O157:H7 Detection Based on Polyaniline/Zinc Oxide (PANI/ZnO) Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of ZnO Nanoparticles
2.3. Preparation of PANI/ZnO Nanocomposite
2.4. Characterization of the Nanostructures
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. X-ray Diffraction (XRD)
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Preparation of Microbial Samples
2.6. Antimicrobial Activity of the Nanostructures
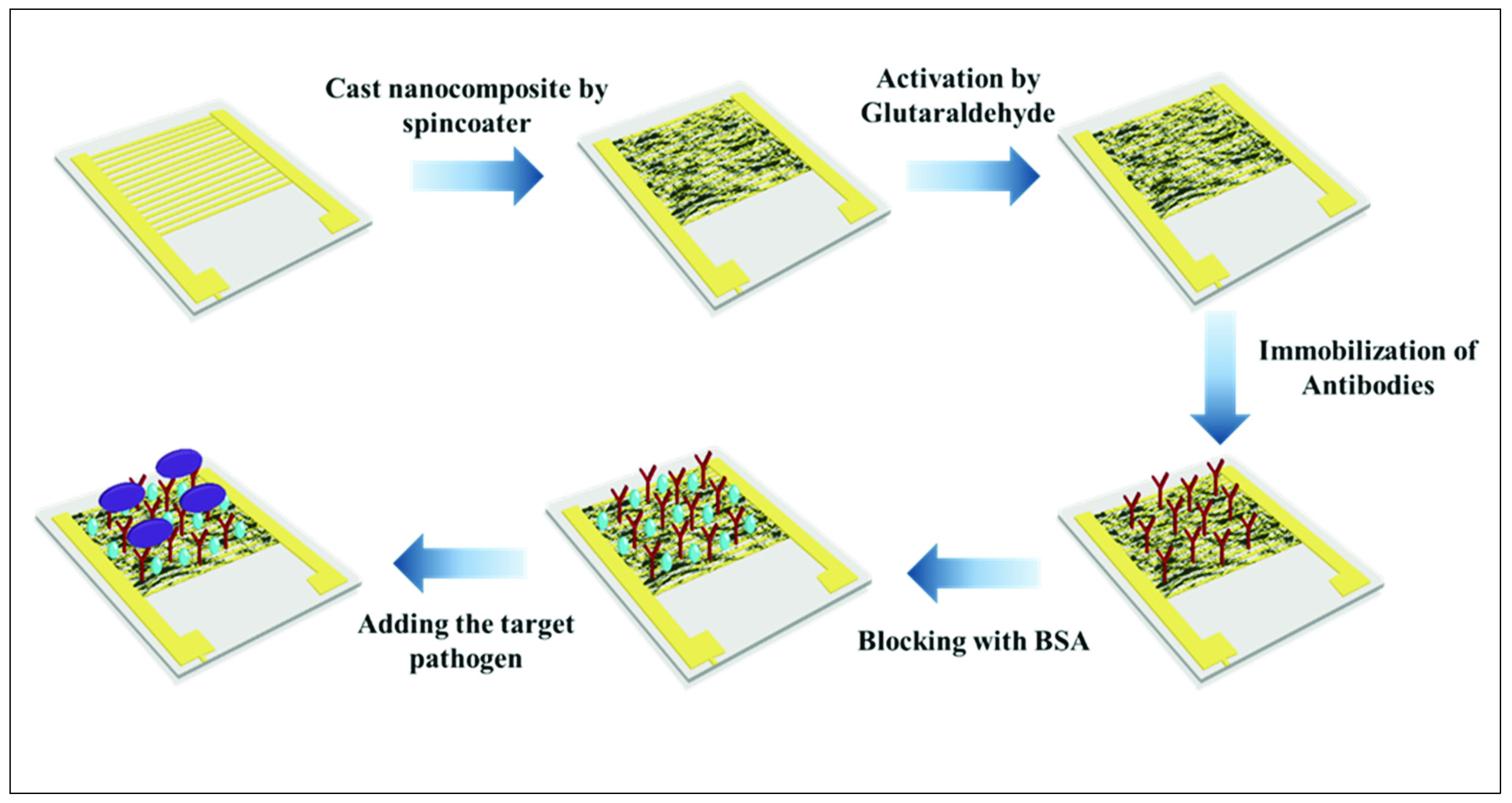
2.7. Preparation of Electrode
2.8. Detection in Real Sample
3. Results and Discussion
3.1. Characterization of the Nanostructures
3.1.1. Scanning Electron Microscopy (SEM)
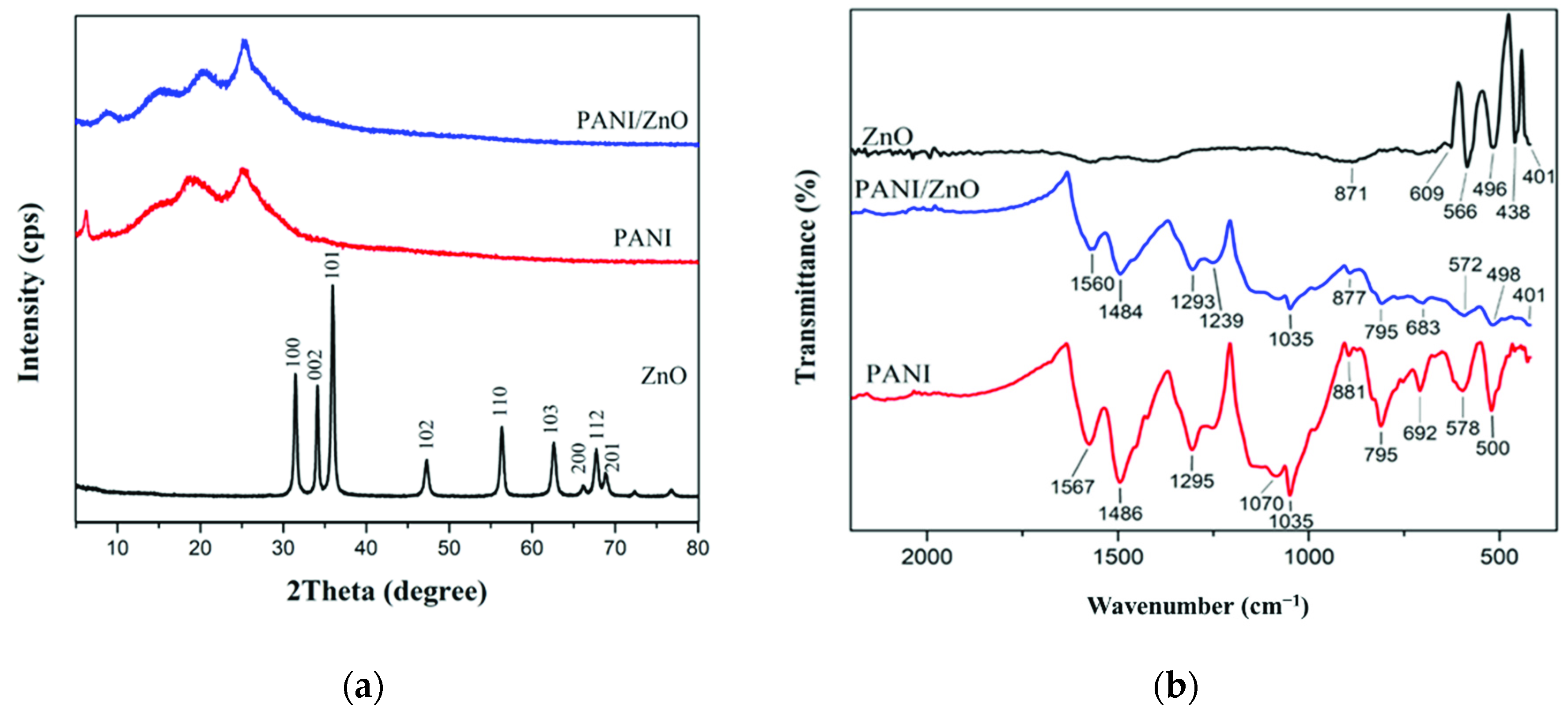
3.1.2. XRD Analysis
3.1.3. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. Minimum Inhibitory Concentration (MIC) of the Nanomaterials
3.3. Electrical Characterization of the Modified Electrode
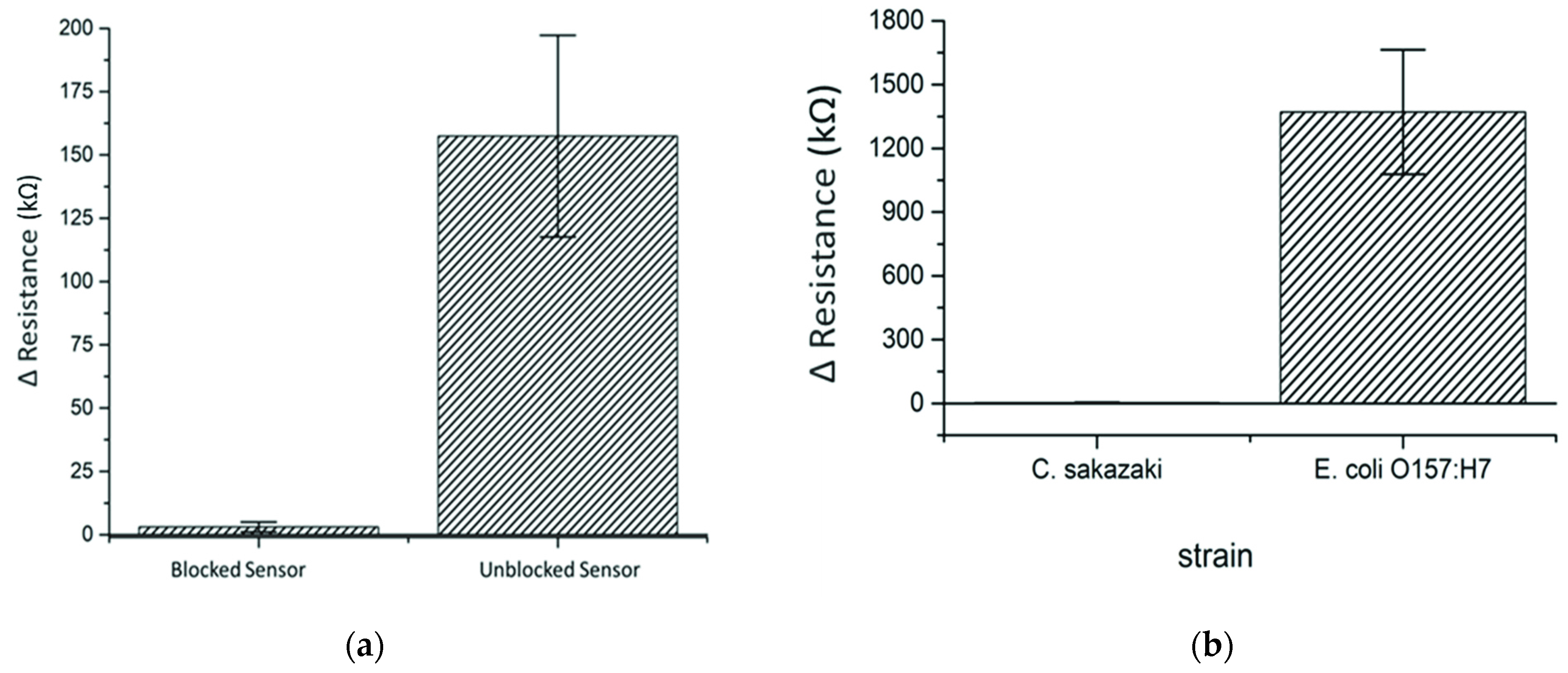
3.4. Optimization of Blocking and Incubation Time
3.5. Specificity and Cross-Reactivity of the Sensors
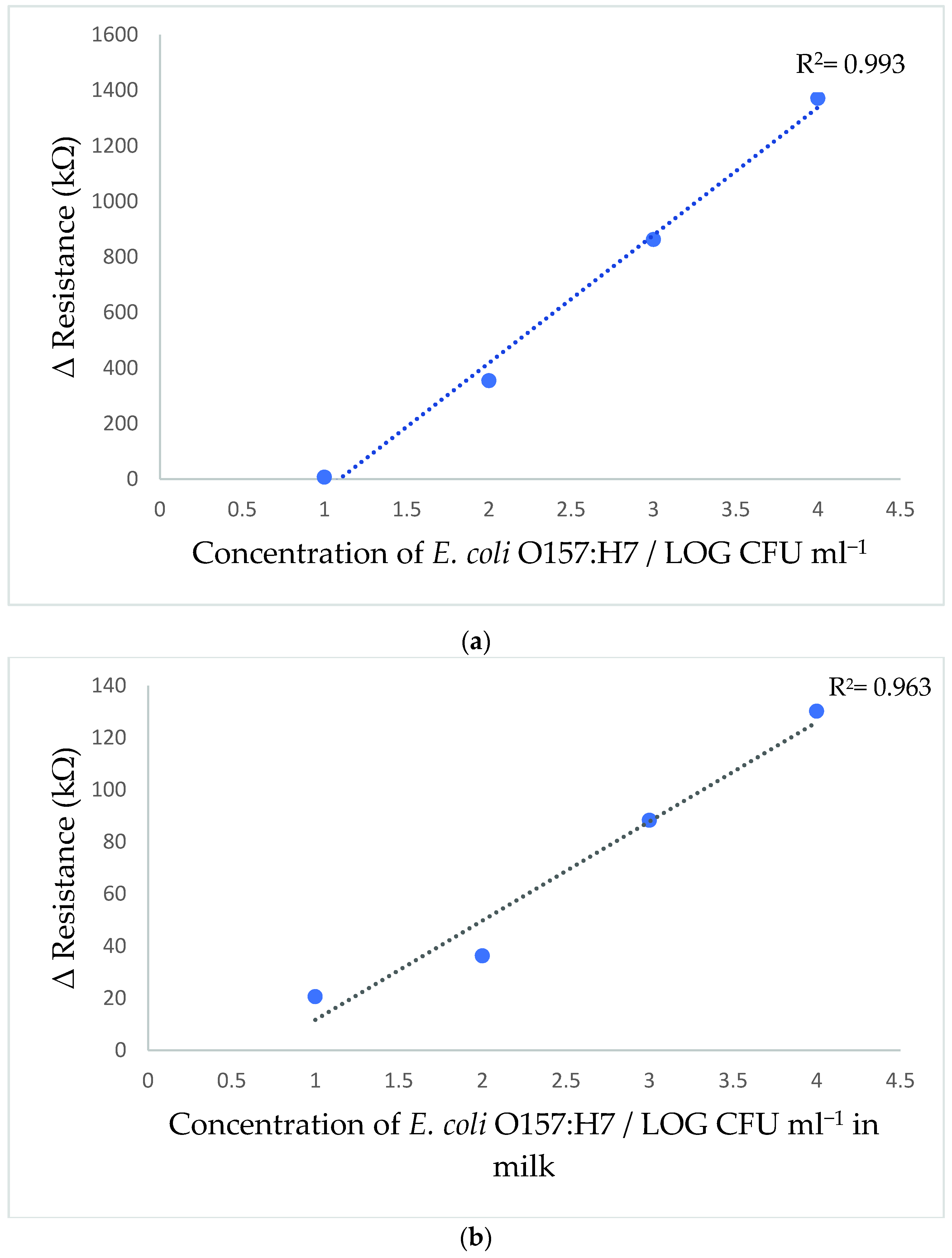
3.6. Calibration Curve of E. coli O157:H7 Sensor
3.7. Application in Milk Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 22 April 2021).
- Cordonnier, C.; Etienne-Mesmin, L.; Thévenot, J.; Rougeron, A.; Rénier, S.; Chassaing, B.; Darfeuille-Michaud, A.; Barnich, N.; Blanquet-Diot, S.; Livrelli, V. Enterohemorrhagic Escherichia coli pathogenesis: Role of Long polar fimbriae in Peyer’s patches interactions. Sci. Rep. 2017, 7, 44655. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L.; Williams, D.W. Chapter Six-Escherichia coli. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: London, UK, 2014; pp. 89–117. [Google Scholar]
- Lim, J.Y.; Yoon, J.W.; Hovde, C.J. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J. Microbiol. Biotechnol. 2010, 20, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, R.K. Escherichia coli O157:H7 (Enterohemorrhagic, E. coli). In Ciottone’s Disaster Medicine, 2nd ed.; Ciottone, G.R., Ed.; Elsevier: Philadelphia, PA, USA, 2016; Chapter 139; pp. 746–749. [Google Scholar]
- Estrada-Garcia, T.; Hodges, K.; Hecht, G.A.; Tarr, P.I. Escherichia coli. In Foodborne Infections and Intoxications, 4th ed.; Morris, J.G., Potter, M.E., Eds.; Academic Press: San Diego, CA, USA, 2013; Chapter 8; pp. 129–164. [Google Scholar]
- Ricke, S.C.; Feye, K.M.; Chaney, W.E.; Shi, Z.; Pavlidis, H.; Yang, Y. Developments in Rapid Detection Methods for the Detection of Foodborne Campylobacter in the United States. Front. Microbiol. 2019, 9, 3280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanh Ngo, V.K.; Nguyen, D.G.; Uyen Nguyen, H.P.; Man Tran, V.; My Nguyen, T.K.; Phat Huynh, T.; Vinh Lam, Q.; Dat Huynh, T.; Lien Truong, T.N. Quartz crystal microbalance (QCM) as biosensor for the detecting of Escherichia coli O157:H7. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 045004. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Si, C.; Ying, Y. Subtractive inhibition assay for the detection of E. coli O157:H7 using surface plasmon resonance. Sensors 2011, 11, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhong, J.; Hu, T.; Zhao, X. Simultaneous detection of Escherichia coli O157:H7, Staphylococcus aureus and Salmonella by multiplex PCR in milk. 3 Biotech 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Güner, A.; Çevik, E.; Şenel, M.; Alpsoy, L. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chem. 2017, 229, 358–365. [Google Scholar] [CrossRef]
- Pangajam, A.; Theyagarajan, K.; Dinakaran, K. Highly sensitive electrochemical detection of E. coli O157:H7 using conductive carbon dot/ZnO nanorod/PANI composite electrode. Sens. Bio-Sens. Res. 2020, 29, 100317. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, Y.; Sun, X.; Wang, X. Amperometric Immunosensor for Carbofuran Detection Based on MWCNTs/GS-PEI-Au and AuNPs-Antibody Conjugate. Sensors 2013, 13, 5286–5301. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.P.; Mohammad, F. Studies on Nanocomposites of Polyaniline and Zinc Oxide Nanoparticles with Supporting Matrix of Polycarbonate. ISRN Mater. Sci. 2012, 2012, 129869. [Google Scholar] [CrossRef] [Green Version]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef] [PubMed]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; De, A.; Chaudhuri, C.R.; Bandyopadhyay, K.; Sen, P. Label free polyaniline based impedimetric biosensor for detection of E. coli O157:H7 Bacteria. Sens. Actuators B 2012, 171–172, 916–923. [Google Scholar] [CrossRef]
- He, Y. A novel emulsion route to sub-micrometer polyaniline/nano-ZnO composite fibers. Appl. Surf. Sci. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Setterington, E.B.; Alocilja, E.C. Rapid electrochemical detection of polyaniline-labeled Escherichia coli O157:H7. Biosens. Bioelectron. 2011, 26, 2208–2214. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.A.; Tsacheva, I. Microbial sensors based on nanostructures. Recent Pat. Nanomed. 2015, 5, 59–65. [Google Scholar] [CrossRef]
- Lee, K.S.; Song, Y.; Kim, C.H.; Kim, Y.T.; Kang, T.; Lee, S.J.; Choi, B.G.; Lee, K.G. Development of zinc oxide-based sub-micro pillar arrays for on-site capture and DNA detection of foodborne pathogen. J. Colloid Interface Sci. 2020, 563, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Antimicrobial Activity and Prevention of Bacterial Biofilm Formation of Silver and Zinc Oxide Nanoparticle-Containing Polyester Surfaces at Various Concentrations for Use. Foods 2020, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Dhole, S.G.; Dake, S.A.; Prajapati, T.A.; Helambe, S.N. Effect of ZnO Filler on Structural and Optical Properties of Polyaniline-ZnO Nanocomposites. Procedia Manuf. 2018, 20, 127–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, C.; Wang, W.; Wang, Q.; Feng, N. Synthesis of uniform polyaniline nanosheets and nanotubes: Dependence of morphology on the pH. Macromol. Res. 2016, 24, 663–669. [Google Scholar] [CrossRef]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.; Amarnath, C.A.; Mangoli, S.H.; Sawant, S.N. Polyaniline nanoparticle based colorimetric sensor for monitoring bacterial growth. Sens. Actuators B 2015, 207, 262–268. [Google Scholar] [CrossRef]
- Gilja, V.; Vrban, I.; Mandić, V.; Žic, M.; Hrnjak-Murgić, Z. Preparation of a PANI/ZnO Composite for Efficient Photocatalytic Degradation of Acid Blue. Polymers 2018, 10, 940. [Google Scholar] [CrossRef] [Green Version]
- Alvi, F.; Ram, M.K.; Gomez, H.; Joshi, R.K.; Kumar, A. Evaluating the chemio-physio properties of novel zinc oxide–polyaniline nanocomposite polymer films. Polym. J. 2010, 42, 935–940. [Google Scholar] [CrossRef] [Green Version]
- Al-Nabulsi, A.; Osaili, T.; Sawalha, A.; Olaimat, A.N.; Albiss, B.A.; Mehyar, G.; Ayyash, M.; Holley, R. Antimicrobial activity of chitosan coating containing ZnO nanoparticles against E. coli O157:H7 on the surface of white brined cheese. Int. J. Food Microbiol. 2020, 334, 108838. [Google Scholar] [CrossRef]
- Jin, T.; Sun, D.; Su, J.Y.; Zhang, H.; Sue, H.J. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157:H7. J. Food Sci. 2009, 74, M46–M52. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucekova, Z.; Kasparkova, V.; Humpolicek, P.; Sevcikova, P.; Stejskal, J. Antibacterial properties of polyaniline-silver films. Chem. Pap. 2013, 67, 1103–1108. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Hu, C.; Dou, W.; Zhao, G. Enzyme immunosensor based on gold nanoparticles electroposition and Streptavidin-biotin system for detection of S. pullorum and S. gallinarum. Electrochim. Acta 2014, 117, 239–245. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Jia, F.; Yu, Y.; Chen, J.; Wang, Z. An aptamer-based electrochemical biosensor for the detection of Salmonella. J. Microbiol. Methods 2014, 98, 94–98. [Google Scholar] [CrossRef]
- Wujcik, E.K.; Wei, H.; Zhang, X.; Guo, J.; Yan, X.; Sutrave, N.; Wei, S.; Guo, Z. Antibody nanosensors: A detailed review. RSC Adv. 2014, 4, 43725–43745. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: A review on recent advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef]
- Wang, Y.; Alocilja, E.C. Gold nanoparticle-labeled biosensor for rapid and sensitive detection of bacterial pathogens. J. Biol. Eng. 2015, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, N.F.D.; Magalhães, J.M.C.S.; Barroso, M.F.; Oliva-Teles, T.; Freire, C.; Delerue-Matos, C. In situ formation of gold nanoparticles in polymer inclusion membrane: Application as platform in a label-free potentiometric immunosensor for Salmonella typhimurium detection. Talanta 2019, 194, 134–142. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, H.; Xu, M.; Ma, Q.; Ai, S. A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-Chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk. Food Chem. 2013, 141, 1980–1986. [Google Scholar] [CrossRef]
- Ranjbar, S.; Shahrokhian, S. Design and fabrication of an electrochemical aptasensor using Au nanoparticles/carbon nanoparticles/cellulose nanofibers nanocomposite for rapid and sensitive detection of Staphylococcus aureus. Bioelectrochemistry 2018, 123, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Arshavsky-Graham, S.; Urmann, K.; Salama, R.; Massad-Ivanir, N.; Walter, J.-G.; Scheper, T.; Segal, E. Aptamers vs. antibodies as capture probes in optical porous silicon biosensors. Analyst 2020, 145, 4991–5003. [Google Scholar] [CrossRef] [PubMed]


| Strain Type | MIC (mg/mL) of the Following Nanostructures | |||
|---|---|---|---|---|
| ZnO-NPs | PANI Nanosheets | PANI/ZnO Nanocomposite | ||
| Escherichia coli O157:H7 | 02:0628 | 0.6250 | >20 | >20 |
| 02:0627 | 0.3125 | >20 | >20 | |
| NCTC 12900 | 0.6250 | >20 | 10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutlaq, S.; Albiss, B.; Al-Nabulsi, A.A.; Jaradat, Z.W.; Olaimat, A.N.; Khalifeh, M.S.; Osaili, T.; Ayyash, M.M.; Holley, R.A. Conductometric Immunosensor for Escherichia coli O157:H7 Detection Based on Polyaniline/Zinc Oxide (PANI/ZnO) Nanocomposite. Polymers 2021, 13, 3288. https://doi.org/10.3390/polym13193288
Mutlaq S, Albiss B, Al-Nabulsi AA, Jaradat ZW, Olaimat AN, Khalifeh MS, Osaili T, Ayyash MM, Holley RA. Conductometric Immunosensor for Escherichia coli O157:H7 Detection Based on Polyaniline/Zinc Oxide (PANI/ZnO) Nanocomposite. Polymers. 2021; 13(19):3288. https://doi.org/10.3390/polym13193288
Chicago/Turabian StyleMutlaq, Sawsan, Borhan Albiss, Anas A. Al-Nabulsi, Ziad W. Jaradat, Amin N. Olaimat, Mohammad S. Khalifeh, Tareq Osaili, Mutamed M. Ayyash, and Richard A. Holley. 2021. "Conductometric Immunosensor for Escherichia coli O157:H7 Detection Based on Polyaniline/Zinc Oxide (PANI/ZnO) Nanocomposite" Polymers 13, no. 19: 3288. https://doi.org/10.3390/polym13193288






