Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications
Abstract
:1. Introduction
2. Polymers with Antimicrobial Properties
3. Strategies for Imparting Antimicrobial Properties to Polymers
4. Covalent Immobilization of Antimicrobial Properties to Polymers
4.1. Radiation-Induced Graft Copolymerization
4.1.1. γ-ray- and Electron-Induced Graft Copolymerization
4.1.2. Photo-Induced Graft Copolymerization
4.1.3. Plasma-Induced Graft Copolymerization
5. Designing Antimicrobial Surfaces by Radiation-Induced Graft Copolymerization
6. Synergizing of Antimicrobial Properties by Metallic Nanoparticles
7. Applications of Grafted Polymers with Antimicrobial Properties
7.1. Health Care
7.1.1. Protective Fabrics
7.1.2. Face Masks
7.2. Medical Devices
7.2.1. Catheters
7.2.2. Surgical Suture
7.2.3. Wound Dressing Hydrogels/Patches
7.2.4. Contact Lenses
7.3. Biomedical Applications
7.3.1. Substrates/Scaffolds for Tissue Engineering
7.3.2. Thermo-Responsive Culture Dish
7.4. Food Packaging Polymers with Antibacterial Properties
7.4.1. Packaging Films with Grafted Biocidal Monomers
7.4.2. Packaging Films with In-Situ Immobilized Silver Nanoparticles
8. Challenges and Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Radovic-Moreno, A.F.; Wu, J.; Langer, R.; Shi, J. Nanomedicine in the management of microbial infection—Overview and perspectives. Nano Today 2014, 9, 478–498. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef]
- Vasilev, K.; Cavallaro, A.; Zilm, P. Special Issue: Antibacterial Materials and Coatings. Molecules 2018, 23, 585. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Crawford, R.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Shin, I.H. Graft copolymerization of GMA and EDMA on PVDF to hydrophilic surface modification by electron beam irradiation. Nucl. Eng. Technol. 2020, 52, 373–380. [Google Scholar] [CrossRef]
- Shintani, H. Bulk and/or Surface Modification of Medical Polymers to Attain Antimicrobial Activity and/or Biocompatibility. Biocontrol. Sci. 2005, 10, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.D.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Francolini, I. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Sangermano, M.; Razza, N. Light induced grafting-from strategies as powerful tool for surface modification. Express Polym. Lett. 2019, 13, 135–145. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Brodus, D.; Hollimon, V.; Hu, H. A brief review of recent developments in the designs that prevent bio-fouling on silicon and silicon-based materials. Chem. Central. J. 2017, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Cranston, R. Recent Advances in Antimicrobial Treatments of Textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Balaji, A.; Vellayappan, M.V.; Subramanian, A.P.; John, A.A.; Asokan, M.K.; Supriyanto, E. Review: Radiation-induced surface modification of polymers for biomaterial application. J. Mater. Sci. 2014, 50, 2007–2018. [Google Scholar] [CrossRef]
- Giannossa, L.C.; Longano, D.; Ditaranto, N.; Nitti, M.A.; Paladini, F.; Pollini, M.; Rai, M.; Sannino, A.; Valentini, A.; Cioffi, N. Metal nanoantimicrobials for textile applications. Nanotechnol. Rev. 2013, 2, 307–331. [Google Scholar] [CrossRef]
- Song, B.; Zhang, E.; Han, X.; Zhu, H.; Shi, Y.; Cao, Z. Engineering and Application Perspectives on Designing an Antimicrobial Surface. ACS Appl. Mater. Interfaces 2020, 12, 21330–21341. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and antiviral functional materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharmacol. Transl. Sci. 2020, 4, 8–54. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.Y.; Cheung, S.O.F.; Huang, L.; Niu, J.; Tao, C.; Ho, C.-M.; Che, C.M.; Tam, P.K.H. Further Evidence of the Anti-inflammatory Effects of Silver Nanoparticles. Chem. Med. Chem. 2009, 4, 1129–1135. [Google Scholar] [CrossRef]
- Waugh, D.; Toccaceli, C.; Gillett, A.; Ng, C.-H.; Hodgson, S.; Lawrence, J. Surface Treatments to Modulate Bioadhesion: A Critical Review. Rev. Adhes. 2016, 4, 69–103. [Google Scholar] [CrossRef]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart Antibacterial Surfaces with Switchable Bacteria-Killing and Bacteria-Releasing Capabilities. ACS Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef]
- Al-Balakocy, N.G.; Shalaby, S.E. Imparting Antimicrobial Properties to Polyester and Polyamide Fibers-State of the Art. J. Text. Assoc. 2017, 78, 179–201. [Google Scholar]
- Greenhalgh, R.; Dempsey-Hibbert, N.C.; Whitehead, K.A. Antimicrobial strategies to reduce polymer biomaterial infections and their economic implications and considerations. Int. Biodeterior. 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Maillard, J.-Y. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002, 92, 16–27. [Google Scholar] [CrossRef]
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro. Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Nagaraja, A.; Puttaiahgowda, Y.M.; Kulal, A.; Parambil, A.M.; Varadavenkatesan, T. Synthesis, Characterization, and Fabrication of Hydrophilic Antimicrobial Polymer Thin Film Coatings. Macromol. Res. 2019, 27, 301–309. [Google Scholar] [CrossRef]
- Badrossama, M.; Sun, G. Enhancing hygiene/antimicrobial properties of polyolefins. Polyolefin Fibers 2009, 262–287. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.C.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, K. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Pinson, J.; Thiry, D. Surface Modification of Polymers: Methods and Applications; Wiley-VCH: Weinheim, Germany, 2019; pp. 1–460. [Google Scholar]
- Goel, N.; Rao, M.; Kumar, V.; Bhardwaj, Y.; Chaudhari, C.; Dubey, K.A.; Sabharwal, S. Synthesis of antibacterial cotton fabric by radiation-induced grafting of [2-(Methacryloyloxy)ethyl]trimethylammonium chloride (MAETC) onto cotton. Radiat. Phys. Chem. 2009, 78, 399–406. [Google Scholar] [CrossRef]
- Nasef, M.M. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog. Polym. Sci. 2004, 29, 499–561. [Google Scholar] [CrossRef]
- Nasef, M.M.E.-S. Radiation-Grafted Membranes for Polymer Electrolyte Fuel Cells: Current Trends and Future Directions. Chem. Rev. 2014, 114, 12278–12329. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M.; Ferrario, S. Sustainable antimicrobial finishing of cotton fabrics by chitosan UV-grafting: From laboratory experiments to semi industrial scale-up. J. Clean. Prod. 2015, 96, 244–252. [Google Scholar] [CrossRef]
- Terada, A.; Yuasa, A.; Tsuneda, S.; Hirata, A.; Katakai, A.; Tamada, M. Elucidation of dominant effect on initial bacterial adhesion onto polymer surfaces prepared by radiation-induced graft polymerization. Colloids Surf. B. Biointerfaces 2005, 43, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Terada, A.; Yuasa, A.; Kushimoto, T.; Tsuneda, S.; Katakai, A.; Tamada, M. Bacterial adhesion to and viability on positively charged polymer surfaces. Microbiology 2006, 152, 3575–3583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, N.; Bellon-Fontaine, M.N.; Herry, J.M.; Riquet, A.M. A novel process to develop modified polymeric surfaces for the analysis of bacterial adhesion: Surface properties and adhesion test. J. Appl. Polym. Sci. 2008, 109, 1746–1756. [Google Scholar] [CrossRef]
- Chmielewski, A.; Al-Sheikhly, M.; Berejka, A.; Cleland, M.; Antoniak, M. Recent developments in the application of electron accelerators for polymer processing. Radiat. Phys. Chem. 2014, 94, 147–150. [Google Scholar] [CrossRef]
- Nasef, M.M.; Güven, O. Radiation-grafted copolymers for separation and purification purposes: Status, challenges and future directions. Prog. Polym. Sci. 2012, 37, 1597–1656. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gürsel, S.A.; Karabelli, D.; Güven, O. Radiation-grafted materials for energy conversion and energy storage applications. Prog. Polym. Sci. 2016, 63, 1–41. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, K.; Griesser, S.S.; Griesser, H. Antibacterial Surfaces and Coatings Produced by Plasma Techniques. Plasma Process. Polym. 2011, 8, 1010–1023. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.; Crawford, R.; Ivanova, E.P. Plasma-assisted surface modification of organic biopolymers to prevent bacterial attachment. Acta Biomater. 2011, 7, 2015–2028. [Google Scholar] [CrossRef]
- Denes, F.S.; Manolache, S. Macromolecular plasma-chemistry: An emerging field of polymer science. Prog. Polym. Sci. 2004, 29, 815–885. [Google Scholar] [CrossRef]
- Kale, K.H. Atmospheric pressure plasma treatment of textiles using non-polymerising gases. Indian J. Fibre Text. Res. 2011, 36, 289–299. [Google Scholar]
- Simionescu, C.I.; Denes, F.; Macoveanu, M.M.; Negulescu, I. Surface modification and grafting of natural and synthetic fibres and fabrics under cold plasma conditions. Die Makromol. Chem. 1984, 8, 17–36. [Google Scholar] [CrossRef]
- Anjum, S.; Singh, S.; Benedicte, L.; Roger, P.; Panigrahi, M.; Gupta, B. Biomodification Strategies for the Development of Antimicrobial Urinary Catheters: Overview and Advances. Glob. Chall. 2018, 2, 1700068. [Google Scholar] [CrossRef]
- Kumar, V.; Bhardwaj, Y.; Rawat, K.; Sabharwal, S. Radiation-induced grafting of vinylbenzyltrimethylammonium chloride (VBT) onto cotton fabric and study of its anti-bacterial activities. Radiat. Phys. Chem. 2005, 73, 175–182. [Google Scholar] [CrossRef]
- Teper, P.; Chojniak-Gronek, J.; Hercog, A.; Oleszko-Torbus, N.; Płaza, G.; Kubacki, J.; Balin, K.; Kowalczuk, A.; Mendrek, B. Nanolayers of Poly (N, N′-Dimethylaminoethyl Methacrylate) with a Star Topology and Their Antibacterial Activity. Polymers 2020, 12, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Rojas, G.G.; Lopez-Saucedo, F.; Vázquez, E.; Hernández-Mecinas, E.; Huerta, L.; Cedillo, G.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Synthesis of polyamide-6@cellulose microfilms grafted with N-vinylcaprolactam using gamma-rays and loading of antimicrobial drugs. Cellulose 2020, 27, 2785–2801. [Google Scholar] [CrossRef]
- Singh, H.; Tyagi, P. Radiation induced grafting of methacrylic acid onto silk for the immobilization of antimicrobial drug for sustained delivery. Die Angewandte Makromolekulare Chemie: Appl. Macromol. Chem. Phys. 1989, 172, 87–102. [Google Scholar] [CrossRef]
- Huang, C.; Wang, H.; Xu, Y.H. Functional Finishing on Silk Fabric with Acrylamide Monomer and Chitosan. Adv. Mater. Res. 2011, 175–176, 696–702. [Google Scholar] [CrossRef]
- Aoki, S.; Fujiwara, K.; Sugo, T.; Suzuki, K. Antimicrobial fabric adsorbed iodine produced by radiation-induced graft polymerization. Radiat. Phys. Chem. 2013, 84, 242–245. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Burillo, G.; Magariños, B.; Concheiro, A.; Bucio, E. Radiation-grafting of N-vinylimidazole onto silicone rubber forantimicrobial properties. Radiat. Phys. Chem. 2015, 110, 59–66. [Google Scholar] [CrossRef]
- Nava-Ortiz, C.A.; Burillo, G.; Concheiro, A.; Bucio, E.; Matthijs, N.; Nelis, H.; Coenye, T.; Alvarez-Lorenzo, C. Cyclodextrin-functionalized biomaterials loaded with miconazole prevent Candida albicans biofilm formation in vitro. Acta Biomater. 2010, 6, 1398–1404. [Google Scholar] [CrossRef]
- Maitz, M. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res. Lett. 2015, 10, 501. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, M.; De, S. Antibacterial polymeric membranes: A short review. Environ. Sci. Water Res. Technol. 2018, 4, 1078–1104. [Google Scholar] [CrossRef]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chen, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, K. Nanoengineered antibacterial coatings and materials: A perspective. Coatings 2019, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Yañez-Macías, R.; Muñoz-Bonilla, A.; De Jesús-Tellez, M.A.; Maldonado-Textle, H.; Guerrero-Sánchez, C.; Schubert, U.S.; Guerrero-Santos, R. Combinations of Antimicrobial Polymers with Nanomaterials and Bioactives to Improve Biocidal Therapies. Polymers 2019, 11, 1789. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Park, H.; Seo, S.W. In situ synthesis of silver nanoparticles on the surface of PDMS with high antibacterial activity and biosafety toward an implantable medical device. Nano Converg. 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.; Dhiman, N.K. Development of nano-antimicrobial biomaterials for biomedical applications. In Advances in Biomaterials for Biomedical Applications; Springer: Singapore, 2017; Volume 66, pp. 479–545. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [Green Version]
- Ping, X.; Wang, M.; Xuewu, G. Surface modification of poly(ethylene terephthalate) (PET) film by gamma-ray induced grafting of poly(acrylic acid) and its application in antibacterial hybrid film. Radiat. Phys. Chem. 2011, 80, 567–572. [Google Scholar] [CrossRef]
- Saxena, S.; Ray, A.R.; Kapil, A.; Pavon-Djavid, G.; Letourneur, D.; Gupta, B.; Meddahi-Pellé, A. Development of a new polypropylene-based suture: Plasma grafting, surface treatment, characterization, and biocompatibility studies. Macromol. Biosci. 2011, 11, 373–382. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Flores-Rojas, G.G.; López-Saucedo, J.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Antimicrobial silver-loaded polypropylene sutures modified by radiation-grafting. Eur. Polym. J. 2018, 100, 290–297. [Google Scholar] [CrossRef]
- Hosny, A.E.-D.M.; Farrag, H.A.; Helmy, O.M.; Hagras, S.A.A.; Ali, A.E.-H. In-vitro evaluation of antibacterial and antibiofilm efficiency of radiation-modified polyurethane–ZnO nanocomposite to be used as a self-disinfecting catheter. J. Radiat. Res. Appl. Sci. 2020, 13, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Rigo, S.; Cai, C.; Gunkel-Grabole, G.; Maurizi, L.; Zhang, X.; Xu, J.; Palivan, C.G. Nanoscience-Based Strategies to Engineer Antimicrobial Surfaces. Adv. Sci. 2018, 5, 1700892. [Google Scholar] [CrossRef]
- Seino, S.; Imoto, Y.; Kitagawa, D.; Kubo, Y.; Kosaka, T.; Kojima, T.; Nitani, H.; Nakagawa, T.; Yamamoto, T.A. Radiochemical synthesis of silver nanoparticles onto textile fabrics and their antibacterial activity. J. Nucl. Sci. Technol. 2015, 53, 1–7. [Google Scholar] [CrossRef]
- Chang, S.; Kang, B.; Dai, Y.; Chen, D. Synthesis of antimicrobial silver nanoparticles on silk fibers via γ-radiation. J. Appl. Polym. Sci. 2009, 112, 2511–2515. [Google Scholar] [CrossRef]
- El Sayed, S.S.; El-Naggar, A.A.; Ibrahim, S.M. Gamma irradiation induced surface modification of silk fabrics for antibacterial application. J. Part. Sci. Technol. 2017, 3, 71–77. [Google Scholar]
- Flores-Rojas, G.; López-Saucedo, F.; Bucio, E. Gamma-irradiation applied in the synthesis of metallic and organic nanoparticles: A short review. Radiat. Phys. Chem. 2020, 169, 107962. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chiang, C.-L. Preparation of cotton fibers with antibacterial silver nanoparticles. Mater. Lett. 2008, 62, 3607–3609. [Google Scholar] [CrossRef]
- Hassan, M.S.; Ibrahim, H.M.M. Characterization and antimicrobial properties of metal complexes of polypropylene fibers grafted with acrylic acid using gamma irradiation. Polym. Adv. Technol. 2015, 27, 532–541. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.H.; Nho, Y.C.; Kwon, O.H. Antibacterial activities of acrylic acid-grafted polypropylene fabric and its metallic salt. J. Appl. Polym. Sci. 1998, 69, 2213–2220. [Google Scholar] [CrossRef]
- Yang, J.M.; Lin, H.T.; Wu, T.H.; Chen, C.-C. Wettability and antibacterial assessment of chitosan containing radiation-induced graft nonwoven fabric of polypropylene-g-acrylic acid. J. Appl. Polym. Sci. 2003, 90, 1331–1336. [Google Scholar] [CrossRef]
- He, C.; Gu, Z. Studies on acrylic acid-grafted polyester fabrics by electron beam preirradiation method. I. Effects of process parameters on graft ratio and characterization of grafting products. J. Appl. Polym. Sci. 2003, 89, 3931–3938. [Google Scholar] [CrossRef]
- Zhao, J.; Song, L.; Shi, Q.; Luan, S.; Yin, J. Antibacterial and Hemocompatibility Switchable Polypropylene Nonwoven Fabric Membrane Surface. ACS Appl. Mater. Interfaces 2013, 5, 5260–5268. [Google Scholar] [CrossRef] [PubMed]
- Mazloumpour, M.; Malshe, P.; El-Shafei, A.; Hauser, P. Conferring durable antimicrobial properties on nonwoven polypropylene via plasma-assisted graft polymerization of DADMAC. Surf. Coat. Technol. 2013, 224, 1–7. [Google Scholar] [CrossRef]
- Villegas, K.A.M.; Ramírez-Jiménez, A.; Licea-Claverie, Á.; Pérez-Sicairos, S.; Bucio, E.; Bernáldez-Sarabia, J.; Licea-Navarro, A.F. Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods. Materials 2019, 12, 3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, F.; Huang, C.; Jiang, X.; He, W.; Gao, X.; Ma, L.; Ao, J.; Xu, L.; Wang, Z.; Li, Q.; et al. Reusable fibrous adsorbent prepared via Co-radiation induced graft polymerization for iodine adsorption. Ecotoxicol. Environ. Saf. 2020, 203, 111021. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazaka, K.; Jacob, M.V.; Chrzanowski, W.; Ostrikov, K. Anti-bacterial surfaces: Natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015, 5, 48739–48759. [Google Scholar] [CrossRef] [Green Version]
- McKeen, L.W. Plastics Used in Medical Devices. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier/William Andrew: Oxford, UK, 2014; pp. 21–53. [Google Scholar] [CrossRef]
- Costoya, A.; Becerra, L.E.V.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Mayer, C.; Otero, A.; Concheiro, A.; Bucio, E.; Alvarez-Lorenzo, C. Immobilization of antimicrobial and anti-quorum sensing enzymes onto GMA-grafted poly (vinyl chloride) catheters. Int. J. Pharm. 2019, 558, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Magaña, H.; Becerra, C.D.; Serrano-Medina, A.; Palomino, K.; Palomino-Vizcaíno, G.; Olivas-Sarabia, A.; Bucio, E.; Cornejo-Bravo, J.M. Radiation Grafting of a Polymeric Prodrug onto Silicone Rubber for Potential Medical/Surgical Procedures. Polymers 2020, 12, 1297. [Google Scholar] [CrossRef]
- Zuñiga-Zamorano, I.; Meléndez-Ortiz, H.I.; Costoya, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Poly (vinyl chloride) catheters modified with pH-responsive poly (methacrylic acid) with affinity for antimicrobial agents. Radiat. Phys. Chem. 2018, 142, 107–114. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Concheiro, A.; Jiménez-Páez, V.M.; Bucio, E. Modification of medical grade PVC with N-vinylimidazole to obtain bactericidal surface. Radiat. Phys. Chem. 2016, 119, 37–43. [Google Scholar] [CrossRef]
- Valencia-Mora, R.A.; Zavala-Lagunes, E.; Bucio, E. Grafting of thermo-sensitive N-vinylcaprolactam onto silicone rubber through the direct radiation method. Radiat. Phys. Chem. 2016, 124, 155–158. [Google Scholar] [CrossRef]
- Tajirian, A.L.; Goldberg, D.J. A review of sutures and other skin closure materials. J. Cosmet. Laser Ther. 2010, 12, 296–302. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Anjum, S.; Kumari, S.; Gupta, B. Antimicrobial Surgical Sutures: Recent Developments and Strategies. Polym. Rev. 2016, 56, 607–630. [Google Scholar] [CrossRef]
- Arora, A.; Aggarwal, G.; Chander, J.; Maman, P.; Nagpal, M. Drug eluting sutures: A recent update. J. Appl. Pharm. Sci. 2019, 9, 111–123. [Google Scholar]
- Anjum, S.; Gupta, A.; Sharma, D.; Kumari, S.; Sahariah, P.; Bora, J.; Bhan, S.; Gupta, B. Antimicrobial nature and healing behavior of plasma functionalized polyester sutures. J. Bioact. Compat. Polym. 2017, 32, 263–279. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gupta, B. Radiation-induced graft copolymerization of methacrylic acid onto polypropylene fibers. I. Effect of synthesis conditions. J. Appl. Polym. Sci. 1985, 30, 2643–2653. [Google Scholar] [CrossRef]
- Tyagi, P.K.; Gupta, B.; Singh, H. Radiation-Induced Grafting of 2-Hydroxyethyl Methacrylate onto Polypropylene for Biomedical Applications. II. Evaluation as Antimicrobial Suture. J. Macromol. Sci. Part A 1993, 30, 303–313. [Google Scholar] [CrossRef]
- Plessier, C.; Gupta, B.; Chapiro, A. Modification of polypropylene fiber by radiation-induced graft copolymerization of acrylonitrile monomer. J. Appl. Polym. Sci. 1998, 69, 1343–1348. [Google Scholar] [CrossRef]
- Gupta, B.; Anjum, N.; Gulrez, S.K.H.; Singh, H. Development of antimicrobial polypropylene sutures by graft copolymerization. II. Evaluation of physical properties, drug release, and antimicrobial activity. J. Appl. Polym. Sci. 2006, 103, 3534–3538. [Google Scholar] [CrossRef]
- Yuan, F.; Wei, J.; Tang, E.-Q.; Zhao, K.-Y.; Xue, Y. Synthesis and Modification of Polypropylene by Radiation-induced Grafting. Int. J. Chem. 2009, 1, 75. [Google Scholar] [CrossRef] [Green Version]
- García-Vargas, M.; González-Chomón, C.; Magariños, B.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Acrylic polymer-grafted polypropylene sutures for covalent immobilization or reversible adsorption of vancomycin. Int. J. Pharm. 2014, 461, 286–295. [Google Scholar] [CrossRef] [PubMed]
- López-Saucedo, F.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-grafting of vinyl monomers separately onto polypropylene monofilament sutures. Radiat. Phys. Chem. 2017, 132, 1–7. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Flores-Rojas, G.G.; Bucio, E.; Alvarez-Lorenzo, C.; Concheiro, A.; González-Antonio, O. Achieving antimicrobial activity through poly(N-methylvinylimidazolium) iodide brushes on binary-grafted polypropylene suture threads. MRS Commun. 2017, 7, 938–946. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, B. Designing and nanofunctionalization of infection-resistant polyester suture. In Advance in Polymer Science and Technology; Gupta, B., Ghosh, A.K., Suzuki, A., Rattan, S., Eds.; Springer: Singapore, 2018; pp. 1–12. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Kumari, S.; Gupta, B. Preparation and biological characterization of plasma functionalized poly(ethylene terephthalate) antimicrobial sutures. Int. J. Polym. Mater. 2019, 69, 1034–1042. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog. Biomater. 2014, 3, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.; Agarwal, R.; Alam, M. Textile-based smart wound dressings. Indian J. Fibre Text. Res. 2010, 35, 174–187. [Google Scholar]
- Boateng, J.; Matthews, K.; Stevens, H.N.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Chen, X.; Yu, F.; Zhu, Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr. Polym. 2008, 73, 401–408. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Biomedical evaluation of polyvinyl alcohol–gelatin esterified hydrogel for wound dressing. J. Mater. Sci. Mater. Electron. 2007, 18, 1889–1894. [Google Scholar] [CrossRef]
- Don, T.-M.; King, C.-F.; Chiu, W.-Y.; Peng, C.-A. Preparation and characterization of chitosan-g-poly(vinyl alcohol)/poly(vinyl alcohol) blends used for the evaluation of blood-contacting compatibility. Carbohydr. Polym. 2006, 63, 331–339. [Google Scholar] [CrossRef]
- Yang, X.; Yang, K.; Wu, S.; Chen, X.; Yu, F.; Li, J.; Ma, M.; Zhu, Z. Cytotoxicity and wound healing properties of PVA/ws-chitosan/glycerol hydrogels made by irradiation followed by freeze–thawing. Radiat. Phys. Chem. 2010, 79, 606–611. [Google Scholar] [CrossRef]
- Sung, J.H.; Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int. J. Pharm. 2010, 392, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mitomo, H.; Zhai, M.; Yoshii, F.; Nagasawa, N.; Kume, T. Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohydr. Polym. 2003, 53, 439–446. [Google Scholar] [CrossRef]
- Wu, M.; Bao, B.; Yoshii, F.; Makuuchi, K. Irradiation of Crosslinked, Poly(Vinyl Alcohol) Blended Hydrogel for Wound Dressing. J. Radioanal. Nucl. Chem. 2001, 250, 391–395. [Google Scholar] [CrossRef]
- Taleb, M.A.; Ismail, S.A.; El-Kelesh, N.A. Radiation Synthesis and Characterization of Polyvinyl Alcohol/Methacrylic Acid–Gelatin Hydrogel for Vitro Drug Delivery. J. Macromol. Sci. Part A 2008, 46, 170–178. [Google Scholar] [CrossRef]
- Kaur, I.; Bhati, P.; Sharma, S. Radiation induced synthesis of (gelatin-co-PVA)-g-poly (AAc) copolymer as wound dressing material. Adv. Mater. Res. 2014, 3, 183–197. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, K.S.; Wu, T.H.; Len, C.H.; Tsai, Z.T.; Lin, B. Preparation of Easily Stripped off Temporary Wound Dressing Materials by Radiation Grafting. US Patent 6022330A, 8 February 2000. [Google Scholar]
- Wu, T.H.; Yang, J.M.; Fu, Y.K.; Lin, H.T.; Chen, C.C. Chitosan Based Dressing. US Patent 20,060,292,207A1, 28 December 2006. [Google Scholar]
- Ikram, S.; Kumari, M.; Gupta, B. Thermosensitive membranes by radiation-induced graft polymerization of N-isopropyl acrylamide/acrylic acid on polypropylene nonwoven fabric. Radiat. Phys. Chem. 2011, 80, 50–56. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Turon, P.; Weis, C.; Alemán, C.; Armelin, E. The mechanism of adhesion and graft polymerization of a PNIPAAm thermoresponsive hydrogel to polypropylene meshes. Soft Matter 2019, 15, 3432–3442. [Google Scholar] [CrossRef]
- Small, M.; Faglie, A.; Craig, A.J.; Pieper, M.; Narcisse, V.E.F.; Neuenschwander, P.F.; Chou, S.-F. Nanostructure-Enabled and Macromolecule-Grafted Surfaces for Biomedical Applications. Micromachines 2018, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Mndlovu, H.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Bioplatform Fabrication Approaches Affecting Chitosan-Based Interpolymer Complex Properties and Performance as Wound Dressings. Molecules 2020, 25, 222. [Google Scholar] [CrossRef] [Green Version]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.J.; Liu, F. Imparting antifouling properties of silicone hydrogels by grafting poly(ethylene glycol) methyl ether acrylate initiated by UV light. J. Appl. Polym. Sci. 2011, 125, 548–554. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, F. Photoinduced graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on silicone hydrogels for reducing protein adsorption. J. Mater. Sci. Mater. Electron. 2011, 22, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Austin, B.C.; Walsh, P.R.; Murphy, N.E. Development of UV-curable, hydrophilic gel networks for soft contact lens manufacture. Soc. Plast. Eng. Eurotech. 2013, pp. 514–519. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=12e1592ba3c16ff30e71cd1f6a5d8391&site=xueshu_se&hitarticle=1 (accessed on 5 September 2021).
- Thissen, H.; Gengenbach, T.; du Toit, R.; Sweeney, D.; Kingshott, P.; Griesser, H.; Meagher, L. Clinical observations of biofouling on PEO coated silicone hydrogel contact lenses. Biomaterials 2010, 31, 5510–5519. [Google Scholar] [CrossRef]
- Dutta, D.; Kamphuis, B.; Ozcelik, B.; Thissen, H.; Pinarbasi, R.; Kumar, N.; Willcox, M.D.P. Development of Silicone Hydrogel Antimicrobial Contact Lenses with Mel4 Peptide Coating. Optom. Vis. Sci. 2018, 95, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Cole, N.; Kumar, N.; Willcox, M. Broad Spectrum Antimicrobial Activity of Melimine Covalently Bound to Contact Lenses. Investig. Opthalmology Vis. Sci. 2013, 54, 175–182. [Google Scholar] [CrossRef]
- Xiao, A.; Dhand, C.; Leung, C.M.; Beuerman, R.W.; Ramakrishna, S.; Lakshminarayanan, R. Strategies to design antimicrobial contact lenses and contact lens cases. J. Mater. Chem. B 2018, 6, 2171–2186. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Toffoli, A.; Ghiacci, G.; Macaluso, G.M. Tailoring the Interface of Biomaterials to Design Effective Scaffolds. J. Funct. Biomater. 2018, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Ma, J.; Bei, J.; Wang, S. Surface Modification of Aliphatic Polyester to Enhance Biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Sreearunothai, P.; Opaprakasit, P. Development and Characterization of Photoinduced Acrylamide-Grafted Polylactide Films for Biomedical Applications. Int. J. Polym. Sci. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nugroho, R.W.N.; Pettersson, T.; Odelius, K.; Höglund, A.; Albertsson, A.-C. Force Interactions of Nonagglomerating Polylactide Particles Obtained through Covalent Surface Grafting with Hydrophilic Polymers. Langmuir 2013, 29, 8873–8881. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Shen, J. Surface modification of polycaprolactone with poly(methacrylic acid) and gelatin covalent immobilization for promoting its cytocompatibility. Biomaterials 2002, 23, 4889–4895. [Google Scholar] [CrossRef]
- Luk, J.Z.; Cooper-White, J.; Rintoul, L.; Taran, E.; Grøndahl, L. Functionalised polycaprolactone films and 3D scaffolds via gamma irradiation-induced grafting. J. Mater. Chem. B 2013, 1, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-Q.; Lu, H.-J.; Leach, M.K.; Huang, N.-P.; Wang, Y.-C.; Liu, C.-J.; Gu, Z.-Z. The influence of type-I collagen-coated PLLA aligned nanofibers on growth of blood outgrowth endothelial cells. Biomed. Mater. 2010, 5, 065011. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-M.; Choi, D.-Y.; Jung, S.-C.; Kim, B.-H. Characteristics of Plasma Treated Electrospun Polycaprolactone (PCL) Nanofiber Scaffold for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2015, 15, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chemie. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Two-Dimensional Manipulation of Cardiac Myocyte Sheets Utilizing Temperature-Responsive Culture Dishes Augments the Pulsatile Amplitude. Tissue Eng. 2001, 7, 141–151. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Okano, T. Recent development of temperature-responsive surfaces and their application for cell sheet engineering. Regen. Biomater. 2014, 1, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Yamato, M.; Akiyama, Y.; Kobayashi, J.; Yang, J.; Kikuchi, A.; Okano, T. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog. Polym. Sci. 2007, 32, 1123–1133. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
- Ito, Y.; Chen, G.; Guan, A.Y.; Imanishi, Y. Patterned Immobilization of Thermoresponsive Polymer. Langmuir 1997, 13, 2756–2759. [Google Scholar] [CrossRef]
- Chen, G.; Ito, Y.; Imanishi, Y. Regulation of growth and adhesion of cultured cells by insulin conjugated with thermoresponsive polymers. Biotechnol. Bioeng. 1997, 53, 339–344. [Google Scholar] [CrossRef]
- Von Recum, H.A.; Kim, S.W.; Kikuchi, A.; Okuhara, M.; Sakurai, Y.; Okano, T. Novel thermally reversible hydrogel as detachable cell culture substrate. J. Biomed. Mater. Res. 1998, 40, 631–639. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin Poly(N-isopropylacrylamide) Grafted Layer on Polystyrene Surfaces for Cell Adhesion/Detachment Control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef]
- Kumar, P.A.; Sreenivasan, K.; Kumary, T. Alternate method for grafting thermoresponsive polymer for transferring in vitro cell sheet structures. J. Appl. Polym. Sci. 2007, 105, 2245–2251. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Yamato, M.; Kobayashi, J.; Sakai, K.; Okano, T. Temperature-responsive glass coverslips with an ultrathin poly(N-isopropylacrylamide) layer. Acta Biomater. 2009, 5, 470–476. [Google Scholar] [CrossRef]
- Galperin, A.; Long, T.J.; Ratner, B.D. Degradable, Thermo-Sensitive Poly(N-isopropyl acrylamide)-Based Scaffolds with Controlled Porosity for Tissue Engineering Applications. Biomacromolecules 2010, 11, 2583–2592. [Google Scholar] [CrossRef] [Green Version]
- Nash, M.E.; Carroll, W.M.; Foley, P.J.; Maguire, G.; Connell, C.O.; Gorelov, A.V.; Beloshapkin, S.; Rochev, Y.A. Ultra-thin spin coated crosslinkable hydrogels for use in cell sheet recovery—Synthesis, characterisation to application. Soft Matter 2012, 8, 3889–3899. [Google Scholar] [CrossRef]
- Akiyama, Y.; Yamato, M.; Okano, T. Preparation of Poly(N-isopropylacrylamide) Grafted Polydimethylsiloxane by Using Electron Beam Irradiation. J. Robot. Mechatron. 2013, 25, 631–636. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Yamato, M.; Okano, T. A Facile Method for Preparing Temperature-Responsive Cell Culture Surfaces by Using a Thioxanthone Photoinitiator Immobilized on a Polystyrene Surface. ChemNanoMat 2016, 2, 454–460. [Google Scholar] [CrossRef]
- Mizutani, A.; Kikuchi, A.; Yamato, M.; Kanazawa, H.; Okano, T. Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials 2008, 29, 2073–2081. [Google Scholar] [CrossRef]
- Tekin, H.; Sanchez, J.G.; Tsinman, T.; Langer, R.; Khademhosseini, A. Thermoresponsive platforms for tissue engineering and regenerative medicine. AIChE J. 2011, 57, 3249–3258. [Google Scholar] [CrossRef]
- Kobayashi, J.; Okano, T. Fabrication of a thermoresponsive cell culture dish: A key technology for cell sheet tissue engineering. Sci. Technol. Adv. Mater. 2010, 11, 014111. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Okano, T. Temperature-Responsive Polymer Modified Surface for Cell Sheet Engineering. Polymers 2012, 4, 1478–1498. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [Green Version]
- Alavi, S.; Thomas, S.; Sandeep, K.; Kalarikkal, N.; Varghese, J.; Yaragalla, S. Polymers for Packaging Applications; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Biji, K.; Ravishankar, C.; Mohan, C.; Gopal, T.S. Smart packaging systems for food applications: A Review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, N.; Gupta, B.; Riquet, A.M. Surface designing of polypropylene by critical monitoring of the grafting conditions: Structural investigations. J. Appl. Polym. Sci. 2006, 101, 772–778. [Google Scholar] [CrossRef]
- Riquet, A.-M.; Delattre, J.; Vitrac, O.; Guinault, A. Design of modified plastic surfaces for antimicrobial applications: Impact of ionizing radiation on the physical and mechanical properties of polypropylene. Radiat. Phys. Chem. 2013, 91, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Anjum, N.; Riquet, A.-M. Development and physicochemical characterization of modified polymeric surfaces for bacterial adhesion. J. Appl. Polym. Sci. 2010, 119, 1307–1315. [Google Scholar] [CrossRef]
- Riquet, A.M.; Rohman, G.; Guinault, A.; Demilly, M. Surface modification of polypropylene by radiation grafting of hydrophilic monomers: Physicochemical properties. Surf. Eng. 2011, 27, 234–241. [Google Scholar] [CrossRef]
- Roman, M.J.; Decker, E.A.; Goddard, J.M. Metal-Chelating Active Packaging Film Enhances Lysozyme Inhibition of Listeria monocytogenes. J. Food Prot. 2014, 77, 1153–1160. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Talbert, J.N.; Hernandez-Munoz, P.; Gavara, R.; Goddard, J.M. Covalent Immobilization of Lysozyme on Ethylene Vinyl Alcohol Films for Nonmigrating Antimicrobial Packaging Applications. J. Agric. Food Chem. 2013, 61, 6720–6727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukri, N.A.; Ghazali, Z.; Fatimah, N.A.; Mohamad, S.F.; Wahit, M.U. Physical, mechanical and oxygen barrier properties of antimicrobial active packaging based on ldpe film incorporated with sorbic acid. Adv. Environ. Biol. 2014, 2748–2753. [Google Scholar]
- Salmieri, S.; Khan, R.; Safrany, A.; Lacroix, M. Gamma rays-induced 2-hydroxyethyl methacrylate graft copolymerization on methylcellulose-based films: Structure analysis and physicochemical properties. Ind. Crop. Prod. 2015, 70, 64–71. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, H.-L.; Du, Z.-H.; Yang, B.; Wu, J.; Wang, R.; Zhang, X.-Q. Hydrophilic and Antibacterial Modification of Poly(lactic acid) Films by γ-ray Irradiation. ACS Omega 2019, 4, 21439–21445. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, H.; Chen, Z.; Chen, Y.; Guo, D.; Ni, M.; Liu, S.; Peng, C. In-situ formation of silver nanoparticles on poly (lactic acid) film by γ-radiation induced grafting of N-vinyl pyrrolidone. Mater. Sci. Eng. C 2016, 63, 142–149. [Google Scholar] [CrossRef]

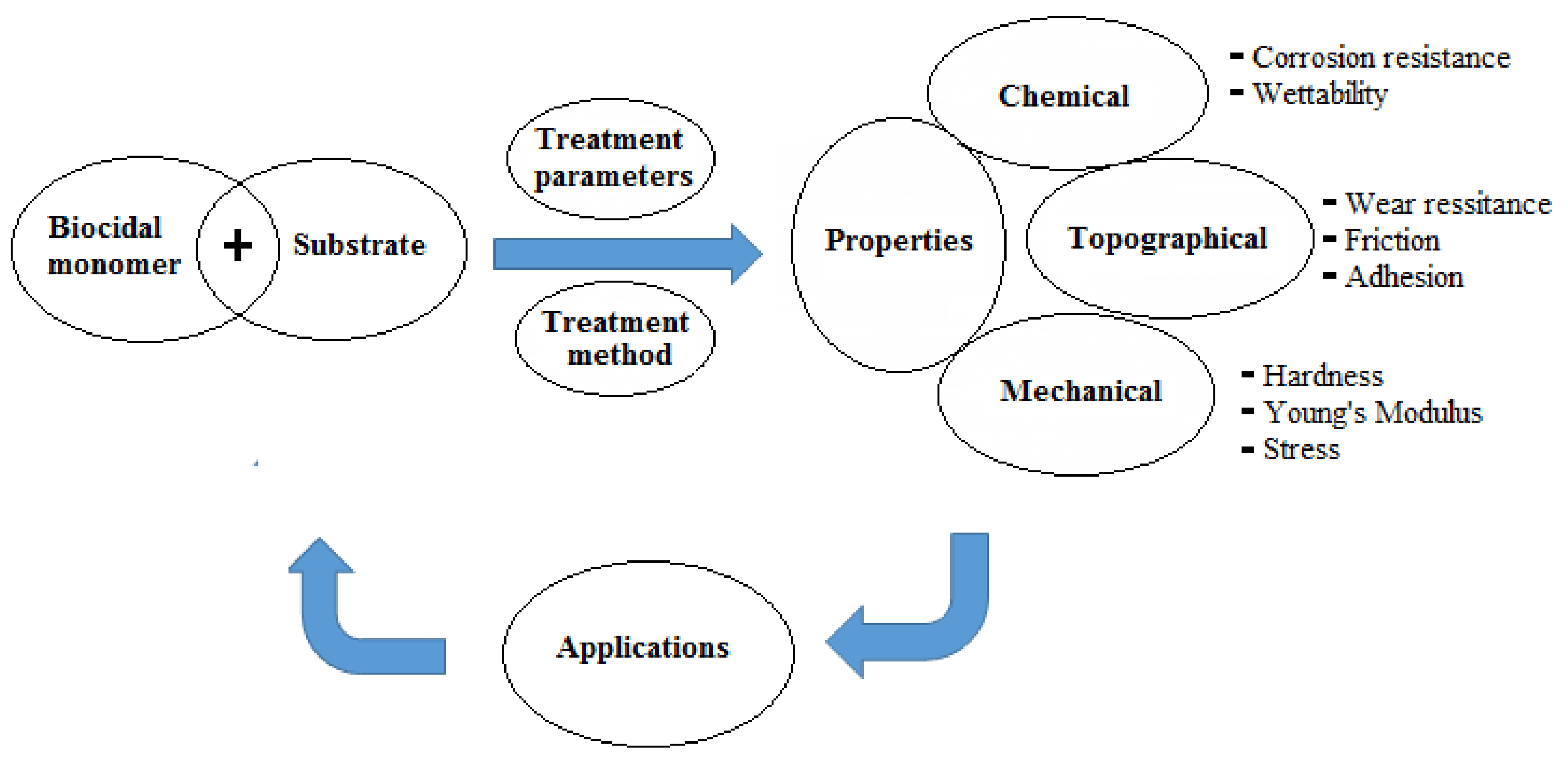
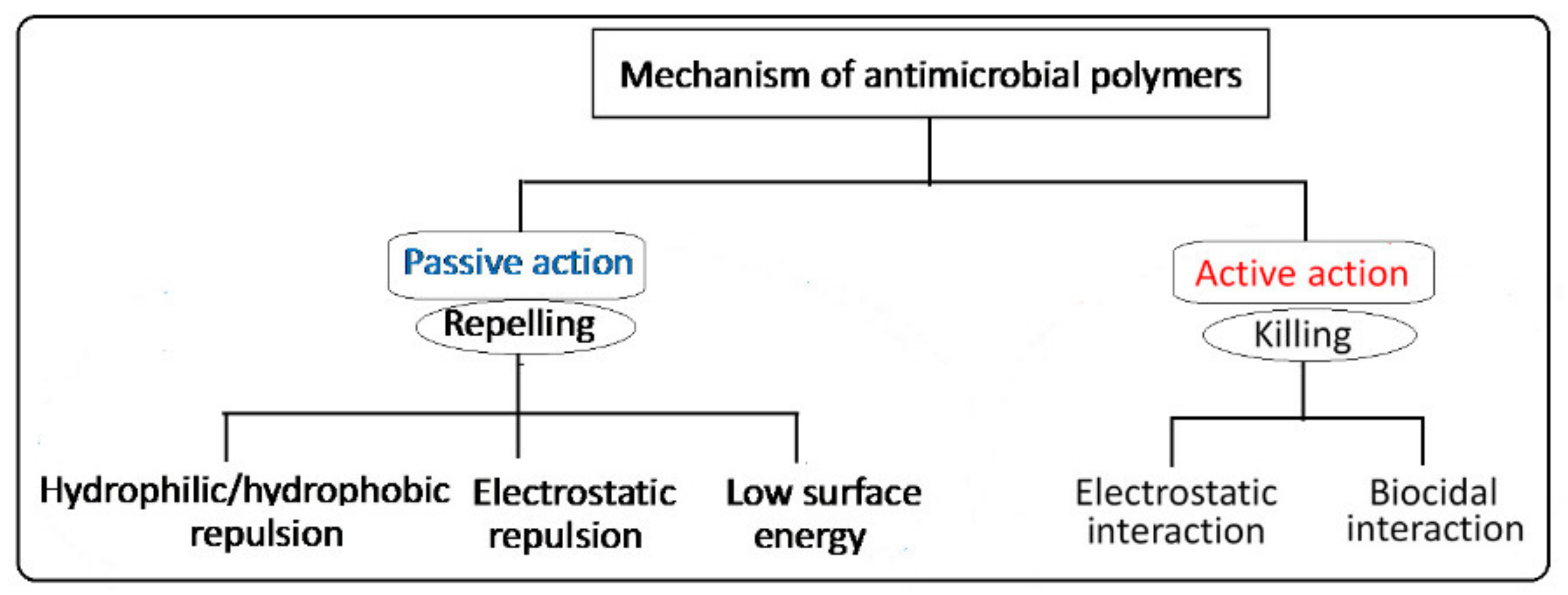


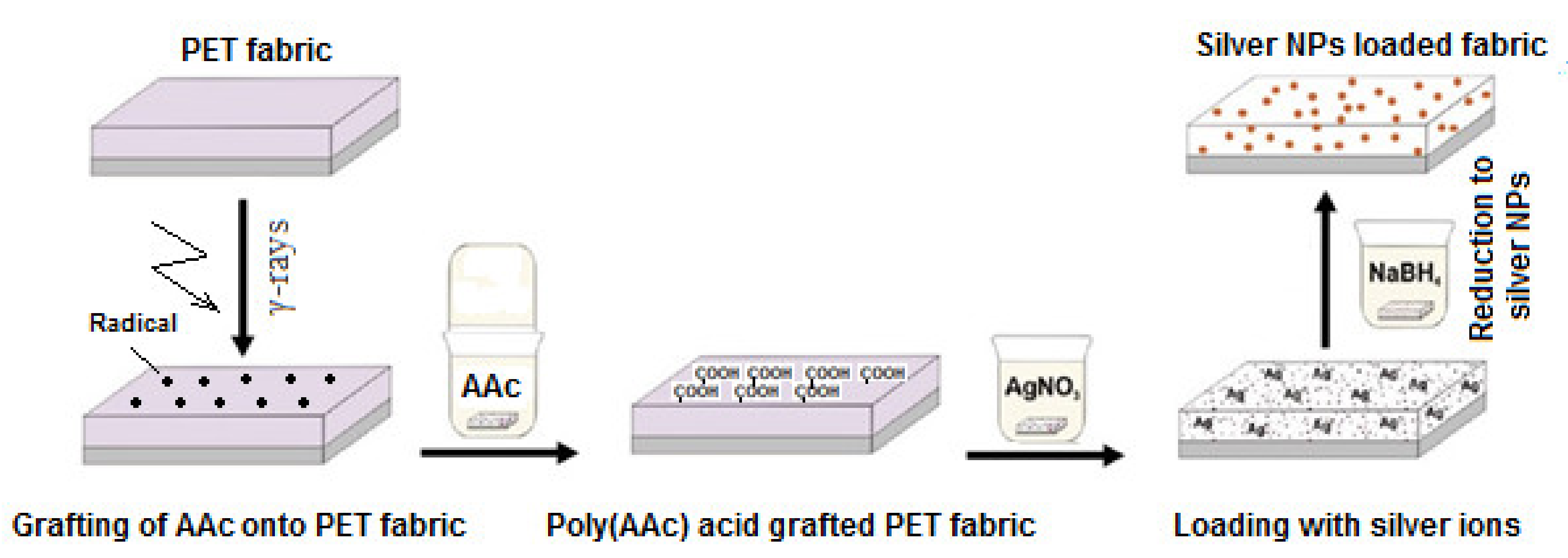


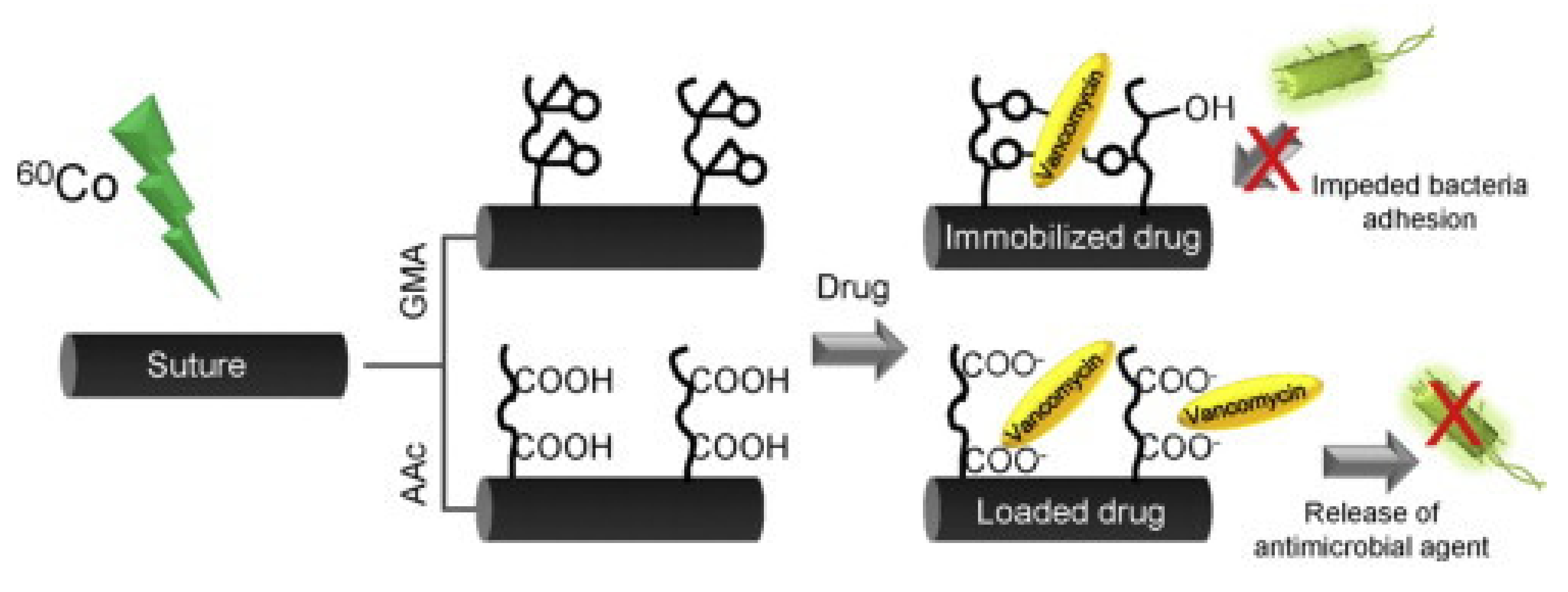


| Graft Copolymerization Methods | Treatment Source | Energetic Species Bombarding Surfaces | Advantages | Disadvantages | Remarks |
|---|---|---|---|---|---|
| EB-induced graft copolymerization | EB | Highly directionalelectrons, of variable energy | Simple and very fast. Allows surface and bulk grafting depending on acceleration energy. Leaves no detrimental residues. Can be initiated with EBs with wide range of energies. | High cost of infrastructure for irradiation Grafted materials are likely to sustain mechanical damage when high doses and dose rates are used | More convenience for practical applications and is more suitable for up-scaling and development of semi-continuous lines for industrial applications. |
| γ-Ray-induced graft copolymerization | Co-60 | Energetic photons | Simple but slower than EB, Allows bulk grafting depending on absorbed dose and dose rate. Widely applied and most suitable for simultaneous grafting in bulk solution. | Grafting takes longer time than EB. The Co-60 source continues to decay and thus the dose rate reduces steadily, requiring adjustment of reaction parameters | Green grafting reactions can be conducted in emulsion media to significantly reduce monomer consumption and absorbed dose and improve the process economy. |
| Photo-induced graft copolymerization | UV | Energetic photons of lower energy compared to those of γ-rays | Simple, inexpensive and can easily modify polymer surfaces. | It yields a low grafting level, which is confined to the surface, takes long treatment time and requires the use of a photo-initiator. Not suitable for large-scale applications. | More suitable for surface modification that can help improve wettability and resistance to bacterial colonization and biofilm formation. |
| Plasma-induced graft copolymerization | DC glow discharge plasma | Energetic species including atoms molecules ions, radicals, photons, electrons | Simple process without any pollution to modify the polymeric surfaces without altering their bulk properties, allowing functionalization with moieties for hosting biocides. | The range of chemical groups available for surface modification is limited, posing a challenge to effectiveness for deterring bacterial adhesion. Not suitable for large-scale applications. | More suitable for biomedical application and suitable for limited surface modification such as catheters and cannulas in addition to bio-medical coatings to various surfaces. |
| Substrate | Monomer | Method | Bioactive Agent | Impact | Refs |
|---|---|---|---|---|---|
| PP | AA | RIGC with γ-rays | Ag, Zn and Cu metal complexes | Anti-microbial activity against S. aureus and E. coli | [85] |
| cotton | GMA | RIGC with γ-rays | Ag nanoparticle loaded on iminodiacetic acid | Anti-microbial activity against E. coli | [83] |
| PP | AA | RIGC with γ-rays | Chelated Ag+ and Zn2+ions | Anti-microbial activity against S. aureus and E. coli | [84] |
| PP nonwoven fabric | AA | RIGC with γ-rays | Chitosan immobilized with1-ethyl-3-(3-dimethyamino propyl) carbidiimide | Anti-microbial activity against P. aeruginosa | [86] |
| PP nonwoven fabric | CABA-1-ester | Plasma and UV treatments | Poly(CABA-1-ester) | Anti-microbial activity against S. aureus | [88] |
| Spun-bond nonwoven PP | PDDA | Plasma treatment | Poly(diallyldimethylamm-onium chloride) | Anti-microbial activity against K. pneumoniae and S. aureus | [89] |
| PET fabric | HEMA or HEMA/EGDMA | RIGC with γ-rays and UV | In-situ AgNPs | Anti-microbial activity against S. aureus and E. coli | [90] |
| Cotton fabric | VBTC | RIGC with γ-rays (simultaneous irradiation) | Poly(VBTC) | Anti-microbial activity against S. aureus and E. coli | [55] |
| Cotton fabric | MAETC | RIGC with γ-rays (simultaneous irradiation) | Poly(MAETC) | Anti-microbial activity against following bacteria sequence: S. aureus < E. coli < B. cereus < P. fluorescens | [38] |
| PP sheets | VBTC/AA | RIGC with EB (pre-irradiation) | Poly(VBTC/AA) | Anti-microbial activity against Listeria monocytogenes | [44] |
| PE nonwoven fabric | NVP | RIGC with EB pre-irradiation method | Poly(NVP)/I2/KI | Anti-microbial activity against S. aureus, E. coli, P. aeruginosa | [60] |
| Monomer | Substrate | Method | Bioactive Agent | Impact | Other Functions | Refs |
|---|---|---|---|---|---|---|
| MAA | PVC | RIGC with pre-irradiation from γ-rays | Benzalkonium chloride and ciprofloxacin | Anti-microbial activity against E. coli, P. aeruginosa and S. aureus | pH stimuli responsive character | [98] |
| GMA | PVC | RIGC with pre-irradiation from γ-rays | lysozyme and acylase | Anti-fouling against S. aureus | anti-quorum sensing character | [96] |
| 1-VIm | PVC | RIGC with simultaneous irradiation from γ-rays | Methyl iodide | Anti-microbial activity against S. aureus | - | [99] |
| 1-VIm | Silicone rubber | RIGC with simultaneous irradiation from γ-rays | Poly(1-vinylimidazole) | Antimicrobial activity against P. aeruginosa | - | [61] |
| NVCL | Silicone rubber | RIGC with simultaneous irradiation from γ-rays | Poly(NVCL)/lysozyme | Antimicrobial activity | Thermo stimuli responsive character leading to release of lysozyme | [100] |
| Monomer | Substrate | Method | Bioactive Agent | Impact | Refs |
|---|---|---|---|---|---|
| MAA | PP fibers | RIGC with γ-rays with simultaneous irradiation method | - | - | [105] |
| MAA | Black braided silk and pure mulberry silk twisted yarn | RIGC with γ-rays with simultaneous irradiation method | 8-Hydroxy quinoline hydrochloride | Antimicrobial activity against various Gram-positive and Gram-negative bacteria | [58] |
| HEMA | PP monofilament | RIGC with with simultaneous irradiation from γ-rays | 8-Hydroxy quinoline | Antimicrobial activity against S. aureus | [106] |
| AN | PP fibers | RIGC pre-irradiation method using γ-rays | - | - | [107] |
| VIm | PP monofilament | RIGC with simultaneous irradiation from γ-rays | Ciprofloxacin | Excellent antimicrobial activity against both E. coli | [108] |
| LA and BA | PP fibre | RIGC with simultaneous irradiation from γ-rays | - | - | [109] |
| GMA or AAc | PP monofilament | RIGC pre-irradiation method using γ-rays | Vancomycin | Antimicrobial activity against S. aureus | [110] |
| HEMA or NIPAAm followed by VIm | PP monofilament | RIGC with γ-rays using pre-irradiation method | Methyl iodide | Antimicrobial activity against S. aureus | [112] |
| - | PET sutures | Plasma treatment with CO2 | In-situ immobilized silver nanoparticle | Excellent antimicrobial activity against both E. coli and S. aureus | [104] |
| - | PET sutures | Plasma with CO2 | Triclosan | Excellent antimicrobial activity against both E. coli and S. aureus | [113] |
| - | PET sutures | Plasma with CO2 | In-situ immobilized silver nanoparticle with Alvera | Excellent antimicrobial activity against both E. coli and S. aureus | [114] |
| Monomer | Substrate | Method | Bioactive Agent | Graft Form | Refs |
|---|---|---|---|---|---|
| NIPAAm | Polystyrene Dish | EB irradiation | PNIPAAm | Thin gel on substrate allowing successive subculturing of bovine hepatocytes | [153] |
| NIPAAm/AA | Polystyrene film | UV irradiation | P(NIPAAm/AA)/azidoaniline | Hydrophilic/antimicrobial/thermo-response surface | [160] |
| NIPAAm/AA | Polystyrene film | Glow-discharged | P(NIPAAm/AA)/insulin | Hydrophilic/antimicrobial/thermo-response surface conjugated with insulin | [161] |
| P(NIPAAm-co-CCMS) | Tissue culture polystyrene surfaces | UV-irradiation-induced crosslinking | P(NIPAAm-co-CCMS)/bovine endothelium or human retinal pigmented epithelium | Hydrophilic/hydrophobic antimicrobial/thermo-response surface dish | [162] |
| NIPAAm | Polystyrene dish | RIGC with EB irradiation | PNIPAAm | Thin gel on substrate allowing control of hydrophilic/hydrophobic property alterations and cell adhesion/detachment behavior | [163] |
| NIPAAm | Substrate | RIGC with γ-rays | PNIPAAm | Thin gel on substrate allowing control of hydrophilic/hydrophobic property alterations and cell adhesion/detachment behavior | [164] |
| NIPAAm | Glass coverslips | RIGC with EB irradiation | PNIPAAm | Thin gel on substrate allowing control of hydrophilicity and allowing temperature-dependent cell adhesion/detachment when the grafted density was around 2mg/cm2 | [165] |
| NIPAAm with MDO CLDMA | - | Coplymerization with UV | P(NIPAAm/MDO/CLDMA) | Hydrogel scaffolds with controllable pore size, fully degradable and thermo-responsive properties | [166] |
| PNIPAAm solution | Thin film of P(NIPAAm) | Irradiation-induced crosslinking | - | - | [167] |
| NIPAAm | PDMS | Initial activation with silanol groups with conventional O2 plasma or hydrochloric acid (HCl) followed by RIGC of NIPPAmwith EB irradiation | PNIPAAm/PDMS | Gel on substrate with temperature-responsive cell culture surface | [168] |
| NIPAAm | Polystyrene dish | UV with photo-initiator | PNIPAAm | Hydrophilic/thermo-response surface dish | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasef, M.M.; Gupta, B.; Shameli, K.; Verma, C.; Ali, R.R.; Ting, T.M. Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications. Polymers 2021, 13, 3102. https://doi.org/10.3390/polym13183102
Nasef MM, Gupta B, Shameli K, Verma C, Ali RR, Ting TM. Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications. Polymers. 2021; 13(18):3102. https://doi.org/10.3390/polym13183102
Chicago/Turabian StyleNasef, Mohamed Mahmoud, Bhuvanesh Gupta, Kamyar Shameli, Chetna Verma, Roshafima Rasit Ali, and Teo Ming Ting. 2021. "Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications" Polymers 13, no. 18: 3102. https://doi.org/10.3390/polym13183102








