The Development of Light-Curable Calcium-Silicate-Containing Composites Used in Odontogenic Regeneration
Abstract
:1. Introduction
2. Materials and Methods
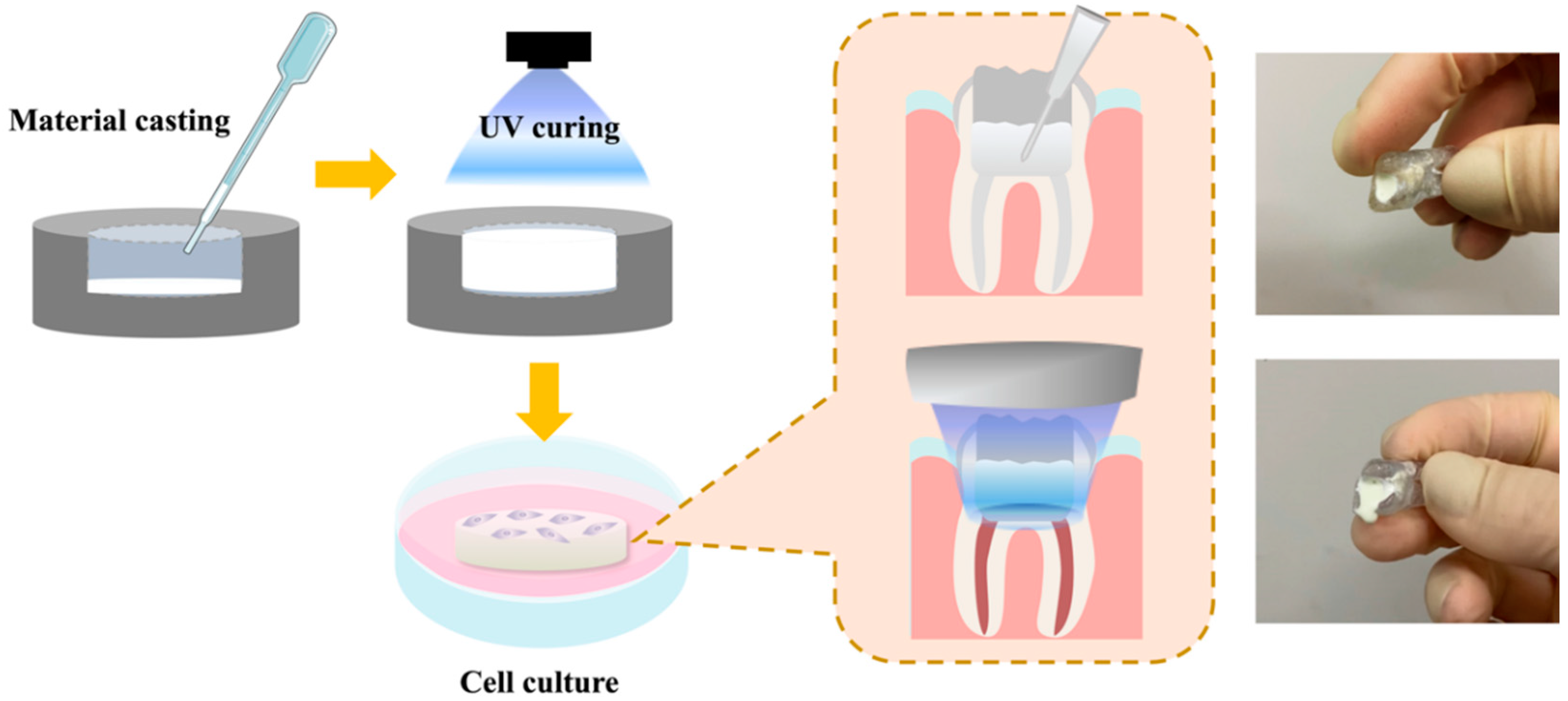
2.1. Preparation of the Light-Curable CS Specimens
2.2. Physicochemical Properties
2.3. Swelling Analysis
2.4. In Vitro Immersion Test
2.5. Cell Viability and Morphology
2.6. Odontogenesis Differentiation Assay
2.7. Data Analysis
3. Results and Discussion
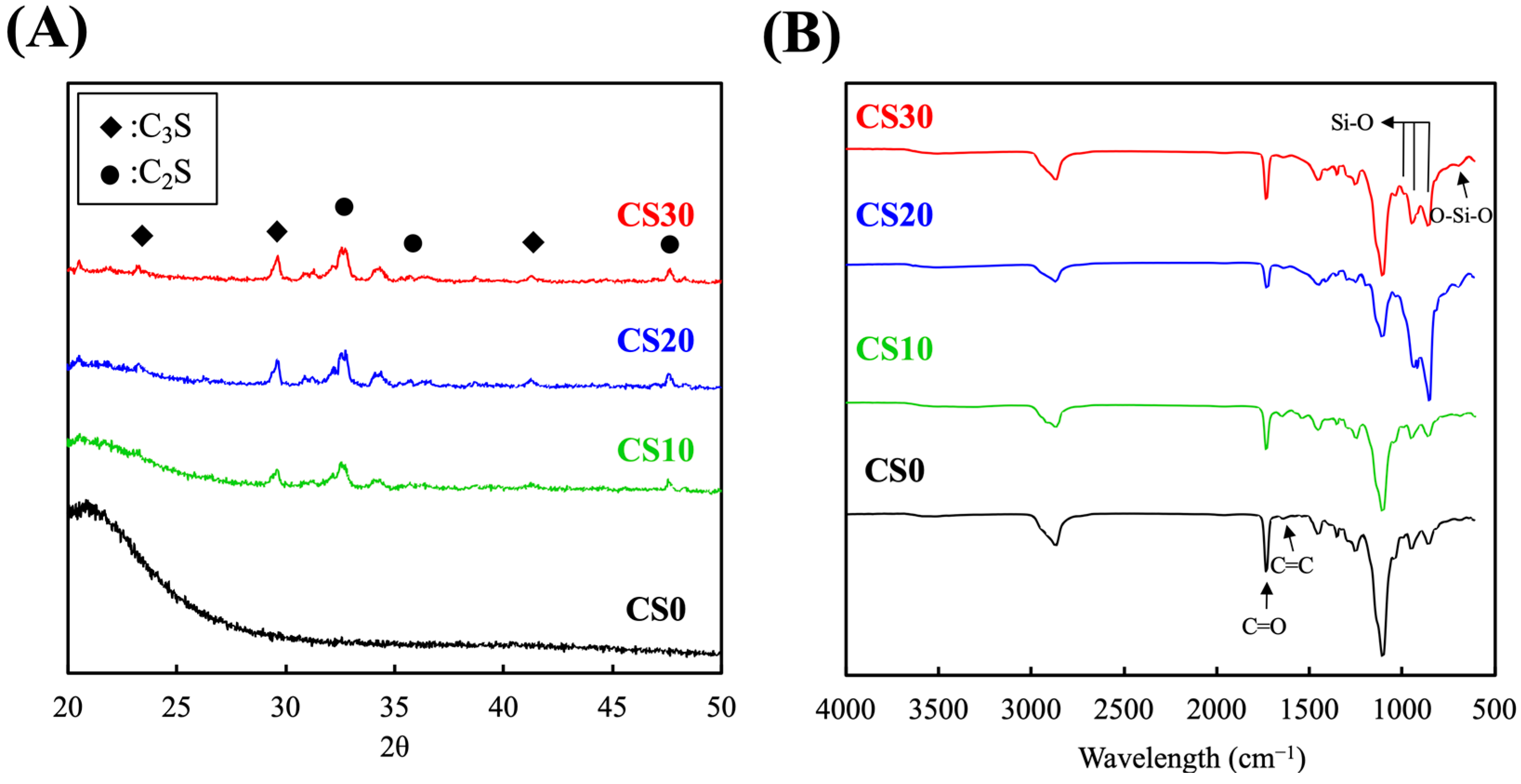
3.1. Synthesis and Characterization of the Light-Curable CS Composite
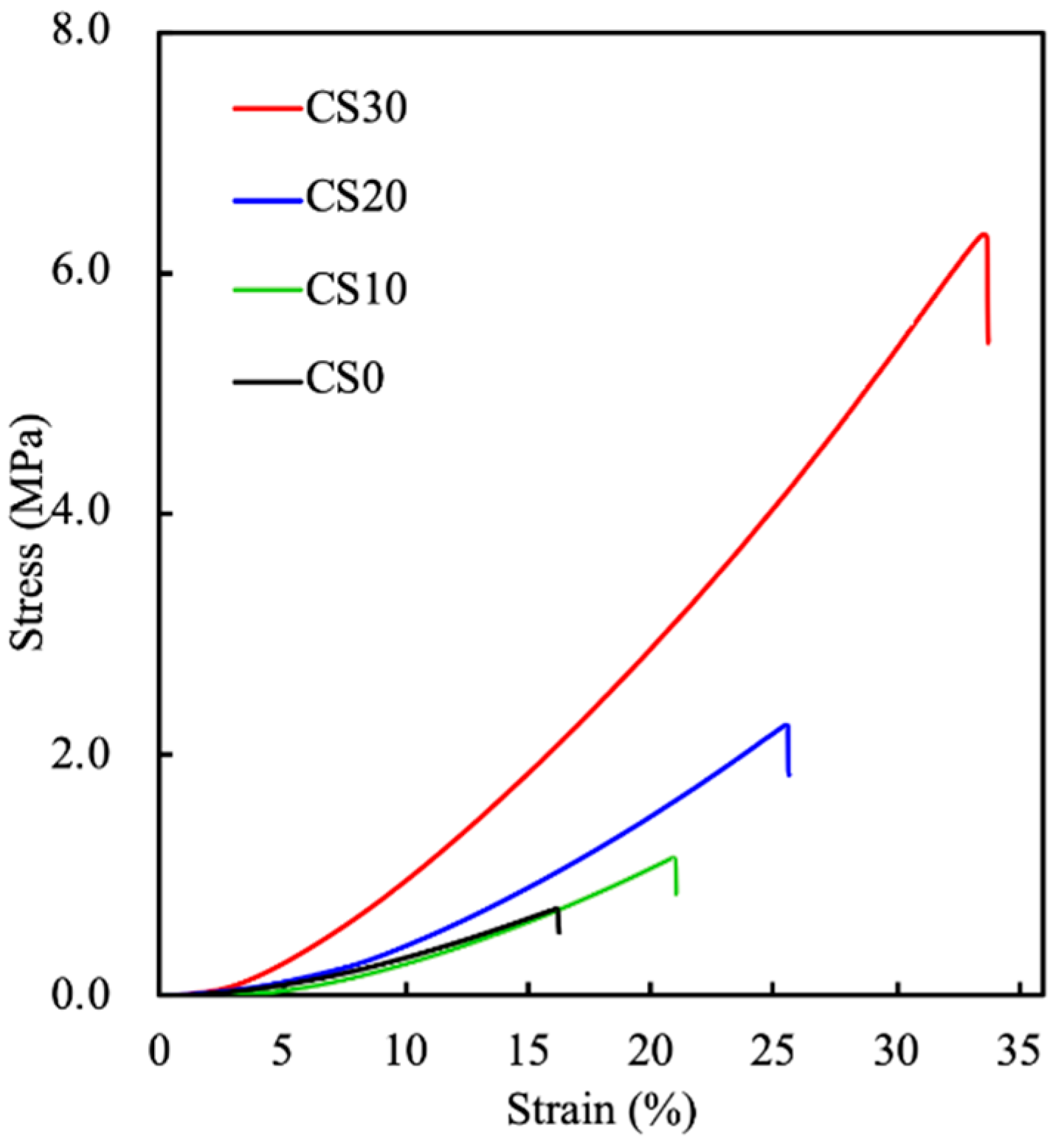
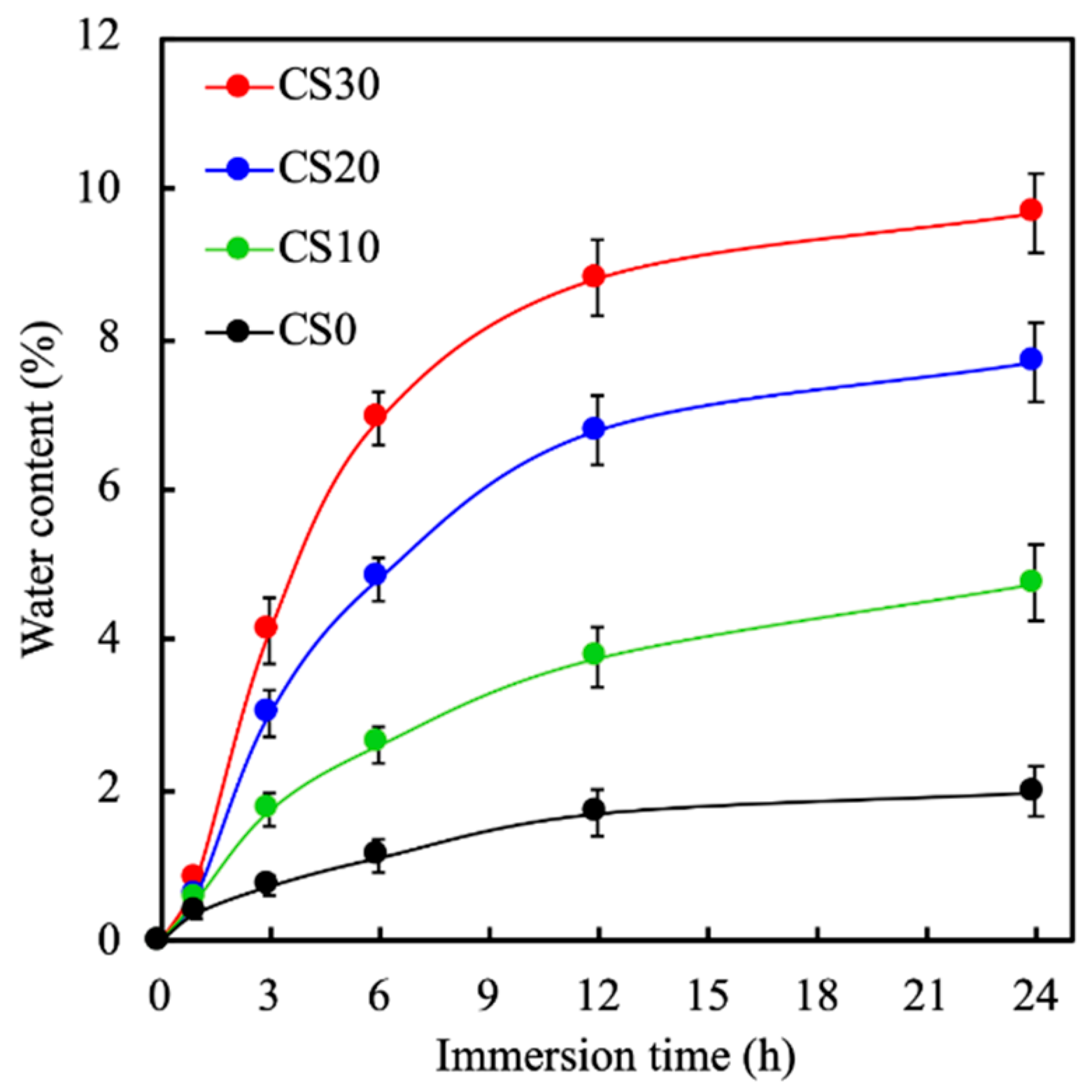
3.2. The Mechanical Properties and the Swelling Behavior of the Light-Curable CS Composite
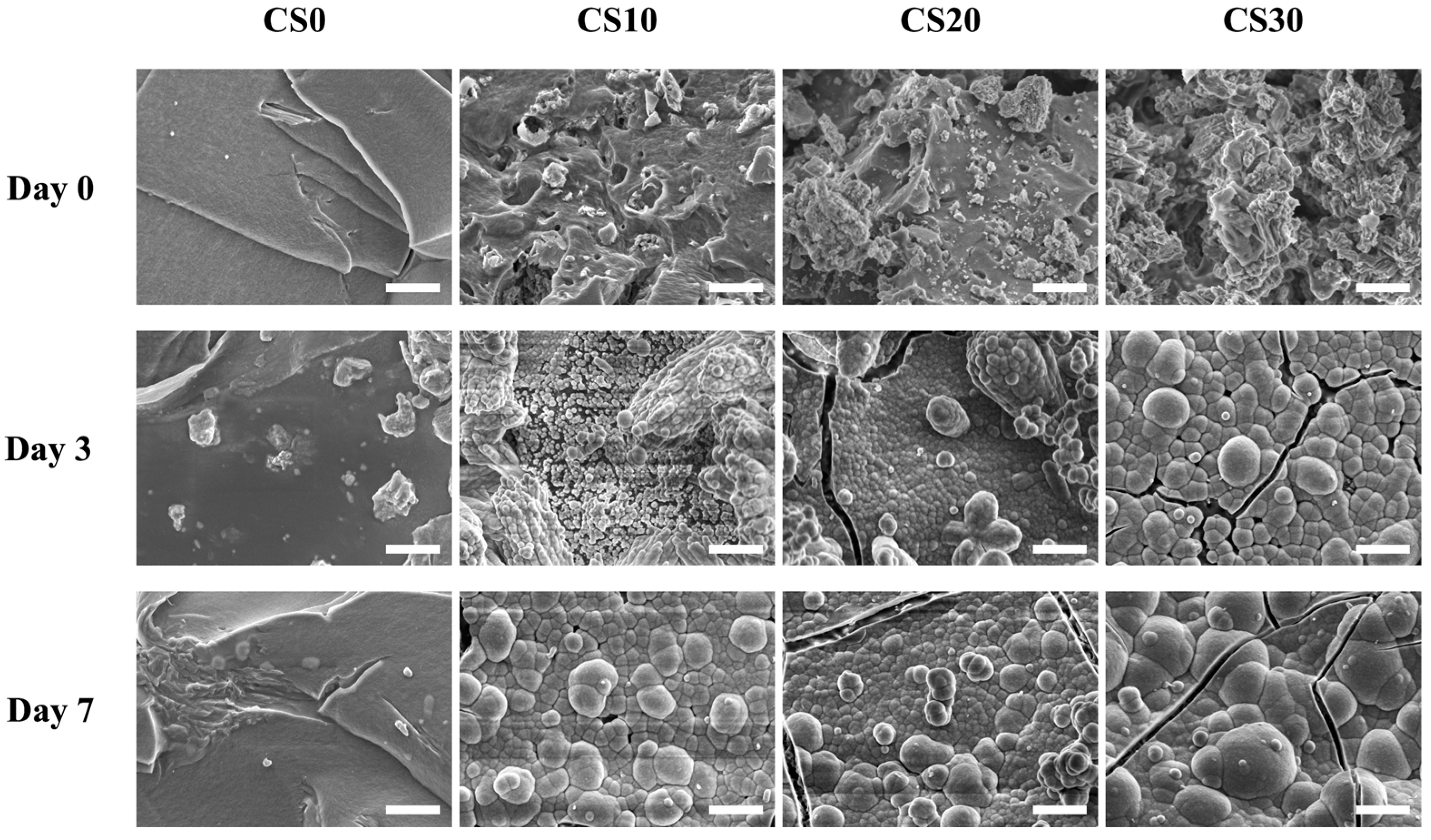
3.3. Effects of Degradation Properties on the Soaking Experiments
3.4. In Vitro hDPSCs Culture
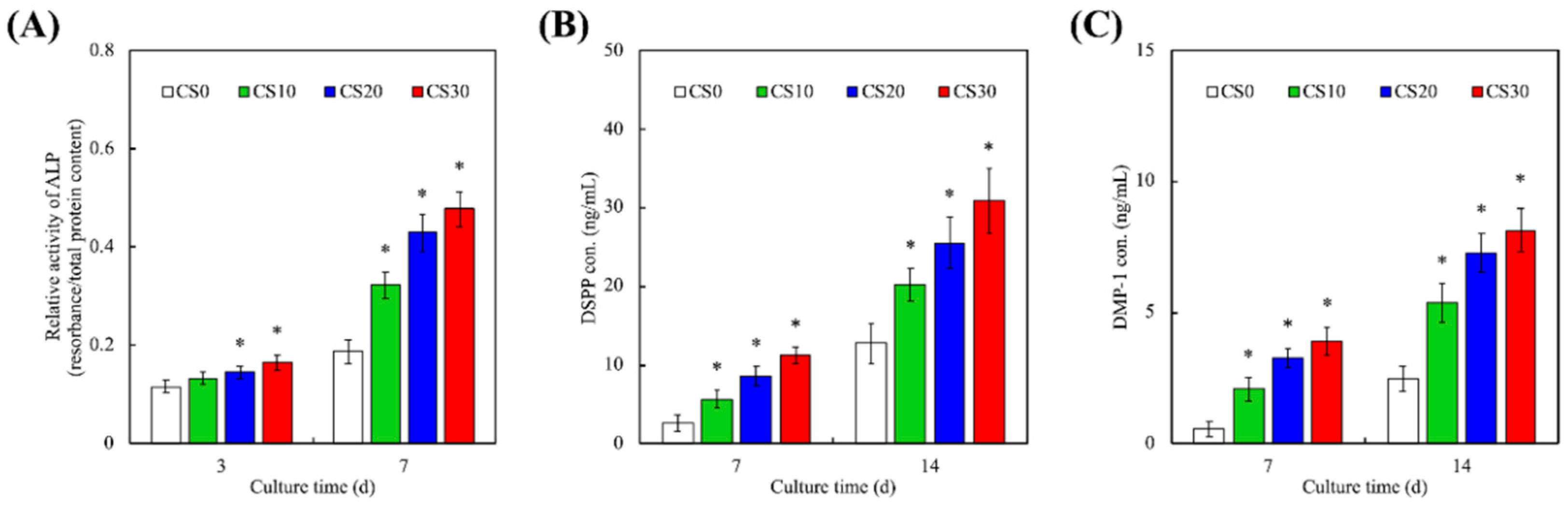
3.5. Odontogenic Behaviors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Rao, Z.; Zhao, Y.; Xu, Y.; Chen, L.; Shen, Z.; Bai, Y.; Lin, Z.; Huang, Q. A Decellularized Matrix Hydrogel Derived from Human Dental Pulp Promotes Dental Pulp Stem Cell Proliferation, Migration, and Induced Multidirectional Differentiation In Vitro. J. Endod. 2020, 46, 1438–1447.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, G.; Dong, Y. Directional Migration and Odontogenic Differentiation of Bone Marrow Stem Cells Induced by Dentin Coated with Nanobioactive Glass. J. Endod. 2019, 46, 216–223. [Google Scholar] [CrossRef]
- Tu, M.G.; Lee, K.X.; Lin, Y.H.; Huang, T.H.; Ho, C.C.; Shie, M.Y. Caffeic acid-coated nanolayer on Mineral Trioxide Aggregate potentiate the host immune responses, angiogenesis, and odontogenesis. J. Endod. 2020, 46, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Tao, Y.; Lin, D.; An, S. Identification of a Calcium-sensing Receptor in Human Dental Pulp Cells That Regulates Mineral Trioxide Aggregate–induced Mineralization. J. Endod. 2019, 45, 907–916. [Google Scholar] [CrossRef]
- Ling, Z.; He, Y.; Huang, H.; Xie, X.; Li, Q.-L.; Cao, C.Y. Effects of oligopeptide simulating DMP-1/mineral trioxide aggregate/agarose hydrogel biomimetic mineralisation model for the treatment of dentine hypersensitivity. J. Mater. Chem. B 2019, 7, 5825–5833. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-T.; Chen, Y.-J.; Huang, T.-H.; Lin, Y.-H.; Hsu, T.-T.; Ho, C.-C. Assessment of the Release Profile of Fibroblast Growth Factor-2-Load Mesoporous Calcium Silicate/Poly-ε-caprolactone 3D Scaffold for Regulate Bone Regeneration. Processes 2020, 8, 1249. [Google Scholar] [CrossRef]
- Gaudin, A.; Tolar, M.; Peters, O. Cytokine Production and Cytotoxicity of Calcium Silicate–based Sealers in 2- and 3-dimensional Cell Culture Models. J. Endod. 2020, 46, 818–826. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Ma, B.; Wu, J.; Chang, J.; Gelinsky, M.; Wu, C. Black Bioceramics: Combining Regeneration with Therapy. Adv. Mater. 2020, 32, e2005140. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Yao, C.-H. Repair of bone defects with gelatin-based composites: A review. BioMedicine 2011, 1, 29–32. [Google Scholar] [CrossRef]
- Itoh, Y.; Sasaki, J.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef]
- Huang, K.-H.; Wang, C.-Y.; Chen, C.-Y.; Hsu, T.-T.; Lin, C.-P. Incorporation of Calcium Sulfate Dihydrate into a Mesoporous Calcium Silicate/Poly-ε-Caprolactone Scaffold to Regulate the Release of Bone Morphogenetic Protein-2 and Accelerate Bone Regeneration. Biomedicines 2021, 9, 128. [Google Scholar] [CrossRef]
- Tu, M.-G.; Ho, C.-C.; Hsu, T.-T.; Huang, T.-H.; Lin, M.-J.; Shie, M.-Y. Mineral Trioxide Aggregate with Mussel-inspired Surface Nanolayers for Stimulating Odontogenic Differentiation of Dental Pulp Cells. J. Endod. 2018, 44, 963–970. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.; Cheng, L.; Lin, K. Enhancement of osteoporotic bone regeneration by strontium-substituted 45S5 bioglass via time-dependent modulation of autophagy and the Akt/mTOR signaling pathway. J. Mater. Chem. B 2021, 9, 3489–3501. [Google Scholar] [CrossRef]
- Shie, M.-Y.; Ding, S.-J.; Chang, H.-C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yuan, Y.; Lin, D.; Niu, H.; Liu, C. Kaolin-reinforced 3D MBG scaffolds with hierarchical architecture and robust mechanical strength for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3782–3790. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chiu, Y.-C.; Lee, A.; Lin, Y.-A.; Lin, P.-Y.; Shie, M.-Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-Y.; Chen, Y.-J.; Lee, A.; Lin, Y.-H.; Liu, Y.-W.; Shie, M.-Y. Therapeutic Effects of the Addition of Fibroblast Growth Factor-2 to Biodegradable Gelatin/Magnesium-Doped Calcium Silicate Hybrid 3D-Printed Scaffold with Enhanced Osteogenic Capabilities for Critical Bone Defect Restoration. Biomedicines 2021, 9, 712. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Hsu, T.-T.; Liu, Y.-W.; Kao, C.-T.; Huang, T.-H. Bidirectional Differentiation of Human-Derived Stem Cells Induced by Biomimetic Calcium Silicate-Reinforced Gelatin Methacrylate Bioink for Odontogenic Regeneration. Biomedicines 2021, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Rubí-Sans, G.; Cano-Torres, I.; Pérez-Amodio, S.; Blanco-Fernandez, B.; Mateos-Timoneda, M.; Engel, E. Development and Angiogenic Potential of Cell-Derived Microtissues Using Microcarrier-Template. Biomedicines 2021, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Chiulan, I.; Heggset, E.B.; Voicu, Ș.I.; Chinga-Carrasco, G. Photopolymerization of bio-based polymers in a biomedical engineering perspective. Biomacromolecules 2021, 22, 1795–1814. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial and physical properties of calcium–phosphate and calcium–fluoride nanocomposites with chlorhexidine. Dent. Mater. 2012, 28, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liang, C.; Gao, X.; Huang, H.; Xing, X.; Tang, Q.; Yang, J.; Wu, Y.; Li, M.; Li, H.; et al. Adipose Tissue–derived Microvascular Fragments as Vascularization Units for Dental Pulp Regeneration. J. Endod. 2021, 47, 1092–1100. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Shen, Y.-F.; Ho, C.-C.; Yu, J.; Wu, Y.-H.A.; Wang, K.; Shih, C.-T.; Shie, M.-Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Shie, M.-Y.; Lee, A.; Chou, Y.-T.; Chiang, C.; Lin, C.-P. 3D-Printed Ginsenoside Rb1-Loaded Mesoporous Calcium Silicate/Calcium Sulfate Scaffolds for Inflammation Inhibition and Bone Regeneration. Biomedicines 2021, 9, 907. [Google Scholar] [CrossRef]
- Kao, C.-T.; Chiu, Y.-C.; Lee, A.K.-X.; Lin, Y.-H.; Huang, T.-H.; Liu, Y.-C.; Shie, M.-Y. The synergistic effects of Xu Duan combined Sr-contained calcium silicate/poly-ε-caprolactone scaffolds for the promotion of osteogenesis marker expression and the induction of bone regeneration in osteoporosis. Mater. Sci. Eng. C 2020, 119, 111629. [Google Scholar] [CrossRef]
- Zidan, G.; Greene, C.A.; Etxabide, A.; Rupenthal, I.D.; Seyfoddin, A. Gelatine-based drug-eluting bandage contact lenses: Effect of PEGDA concentration and manufacturing technique. Int. J. Pharm. 2021, 599, 120452. [Google Scholar] [CrossRef]
- Oh, T.; Choi, C.-K. Comparison between SiOC thin films fabricated by using plasma enhanced chemical vapor deposition and SiO2 thin films by using Fourier transform infrared spectroscopy. J. Korean Phys. Soc. 2010, 56, 1150–1155. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Lei, X.; Cheng, P.; Song, Y.; Gao, Y.; Hu, J.; Wang, C.; Zhang, S.; Li, D.; et al. 3D-bioprinted functional and biomimetic hydrogel scaffolds incorporated with nanosilicates to promote bone healing in rat calvarial defect model. Mater. Sci. Eng. C 2020, 112, 110905. [Google Scholar] [CrossRef]
- Bernardi, A.; Bortoluzzi, E.A.; Felippe, W.T.; Felippe, M.C.S.; Wan, W.S.; Teixeira, C. Effects of the addition of nanoparticulate calcium carbonate on setting time, dimensional change, compressive strength, solubility and pH of MTA. Int. Endod. J. 2016, 50, 97–105. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.; Wang, S.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Health Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Gehrke, S.H.; Detamore, M.S. The bioactivity of agarose–PEGDA interpenetrating network hydrogels with covalently immobilized RGD peptides and physically entrapped aggrecan. Biomaterials 2014, 35, 3558–3570. [Google Scholar] [CrossRef] [Green Version]
- Shie, M.-Y.; Chen, D.C.-H.; Wang, C.-Y.; Chiang, T.-Y.; Ding, S.-J. Immersion behavior of gelatin-containing calcium phosphate cement. Acta Biomater. 2008, 4, 646–655. [Google Scholar] [CrossRef]
- Dai, B.; Li, X.; Xu, J.; Zhu, Y.; Huang, L.; Tong, W.; Yao, H.; Chow, D.H.-K.; Qin, L. Synergistic effects of magnesium ions and simvastatin on attenuation of high-fat diet-induced bone loss. Bioact. Mater. 2021, 6, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Hu, C.-C.; Tseng, Y.-Y.; Sakthivel, R.; Fan, K.-S.; Wang, A.-N.; Wang, Y.-M.; Chung, R.-J. Study on strontium doped tricalcium silicate synthesized through sol-gel process. Mater. Sci. Eng. C 2019, 108, 110431. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chang, J.; Wu, C. Silicate bioceramics: From soft tissue regeneration to tumor therapy. J. Mater. Chem. B 2019, 7, 5449–5460. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Highly porous polycaprolactone scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for bone regeneration. Mater. Sci. Eng. C 2019, 102, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Xi, W.; Ji, M.; Chen, H.; Zhang, Q.; Yan, Y. Developing a biodegradable tricalcium silicate/glucono-delta-lactone/calcium sulfate dihydrate composite cement with high preliminary mechanical property for bone filling. Mater. Sci. Eng. C 2020, 119, 111621. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, Y.-N.; Wang, J.; Cui, R.; Yu, X.; Zhao, G.; Lin, K. A novel multifunctional carbon aerogel-coated platform for osteosarcoma therapy and enhanced bone regeneration. J. Mater. Chem. B 2019, 8, 368–379. [Google Scholar] [CrossRef]
- Ma, P.; Wu, W.; Wei, Y.; Ren, L.; Lin, S.; Wu, J. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Mater. Des. 2021, 207, 109865. [Google Scholar] [CrossRef]
- Shie, M.-Y.; Chang, H.-C.; Ding, S.-J. Composition-dependent protein secretion and integrin level of osteoblastic cell on calcium silicate cements. J. Biomed. Mater. Res. Part A 2013, 102, 769–780. [Google Scholar] [CrossRef]
- Posa, F.; Grab, A.L.; Martin, V.; Hose, D.; Seckinger, A.; Mori, G.; Vukicevic, S.; Cavalcanti-Adam, E.A. Copresentation of BMP-6 and RGD Ligands Enhances Cell Adhesion and BMP-Mediated Signaling. Cells 2019, 8, 1646. [Google Scholar] [CrossRef] [Green Version]
- Schatkoski, V.M.; Montanheiro, T.L.D.A.; de Menezes, B.R.C.; Pereira, R.M.; Rodrigues, K.F.; Ribas, R.G.; da Silva, D.M.; Thim, G.P. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceram. Int. 2020, 47, 2999–3012. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chuang, T.-Y.; Chiang, W.-H.; Chen, I.-W.P.; Wang, K.; Shie, M.-Y.; Chen, Y.-W. The synergistic effects of graphene-contained 3D-printed calcium silicate/poly-ε-caprolactone scaffolds promote FGFR-induced osteogenic/angiogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C 2019, 104, 109887. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.-Y.; Ding, S.-J. Integrin binding and MAPK signal pathways in primary cell responses to surface chemistry of calcium silicate cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef]
- Kao, C.-T.; Lin, C.-C.; Chen, Y.-W.; Yeh, C.-H.; Fang, H.-Y.; Shie, M.-Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 56, 165–173. [Google Scholar] [CrossRef]
- Lee, A.; Lin, Y.-H.; Tsai, C.-H.; Chang, W.-T.; Lin, T.-L.; Shie, M.-Y. Digital Light Processing Bioprinted Human Chondrocyte-Laden Poly (γ-Glutamic Acid)/Hyaluronic Acid Bio-Ink towards Cartilage Tissue Engineering. Biomedicines 2021, 9, 714. [Google Scholar] [CrossRef]
- Mu, C.; He, Y.; Hu, Y.; Li, M.; Chen, M.; Wang, R.; Xiang, Y.; Luo, Z.; Cai, K. Construction of chemokine substance P-embedded biomimetic multilayer onto bioactive magnesium silicate-titanium implant for bone regeneration. Appl. Mater. Today 2020, 20, 100777. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, D.; Ni, Z.; Ou, T.; Wang, Y.; Zhang, H.; Mao, L.; Lin, K.; Xu, S.; Liu, J. Calcium silicate bioactive ceramics induce osteogenesis through oncostatin M. Bioact. Mater. 2020, 6, 810–822. [Google Scholar] [CrossRef]
- Stamnitz, S.; Klimczak, A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, K.-G.; Hwang, J.-H.; Cho, Y.S.; Jeong, H.-J.; Park, S.-H.; Park, Y.; Cho, Y.-S.; Lee, B.-K. Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater. Sci. Eng. C 2019, 98, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Fageeh, H.N. Preliminary Evaluation of Proliferation, Wound Healing Properties, Osteogenic and Chondrogenic Potential of Dental Pulp Stem Cells Obtained from Healthy and Periodontitis Affected Teeth. Cells 2021, 10, 2118. [Google Scholar] [CrossRef] [PubMed]


| CS0 | CS10 | CS20 | CS30 | |
|---|---|---|---|---|
| Yield strength (MPa) | 0.72 ± 0.06 | 1.13 ± 0.11 | 2.22 ± 0.17 | 6.32 ± 0.42 |
| Young’s modulus (MPa) | 5.55 ± 0.33 | 6.08 ± 0.25 | 14.14 ± 0.96 | 23.22 ± 1.42 |
| Toughness (J·m−3) | 4.36 | 8.56 | 21.31 | 84.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Shie, M.-Y.; Lin, Y.-H.; Ho, C.-C.; Kao, C.-T.; Huang, T.-H. The Development of Light-Curable Calcium-Silicate-Containing Composites Used in Odontogenic Regeneration. Polymers 2021, 13, 3107. https://doi.org/10.3390/polym13183107
Lin Y-T, Shie M-Y, Lin Y-H, Ho C-C, Kao C-T, Huang T-H. The Development of Light-Curable Calcium-Silicate-Containing Composites Used in Odontogenic Regeneration. Polymers. 2021; 13(18):3107. https://doi.org/10.3390/polym13183107
Chicago/Turabian StyleLin, Yi-Ting, Ming-You Shie, Yen-Hong Lin, Chia-Che Ho, Chia-Tze Kao, and Tsui-Hsien Huang. 2021. "The Development of Light-Curable Calcium-Silicate-Containing Composites Used in Odontogenic Regeneration" Polymers 13, no. 18: 3107. https://doi.org/10.3390/polym13183107






