Crystallization and Morphology of Triple Crystalline Polyethylene-b-poly(ethylene oxide)-b-poly(?-caprolactone) PE-b-PEO-b-PCL Triblock Terpolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. Small-Angle and Wide-Angle X-ray Scattering (SAXS/WAXS)
2.2.3. Polarized Light Optical Microscopy (PLOM)
3. Results and Discussion
3.1. Small-Angle X-ray Scattering (SAXS)
3.2. Non-Isothermal Crystallization by DSC
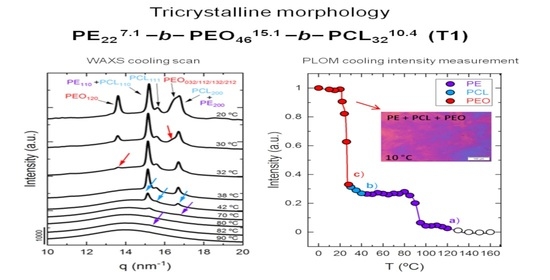
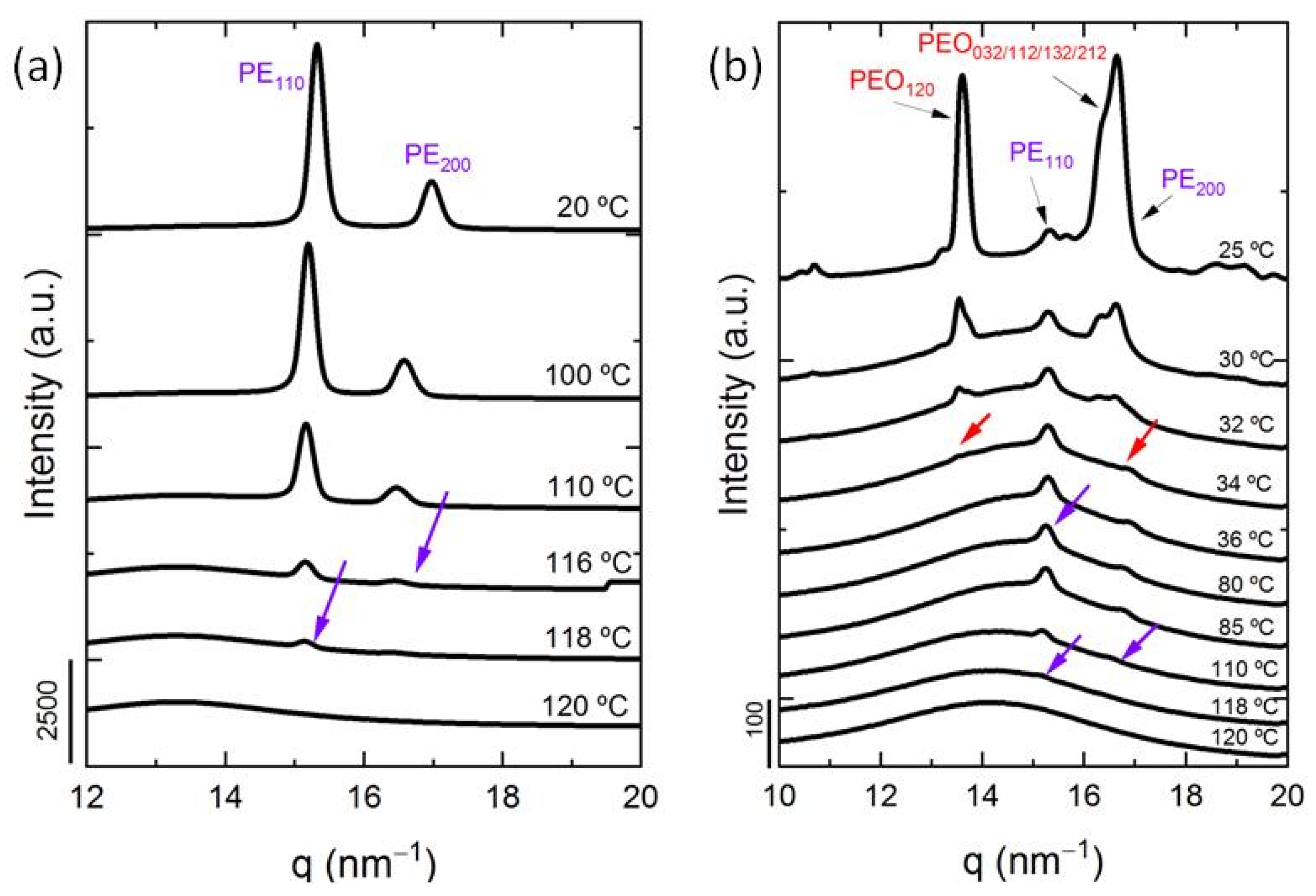
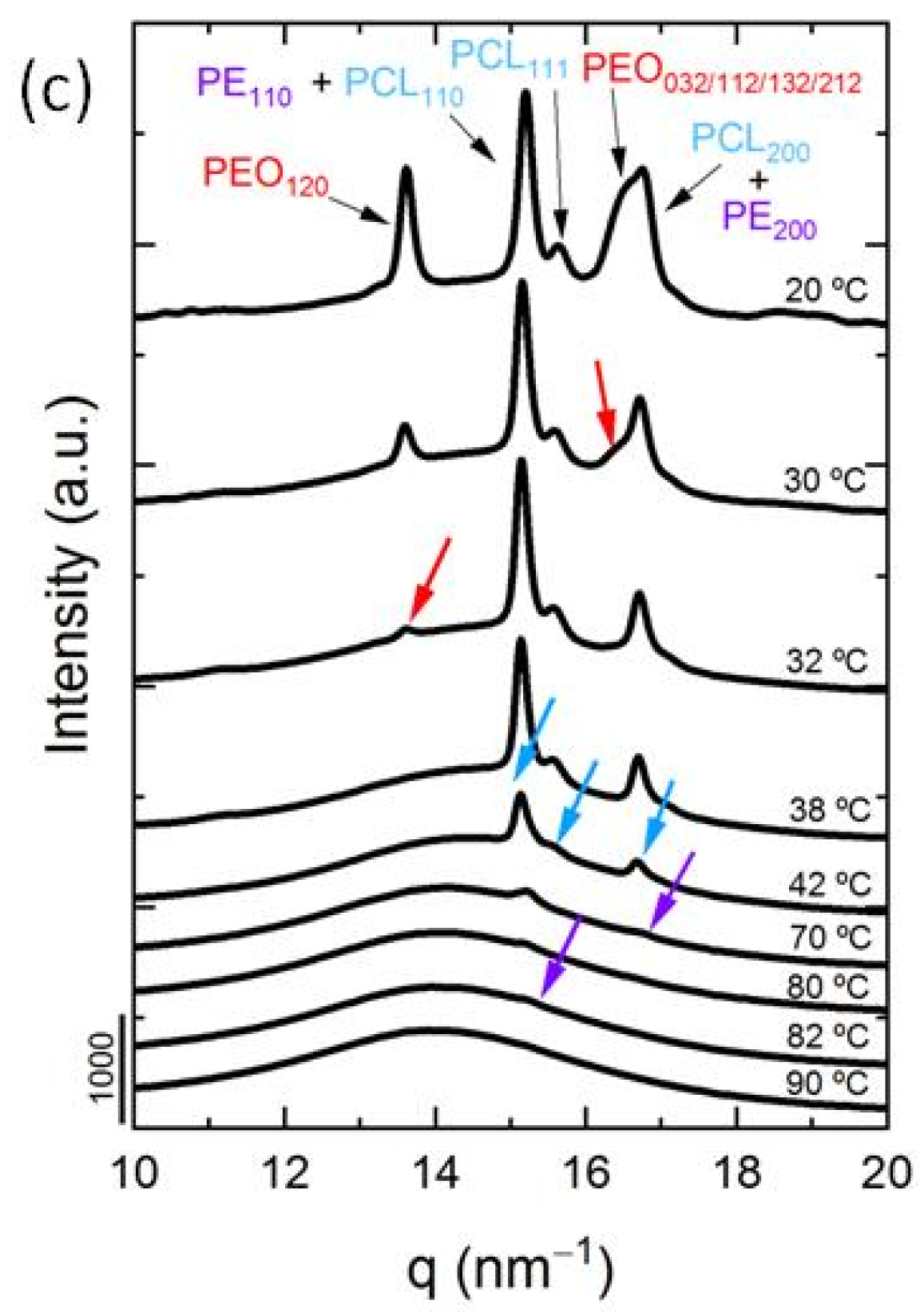
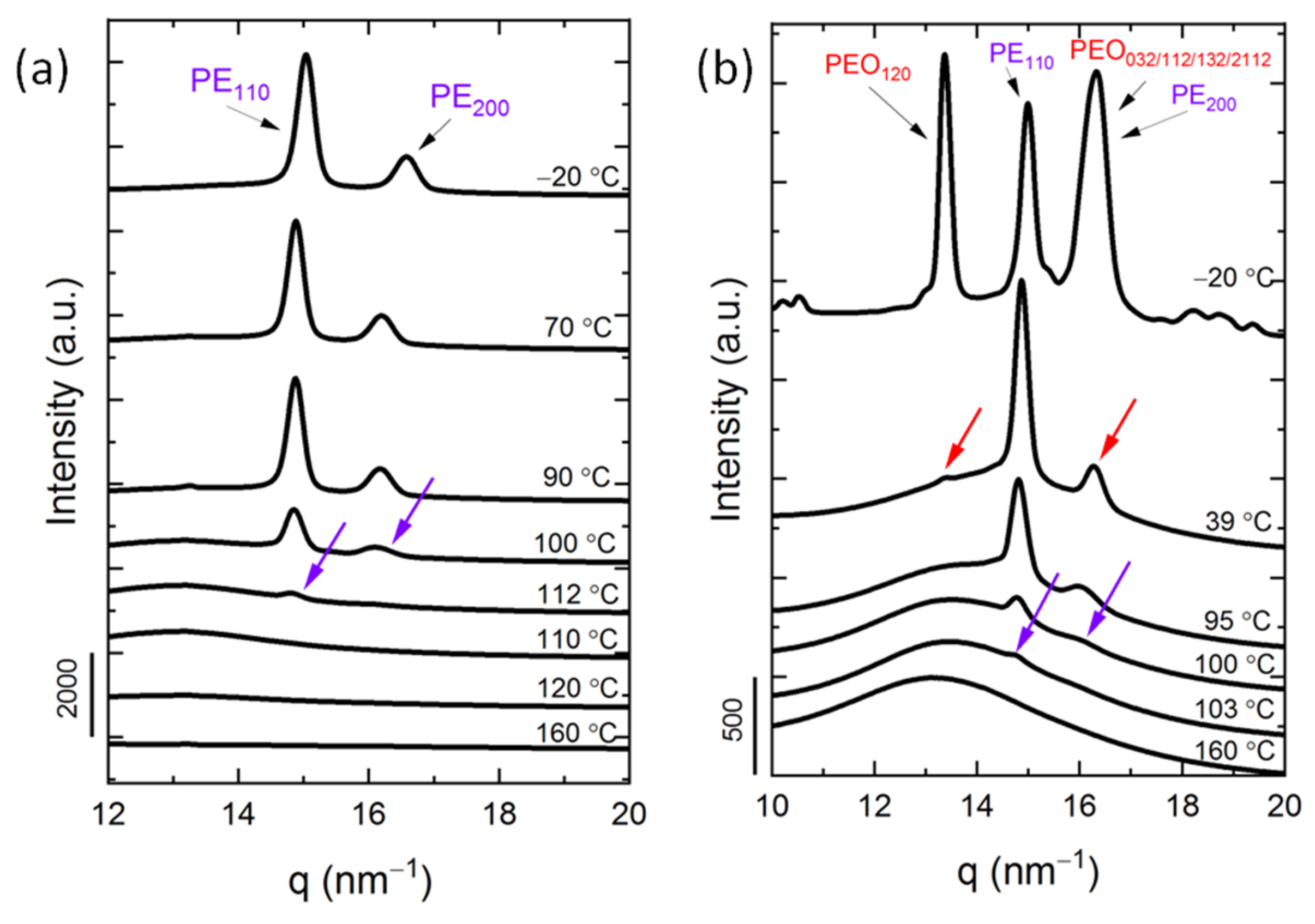
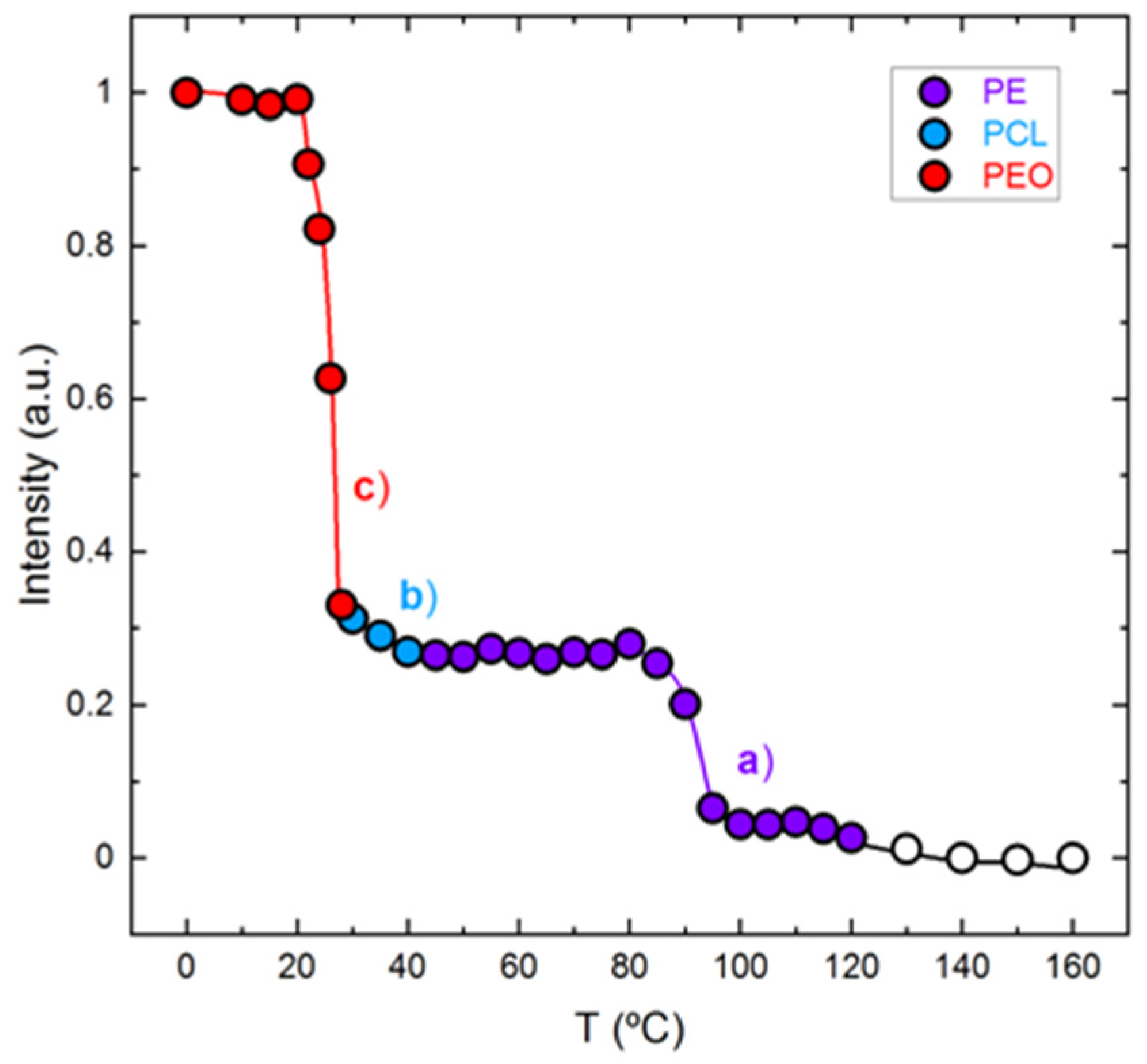
3.3. In Situ Wide Angle X-ray Scattering (WAXS) Real-Time Synchrotron Results
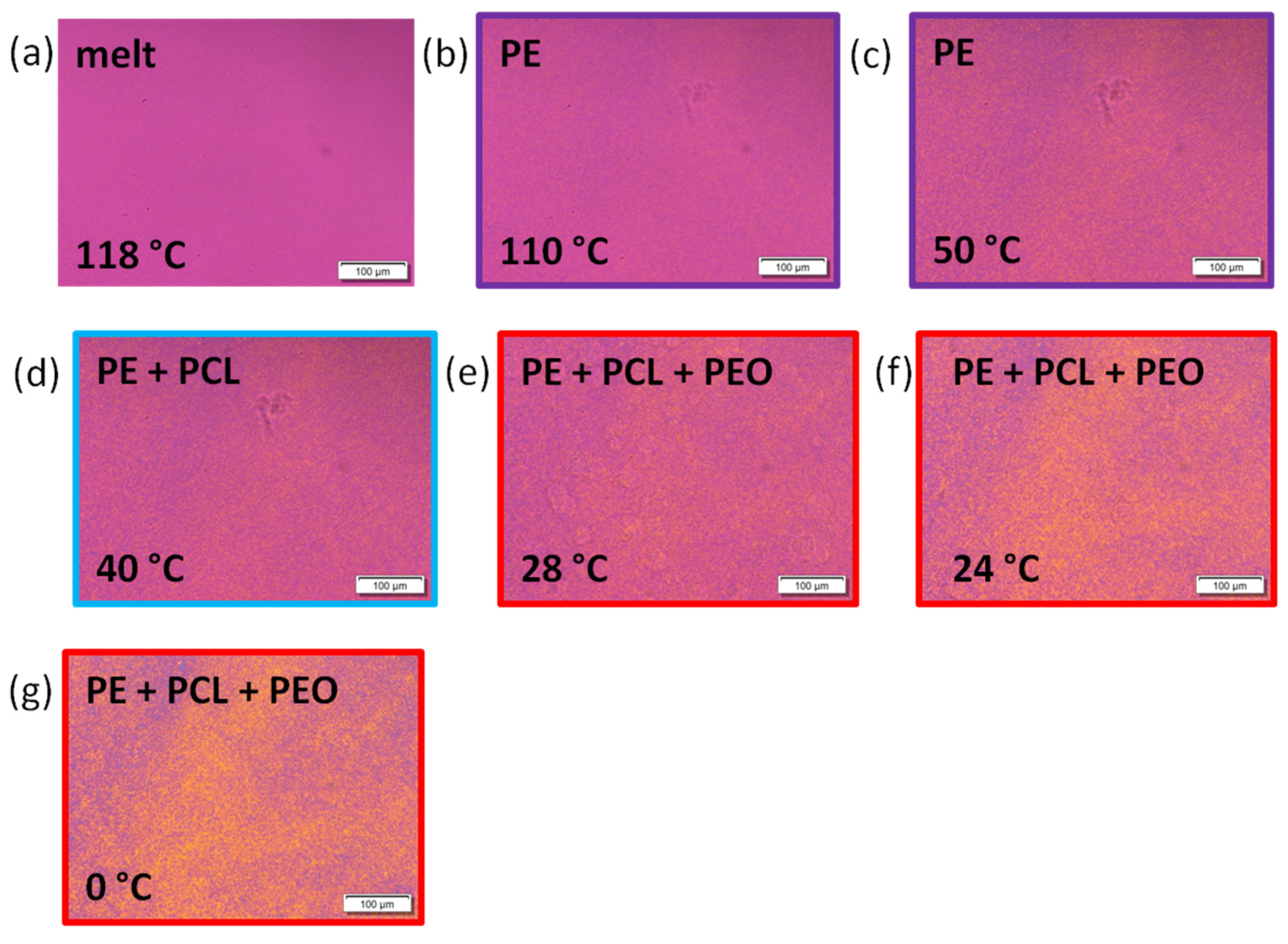
3.4. Polarized Light Optical Microscopy (PLOM) Observations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamley, I. Crystallization in Block Copolymers; Advances in Polymer Science; Springer: Cham, Switzerland, 1999; Volume 148. [Google Scholar]
- Abetz, V.; Simon, P.F.W. Phase Behavior and Morphologies of Block Copolymers; Advances in Polymer Science; Springer: Cham, Switzerland, 2005; Volume 189, pp. 125–212. [Google Scholar]
- Müller, A.J.; Balsamo, V.; Arnal, M.L. Nucleation and crystallization in diblock and triblock copolymers. In Block Copolymers II; Abetz, V., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2005; Volume 190, pp. 1–63. [Google Scholar]
- Müller, A.J.; Arnal, M.L.; Balsamo, V. Crystallization in block copolymers with more than one crystallizable block. In Progress in Understanding of Polymer Crystallization; Reiter, G., Strobl, G.R., Eds.; Lecture Notes in Physics; Springer: Berlin/Heidelberg, Germany, 2007; Volume 714, pp. 229–259. [Google Scholar]
- Michell, R.M.; Müller, A.J. Confined crystallization of polymeric materials. Prog. Polym. Sci. 2016, 54–55, 183–216. [Google Scholar] [CrossRef]
- Nakagawa, S.; Marubayashi, H.; Nojima, S. Crystallization of polymer chains confined in nanodomains. Eur. Polym. J. 2015, 70, 262–275. [Google Scholar] [CrossRef]
- Castillo, R.V.; Müller, A.J. Crystallization and morphology of biodegradable or biostable single and double crystalline block copolymers. Prog. Polym. Sci. 2009, 34, 516–560. [Google Scholar] [CrossRef]
- Li, S.; Register, A. Crystallization in Copolymers. In Handbook of Polymer Crystallization; Piorkowska, E., Rutledge, G.C., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2013; p. 327. [Google Scholar]
- Huang, S.; Jiang, S. Structures and morphologies of biocompatible and biodegradable block copolymers. RSC Adv. 2014, 4, 24566–24583. [Google Scholar] [CrossRef]
- Hamley, I.W. The Physics of Block Copolymers; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Arif, M.; Kalarikkal, N.; Thomas, S. Introduction on crystallization in multiphase polymer systems. In Crystallization in Multiphase Polymer Systems; Thomas, S., Arif, M., Gowd, E.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–13. [Google Scholar]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H. Synthesis of Block Copolymers; Advances in Polymer Science; Springer: Cham, Switzerland, 2005; Volume 189, pp. 1–124. [Google Scholar]
- Barthel, M.J.; Schacher, F.H.; Schubert, U.S. Poly (ethylene oxide)(PEO)-based ABC triblock terpolymers—Synthetic complexity vs. application benefits. Polym. Chem. 2014, 5, 2647. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Wei, X.; Zhou, S. Polymer-based drug delivery systems for cancer treatment. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3525–3550. [Google Scholar] [CrossRef]
- Van Horn, R.M.; Steffen, M.R.; O’connor, D. Recent progress in block copolymer crystallization. Polym. Cryst. 2018, 1, e10039. [Google Scholar] [CrossRef]
- Palacios, J.P.; Mugica, A.; Zubitur, M.; Müller, A.J. Crystallization and morphology of block copolymers and terpolymers with more than one crystallizable block. In Crystallization in Multiphase Polymer Systems; Sabu, T., Mohammed, A.P., Bhoje, G.E., Nandajumar, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 123–171. [Google Scholar]
- Palacios, J.K.; Zhang, H.; Zhang, B.; Hadjichristidis, N.; Müller, A.J. Direct identification of three crystalline phases in PEO-b-PCL-b-PLLA triblock terpolymer by in situ hot-stage atomic force microscopy. Polymer 2020, 205, 122863. [Google Scholar] [CrossRef]
- Müller, A.J.; Arnal, M.L.; Lorenzo, A.T. Crystallization in nano-confined polymeric systems. In Handbook of Polymer Crystallization; Piorkowska, E., Rutledge, G.C., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 347–372. [Google Scholar]
- Castillo, R.V.; Müller, A.J.; Lin, M.C.; Chen, H.L.; Jeng, U.S.; Hillmyer, M.A. Confined crystallization and morphology of melt segregated PLLA-b-PE and PLDA-b-PE diblock copolymers. Macromolecules 2012, 45, 4254–4261. [Google Scholar] [CrossRef]
- Müller, A.J.; Castillo, R.V.; Hillmyer, M. Nucleation and crystallization of PLDA-b-PE and PLLA-b-PE diblock copolymers. Macromol. Symp. 2006, 242, 174–181. [Google Scholar] [CrossRef]
- Müller, A.J.; Lorenzo, A.T.; Castillo, R.V.; Arnal, M.L.; Boschetti-de-Fierro, A.; Abetz, V. Crystallization kinetics of homogeneous and melt segregated PE containing diblock copolymers. Macromol. Symp. 2006, 245–246, 154–160. [Google Scholar] [CrossRef]
- Lin, M.C.; Wang, Y.C.; Chen, J.H.; Chen, H.L.; Müller, A.J.; Su, C.J.; Jeng, U.S. Orthogonal crystal orientation in double-crystalline block copolymer. Macromolecules 2011, 44, 6875–6884. [Google Scholar] [CrossRef]
- Bao, J.; Dong, X.; Chen, S.; Lu, W.; Zhang, X.; Chen, W. Confined crystallization, melting behavior and morphology in PEG-b-PLA diblock copolymers: Amorphous versus crystalline PLA. J. Polym. Sci. 2020, 58, 455–456. [Google Scholar] [CrossRef]
- Bao, J.; Dong, X.; Chen, S.; Lu, W.; Zhang, X.; Chen, W. Fractionated crystallization and fractionated melting behaviors of poly (ethylene glycol) induced by poly (lactide) stereocomplex in their block copolymers and blends. Polymer 2020, 190, 122189. [Google Scholar] [CrossRef]
- Ring, J.O.; Thomann, R.; Mülhaupt, R.; Raquez, J.-M.; Degée, P.; Dubois, P. Controlled synthesis and characterization of Poly[ethylene-block-(L,L-lactide)]s by combining catalytic ethylene oligomerization with ’’Coordination-insertion’’ ring-opening polymerization. Macromol. Chem. Phys. 2007, 208, 896–902. [Google Scholar] [CrossRef]
- Müller, A.J.; Albuerne, J.; Marquez, L.; Raquez, J.M.; Degée, P.; Dubois, P.; Hobbs, J.; Hamley, I.W. Self-nucleation and crystallization kinetics of double crystalline poly(p-dioxanone)-b-poly(ɛ-caprolactone) diblock copolymers. Faraday Discuss 2005, 128, 231–252. [Google Scholar] [CrossRef]
- Müller, A.J.; Albuerne, J.; Esteves, L.M.; Marquez, L.; Raquez, J.-M.; Degée, P.; Dubois, P.; Collins, S.; Hamley, I.W. Confinement effects on the crystallization kinetics and self-nucleation of double crystalline poly(p-dioxanone)-b-poly(ɛ-caprolactone) diblock copolymers. Macromol. Symp. 2004, 215, 369–382. [Google Scholar] [CrossRef]
- Albuerne, J.; Máquez, L.; Müller, A.J.; Raquez, J.M.; Degée, P.; Dubois, P.; Castelleto, V.; Hamley, I.W. Nucleation and crystallization in double crystalline poly(p-dioxanone)-b-poly(ɛ-caprolactone) diblock copolymers. Macromolecules 2003, 36, 1633–1644. [Google Scholar] [CrossRef]
- Hamley, I.W.; Parras, P.; Castelletto, V.; Castillo, R.V.; Müller, A.J.; Pollet, E.; Dubois, P.; Martin, C.M. Melt structure and its transformation by sequential crystallization of the two blocks within poly(ʟ-lactide)-block- poly(ɛ-caprolactone) double crystalline diblock copolymers. Macromol. Chem. Phys. 2006, 207, 941–953. [Google Scholar] [CrossRef]
- Castillo, R.V.; Müller, A.J.; Raquez, J.M.; Dubois, P. Crystallization kinetics and morphology of biodegradable double crystalline PLLA-b-PCL diblock copolymers. Macromolecules 2010, 43, 4149–4160. [Google Scholar] [CrossRef]
- Laredo, E.; Prutsky, N.; Bello, A.; Grimau, M.; Castillo, R.V.; Müller, A.J.; Dubois, P. Miscibility in poly(ʟ-lactide)-b-poly(ɛ-caprolactone) double crystalline diblock copolymers. Eur. Phys. J. E 2007, 23, 295–303. [Google Scholar] [CrossRef]
- Hamley, I.W.; Castelletto, V.; Castillo, R.W.; Müller, A.J.; Martin, C.M.; Pollet, E.; Dubois, P. Crystallization in poly(ʟ-lactide)-b-poly(ɛ-caprolactone) double crystalline diblock copolymers: A study using X-ray scattering, differential scanning calorimetry and polarized optical microscopy. Macromolecules 2005, 38, 463–472. [Google Scholar] [CrossRef]
- Myers, S.B.; Register, R.A. Crystalline-crystalline diblock copolymers of linear polyethylene and hydrogenated polynorbornene. Macromolecules 2008, 41, 6773–6779. [Google Scholar] [CrossRef]
- Ponjavic, M.; Nikolic, M.S.; Jevtic, S.; Rogan, J.; Stevanovic, S.; Djonlagic, J. Influence of a low content of PEO segment on the thermal, surface and morphological properties of triblock and diblock PCL copolymers. Macromol. Res. 2016, 24, 323–335. [Google Scholar] [CrossRef]
- Li, L.; Meng, F.; Zhong, Z.; Byelov, D.; De Jeu, W.H.; Feijen, J. Morphology of a highly asymmetric double crystallizable poly(ɛ-caprolactone-b-ethylene oxide) block copolymer. J. Chem. Phys. 2007, 126, 024904. [Google Scholar] [CrossRef] [Green Version]
- Van Horn, R.M.; Zheng, J.X.; Sun, H.J.; Hsiao, M.S.; Zhang, W.B.; Dong, X.H.; Xu, J.; Thomas, E.L.; Lotz, B.; Chen, S.Z.D. Solution crystallization behavior of crystalline-crystalline diblock copolymers of poly(ethylene oxide)-block-poly(ɛ-caprolactone). Macromolecules 2010, 43, 6113–6119. [Google Scholar] [CrossRef]
- Vivas, M.; Contreras, J.; López-Carrasquero, F.; Lorenzo, A.T.; Arnal, M.L.; Balsamo, V.; Müller, A.J.; Laredo, E.; Schmalz, H.; Abetz, V. Synthesis and characterization of triblock terpolymers with three potentially crystallisable blocks: Polyethylene-b-poly(ethylene oxide)-b-poly(ɛ-caprolactone). Macrolecular Symp. 2006, 239, 58–67. [Google Scholar] [CrossRef]
- Jiang, S.; He, C.; An, L.; Chen, X.; Jiang, B. Crystallization and ring-banded spherulite morphology of poly (ethylene oxide)-block-poly (ɛ-caprolactone) diblock copolymer. Macromol. Chem. Phys. 2004, 205, 2229–2234. [Google Scholar] [CrossRef]
- Arnal, M.L.; López-Carrasquero, F.; Laredo, E.; Müller, A.J. Coincident or sequential crystallization of PCL and PEO blocks within polystyrene-b-poly(ethylene oxide)-b-poly(ɛ-caprolactone) linear triblock copolymers. Eur. Polym. J. 2004, 40, 1461–1476. [Google Scholar] [CrossRef]
- Nojima, S.; Ono, M.; Ashida, T. Crystallization of block copolymers II. Morphological study of poly (ethylene glycol)-poly (ɛ-caprolactone) block copolymers. Polym. J. 1992, 24, 1271–1280. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, L.; Yu, F.; Wang, P.; Qi, M. Synthesis and characterization of poly (ɛ-caprolactone)-b-poly (ethylene glycol)-b-poly (ɛ-caprolactone) triblock copolymers with dibutylmagnesium as catalyst. J. Appl. Polym. Sci. 2009, 111, 429–436. [Google Scholar] [CrossRef]
- Arnal, M.L.; Balsamo, V.; López-Carrasquero, F.; Contreras, J.; Carrillo, M.; Schmalz, H.; Abetz, V.; Laredo, E.; Müller, A.J. Synthesis and characterization of polystyrene-b-poly (ethylene oxide)-b-poly (ɛ-caprolactone) block copolymers. Macromolecules 2001, 34, 7973–7982. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Zhang, J.; Gou, Q.; Gu, Q.; Wang, Z. Morphology of poly(ethylene oxide)-b-poly(ɛ-caprolactone) spherulites formed under compressed CO2. J. Macromol. Sci. Part B Phys. 2014, 53, 1137–1144. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Wang, Z.; He, T. Tuning radial lamellar packing and orientation into diverse ring-banded spherulites: Effects of structural feature and crystallization condition. Macromolecules 2014, 47, 1783–1792. [Google Scholar] [CrossRef]
- Xue, F.-F.; Chen, X.-S.; An, L.-J.; Funari, S.S.; Jiang, S.-C. Confined lamella formation in crystalline-crystalline poly(ethylene-oxide)-b-poly(ɛ-caprolactone) diblock copolymers. Chin. J. Polym. Sci. 2013, 31, 1260–1270. [Google Scholar] [CrossRef]
- Xue, F.; Chen, X.; An, L.; Funari, S.S.; Jiang, S. Soft nanoconfinement effects on the crystallization behavior of asymmetric poly(ethylene oxide)-block-poly(ɛ-caprolactone) diblock copolymers. Polym. Int. 2012, 61, 909–917. [Google Scholar] [CrossRef]
- Sun, J.; He, C.; Zhuang, X.; Jing, X.; Chen, X. The crystallization behavior of poly(ethylene glycol)-poly(ɛ-caprolactone) diblock copolymers with asymmetric block compositions. J. Polym. Res. 2011, 18, 2161–2168. [Google Scholar] [CrossRef]
- Hua, C.; Dong, C.-M. Synthesis, characterization, effect of architecture on crystallization of biodegradable poly(ɛ-caprolactone)-b-poly(ethylene oxide) copolymers with different arms and nanoparticles thereof. J. Biomed. Mater. Res. Part A 2007, 82, 689–700. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Zhao, T.; Hong, Z.; Zhuang, X.; Chen, X.; Jin, X. Formation of a unique crystal morphology for the poly(ethylene glycol)-poly(ɛ-caprolactone) diblock copolymer. Biomacromolecules 2006, 7, 252–258. [Google Scholar] [CrossRef]
- Piao, L.; Dai, Z.; Deng, M.; Chen, X.; Jing, X. Synthesis and characterization of PCL/PEG/PCL triblock copolymers by using calcium catalyst. Polymer 2003, 44, 2025–2031. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Deng, C.; Zhao, T.; Deng, M.; Chen, X.; Jing, X. Study of the synthesis, crystallization, and morphology of poly(ethylene glycol)-poly(ɛ-caprolactone) diblock copolymers. Biomacromolecules 2004, 5, 2040–2047. [Google Scholar] [CrossRef]
- Arnal, M.L.; Boissé, S.; Müller, A.J.; Meyer, F.; Raquez, J.M.; Dubois, P.; Prud’homme, R.E. Interplay between poly(ethylene oxide) and poly(ʟ-lactide) blocks during diblock copolymer crystallization. CrystEngComm 2016, 18, 3635–3649. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, J.; Shao, J.; Bian, X.; Huang, S.; Li, G.; Chen, X. Unusual crystallization and melting behavior induced by microphase separation in MPEG-b-PLLA diblock copolymer. Polymer 2015, 80, 123–129. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.; Han, C.C. Effect of crystallization temperature on the interactive crystallization behavior of poly(ʟ-lactide)-block-poly (ethylene glycol) copolymer. Polymer 2015, 79, 56–64. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Jiang, S.; Chen, X.; An, L. Morphologies and structures in poly(ʟ-lactide-b-ethylene oxide) copolymers determined by crystallization, microphase separation and vitrification. Polym. Bull. 2011, 67, 885–902. [Google Scholar] [CrossRef]
- Huang, L.; Kiyofuji, G.; Matsumoto, J.; Fukagawa, Y.; Gong, C.; Nojima, S. Isothermal crystallization of poly (b-propiolactone) blocks starting from lamellar microdomain structures of double crystalline poly (b-propiolactone)-block-polyethylene copolymers. Polymer 2012, 53, 5856–5863. [Google Scholar] [CrossRef]
- Sun, J.; Hong, Z.; Yang, L.; Tang, Z.; Chen, X.; Jing, X. Study on crystalline morphology of poly(ʟ-lactide)-poly(ethylene glycol) diblock copolymer. Polymer 2004, 45, 5969–5977. [Google Scholar] [CrossRef]
- Shin, D.; Shin, K.; Aamer, K.A.; Tew, G.N.; Russell, T.P.; Lee, J.H.; Jho, J.Y. A morphological study of a semicrystalline poly(ʟ-lactic acid-b-ethylene oxide-b-ʟ-lactic acid) triblock copolymer. Macromolecules 2005, 38, 104–109. [Google Scholar] [CrossRef]
- Xue, F.; Chen, X.; An, L.; Funari, S.S.; Jiang, S. Crystallization induced layer-to-layer transitions in symmetric PEO-b-PLLA block copolymer with synchrotron simultaneous SAXS/WAXS investigations. RSC Adv. 2014, 4, 56346–56354. [Google Scholar] [CrossRef]
- Huang, S.; Jiang, S.; An, L.; Chen, X. Crystallization and morphology of poly(ethylene oxide-b-lactide) crystalline-crystalline diblock copolymers. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1400–1411. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, T.; Zhou, Y.; Liu, L.; Li, G.; Zhou, E.; Chen, X. Single crystals of the poly(ʟ-lactide) block and the poly(ethylene glycol) block in poly(ʟ-lactide)-poly(ethylene glycol) diblock copolymer. Macromolecules 2007, 40, 2791–2797. [Google Scholar] [CrossRef]
- Cai, C.; Wang, L.U.; Donc, C.M. Synthesis, characterization, effect of architecture on crystallization, and spherulitic growth of poly(ʟ-lactide)-b-poly(ethylene oxide) copolymers with different branch arms. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2034–2044. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, T.; Cui, J.; Liu, L.; Zhou, Y.; Li, G.; Zhou, E.; Chen, X. Nonisothermal crystallization behavior of the poly(ethylene glycol) block in poly(ʟ-lactide)-poly(ethylene glycol) diblock copolymers: Effect of the poly(ʟ-lactide) block length. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3215–3226. [Google Scholar] [CrossRef]
- Huang, C.I.; Tsai, S.H.; Chen, C.M. Isothermal crystallization behavior of poly(ʟ-lactide) in poly(ʟ-lactide)-block-poly(ethylene glycol) diblock copolymers. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2438–2448. [Google Scholar] [CrossRef]
- Kim, K.S.; Chung, S.; Chin, I.J.; Kim, M.N.; Yoon, J.S. Crystallization behavior of biodegradable amphiphilic poly(ethylene glycol)-poly(ʟ-lactide) block copolymers. J. Appl. Polym. Sci. 1999, 72, 341–348. [Google Scholar] [CrossRef]
- Wang, J.L.; Dong, C.M. Synthesis, sequential crystallization and morphological evolution of well-defined star-shaped poly(ɛ-caprolactone)-b-poly(ʟ-lactide) block copolymer. Macromol. Chem. Phys. 2006, 207, 554–562. [Google Scholar] [CrossRef]
- Liénard, R.; Zaldua, N.; Josse, T.; Winter, J.D.; Zubitur, M.; Mugica, A.; Iturrospe, A.; Arbe, A.; Coulembier, O.; Müller, A.J. Synthesis and characterization of double crystalline cyclic diblock copolymers of poly(ɛ-caprolactone) and poly(L(D)-lactide) (c(PCL-b-PL(D)LA)). Macromol. Rapid Commun. 2016, 37, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Baena, I.; Marcos-Fernández, A.; Fernández-Torres, A.; Kenny, J.M.; Peponi, L. Synthesis of PLLA-b-PCL-b-PLLA linear tri-block copolymers and their corresponding poly(ester-urethane)s: Effect of the molecular weight on their crystallisation and mechanical properties. RSC Adv. 2014, 4, 8510–8524. [Google Scholar] [CrossRef] [Green Version]
- Peponi, L.; Navarro-Baena, I.; Báez, J.E.; Kenny, J.M.; Marcos-Fernández, A. Effect of the molecular weight on the crystallinity of PCL-b-PLLA di-block copolymers. Polymer 2012, 53, 4561–4568. [Google Scholar] [CrossRef] [Green Version]
- Ho, R.-M.; Hsieh, P.-Y.; Tseng, W.-H.; Lin, C.-C.; Huang, B.-H.; Lotz, B. Crystallization-induced orientation for microstructures of Poly(ʟ-lactide)-b-poly(ɛ-caprolactone) diblock copolymers. Macromolecules 2003, 36, 9085–9092. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, D.-J.; Lee, M.-S.; Ihn, K.J. Synthesis and crystallization behavior of poly(ʟ-lactide)-block-poly(ɛ-caprolactone) copolymer. Polymer 2001, 42, 7429–7441. [Google Scholar] [CrossRef]
- Yan, D.; Huang, H.; He, T.; Zhang, F. Coupling of microphase separation and dewetting in weakly segregated di-block copolymer ultrathin films. Langmuir 2011, 27, 11973–11980. [Google Scholar] [CrossRef]
- Casas, M.T.; Puiggalí, J.; Raquez, J.M.; Dubois, P.; Córdova, M.E.; Müller, A.J. Single crystals morphology of biodegradable double crystalline PLLA-b-PCL diblock copolymers. Polymer 2011, 52, 5166–5177. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, S.H.; Kim, S.H.; Lee, Y.M.; Kim, Y.H. Synthesis and characterization of poly(ʟ-lactide)-poly(ɛ-caprolactone) multiblock copolymers. Macromolecules 2003, 36, 5585–5592. [Google Scholar] [CrossRef]
- Danafar, H. Applications of copolymeric nanoparticles in drug delivery systems. Drug Res. 2016, 66, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Ostacolo, L.; Marra, M.; Ungaro, F.; Zappavigna, S.; Maglio, G.; Quaglia, F.; Abbruzzese, A.; Caraglia, M. In vitro anticancer activity of docetaxel-loaded micelles based on poly(ethylene oxide)-poly(ɛ-caprolactone) block copolymers: Do nanocarrier properties have a role? J. Control. Release 2010, 148, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Deng, X.; Yang, H. Biodegradable poly(ɛ-caprolactone)-poly(ethylene glycol) block copolymers: Characterization and their use as drug carriers for a controlled delivery system. Biomaterials 2003, 24, 3563–3570. [Google Scholar] [CrossRef]
- Lim, D.W.; Park, T.G. Stereocomplex formation between enantiomeric PLA-PEG-PLA triblock copolymers: Characterization and use as protein delivery microparticulate carriers. J. Appl. Polym. Sci. 2000, 75, 1615–1623. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Xu, Q.; Tu, Y.; Zhu, X.; Chen, E. PBT-b-PEO-b-PBT triblock copolymers: Synthesis, characterization and double crystalline properties. Polymer 2013, 54, 6725–6731. [Google Scholar] [CrossRef]
- Schmalz, H.; Van Guldener, M.; Gabriëlse, W.; Lange, R.; Abetz, V. Morphology, surface structure, and elastic properties of PBT-based copolyesters with PEO-b-PEB-b-PEO triblock copolymer soft segments. Macromolecules 2002, 35, 5491–5499. [Google Scholar] [CrossRef]
- Voet, V.S.D.; van Ekenstein, G.O.R.A.; Meereboer, N.L.; Hoffman, A.H.; Brinke, G.T.; Loos, K. Double crystalline PLLA-b-PVDF-b-PLLA triblock copolymers: Preparation and crystallization. Polym. Chem. 2014, 5, 2219–2230. [Google Scholar] [CrossRef]
- Balsamo, V.; Müller, A.J.; Von Gyldenfeldt, F.; Stadler, R. Ternary ABC block copolymers based on one glassy and two crystallizable blocks: Polystyrene-block-polyethylene-block-poly(ɛ-caprolactone). Macromol. Chem. Phys. 1998, 199, 1063–1070. [Google Scholar]
- Balsamo, V.; Paolini, Y.; Ronca, G.; Müller, A.J. Crystallization of the polyethylene block in polystyrene-b-polyethylene-b-polycaprolactone triblock copolymers, 1: Self-nucleation behavior. Macromol. Chem. Phys. 2000, 201, 2711–2720. [Google Scholar] [CrossRef]
- Balsamo, V.; Müller, A.J.; Stadler, R. Antinucleation effect of the polyethylene block on the polycaprolactone block in ABC triblock copolymers. Macromolecules 1998, 31, 7756–7763. [Google Scholar] [CrossRef]
- Müller, A.J.; Balsamo, V.; Arnal, M.L.; Jakob, T.; Schmalz, H.; Abetz, V. Homogeneous nucleation and fractionated crystallization in block copolymers. Macromolecules 2002, 35, 3048–3058. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Hu, Y.Y.; Li, J.N.; Huang, S.H.; Kuo, S.W. Trilayered single crystals with epitaxial growth in poly(ethylene oxide)-block-poly(ε-caprolactone)-block-poly(ʟ-lactide)thin films. Macromolecules 2015, 48, 8526–8533. [Google Scholar] [CrossRef]
- Zhao, J.; Pahovnik, D.; Gnanou, Y.; Hadjichristidis, N. Sequential polymerization of ethylene oxide, ɛ-caprolactone and ʟ-lactide: A one-pot metal-free route to tri- and pentablock terpolymers. Polym. Chem. 2014, 5, 3750–3753. [Google Scholar] [CrossRef]
- Guillerm, B.; Lemaur, V.; Ernould, B.; Cornil, J.; Lazzaroni, R.; Gohy, J.F.; Dubois, P.; Coulembier, O. A one-pot two-step efficient metal-free process for the generation of PEO-PCL-PLA amphiphilic triblock copolymers. RSC Adv. 2014, 4, 10028–10038. [Google Scholar] [CrossRef]
- Sun, L.; Shen, L.J.; Zhu, M.Q.; Dong, C.M.; Wei, Y. Synthesis, self-assembly, drug-release behavior, and cytotoxicity of triblock and pentablock copolymers composed of poly(ɛ-caprolactone), poly(ʟ-lactide), and poly(ethylene glycol). J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4583–4593. [Google Scholar] [CrossRef]
- Tamboli, V.; Mishra, G.P.; Mitra, A.K. Novel pentablock copolymer (PLA-PCL-PEG-PCL-PLA)-based nanoparticles for controlled drug delivery: Effect of copolymer composition on the crystallinity of copolymers and in vitro drug release profile from nanoparticles. Colloid Polym. Sci. 2013, 291, 1235–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Pispas, S.; Avgeropoulos, A. Linear and non-linear triblock terpolymers. Synthesis, self-assembly in selective solvents and in bulk. Prog. Polym. Sci. 2005, 30, 725–782. [Google Scholar] [CrossRef]
- Palacios, J.K.; Múgica, A.; Zubitur, M.; Iturrospe, A.; Arbe, A.; Liu, G.; Wang, D.; Zhao, J.; Hadjichristidis, N.; Müller, A.J. Sequential crystallization and morphology of triple crystalline biodegradable PEO-b-PCL-b-PLLA triblock terpolymers. R. Soc. Chem. Adv. 2016, 6, 4739–4750. [Google Scholar] [CrossRef] [Green Version]
- Palacios, J.K.; Zhao, J.; Hadjicrhistidis, N.; Müller, A.J. How the complex interplay between different blocks determines the isothermal crystallization kinetics of triple-crystalline PEO-b-PCL-b-PLLA triblock terpolymers. Macromolecules 2017, 50, 9683–9695. [Google Scholar] [CrossRef]
- Palacios, J.K.; Tercjak, A.; Liu, G.; Wang, D.; Zhao, J.; Hadjicrhistidis, N.; Müller, A.J. Trilayered morphology of an ABC triple crystalline triblock terpolymer. Macromolecules 2017, 50, 7268–7281. [Google Scholar] [CrossRef]
- Palacios, J.K.; Liu, G.; Wang, D.; Hadjichristidis, N.; Müller, A.J. Generating triple crystalline superstructures in melt-miscible PEO-b-PCL-b-PLLA triblock terpolymers by controlling thermal history and sequential crystallization. Macromol. Chem. Phys. 2019, 220, 1900292. [Google Scholar] [CrossRef]
- Matxinandiarena, E.; Múgica, A.; Zubitur, M.; Zhang, B.; Ladelta, V.; Zapsas, G.; Hadjichristidis, N.; Müller, A.J. The effect of the cooling rate on the morphology and crystallization of triple crystalline PE-b-PEO-b-PLLA and PE-b-PCL-b-PLLA triblock terpolymers. ACS Appl. Polym. Mater. 2020, 2, 4952–4963. [Google Scholar] [CrossRef]
- Loo, Y.L.; Register, R.A.; Ryan, A.J. Modes of crystallization in block copolymer microdomains: Breakout, templated and confined. Macromolecules 2002, 35, 2365–2374. [Google Scholar] [CrossRef]
- He, W.N.; Xu, J.T. Crystallization assisted self-assembly of semicrystalline block copolymers. Prog. Polym. Sci. 2012, 37, 1350–1400. [Google Scholar] [CrossRef]
- Schmalz, H.; Knoll, A.; Müller, A.J.; Abetz, V. Synthesis and characterization of ABC triblock copolymers with two different crystalline end blocks: Influence of confinement on crystallization behavior and morphology. Macromolecules 2002, 35, 10004–10013. [Google Scholar] [CrossRef]
- Cui, D.; Tang, T.; Bi, W.; Cheng, J.; Chen, W.; Huang, B. Ring-opening polymerization and block copolymerization of ʟ-lactide with divalent samarocene complex. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2667–2675. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Hadjichristidis, N. C1 polymerization: A unique tool towards polyethylene-based complex macromolecular architectures. Polym. Chem. 2017, 8, 4062–4073. [Google Scholar] [CrossRef] [Green Version]
- Ladelta, V.; Zapsas, G.; Abou-Hamad, E.; Gnanou, Y.; Hadjichristidis, N. Tetracrystalline tetrablock quarterpolymers: Four different crystallites under the same roof. Angew. Chem. Int. Ed. 2019, 58, 16267–16274. [Google Scholar] [CrossRef] [Green Version]
- Carmeli, E.; Wang, B.; Moretti, P.; Tranchida, D.; Cavallo, D. Estimating the nucleation ability of various surfaces towards isotactic polypropylene via light intensity induction time measurements. Entropy 2019, 21, 1068. [Google Scholar] [CrossRef] [Green Version]
- Hiemenz, P.C.; Lodge, T.P. Polymer Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Maglio, G.; Migliozzi, A.; Palumbo, R. Thermal properties of di- and triblock copolymers of poly (ʟ-lactide) with poly(oxyethylene) or poly (ɛ-caprolactone). Polymer 2003, 44, 369–375. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, J. Disorder-to-order phase transition and multiple melting behavior of poly(ʟ-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Ren, M.; Tang, Y.; Gao, D.; Ren, Y.; Yao, X.; Shi, H.; Zhang, T.; Wu, C. Recrystallization of biaxially oriented polyethylene film from partially melted state within crystallite networks. Polymer 2020, 191, 122291. [Google Scholar] [CrossRef]
- Izquierdo, R.; Garcia-Giralt, N.; Rodríguez, M.T.; Cáceres, E.; García, S.J.; Gómez Ribelles, J.M.; Monleón, M.; Monllau, J.C.; Suay, J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J. Biomed. Mater. Res. 2007, 85, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ebers, L.S.; Auvergne, R.; Boutevin, B.; Laborie, M.P. Impact of PEO structure and formulation on the properties of a Lignin/PEO blend. Ind. Crop. Prod. 2020, 143, 111883. [Google Scholar] [CrossRef]













| Sample | Mn PE (g/mol) | Mn PEO (g/mol) | Mn PCL (g/mol) | Ɖ a |
|---|---|---|---|---|
| PE7.1 | 7100 | - | - | 1.32 |
| PE327.1-b-PEO6815.1 | 7100 | 15,100 | - | <1.3 |
| PE227.1-b-PEO4615.1-b-PCL3210.4 (T1) | 7100 | 15,100 | 10,400 | <1.3 |
| PE9.5 | 9500 | - | - | 1.28 |
| PE529.5-b-PEO488.8 | 9500 | 8800 | - | <1.3 |
| PE379.5-b-PEO348.8-b-PCL297.6 (T2) | 9500 | 8800 | 7600 | <1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matxinandiarena, E.; Múgica, A.; Zubitur, M.; Ladelta, V.; Zapsas, G.; Cavallo, D.; Hadjichristidis, N.; Müller, A.J. Crystallization and Morphology of Triple Crystalline Polyethylene-b-poly(ethylene oxide)-b-poly(?-caprolactone) PE-b-PEO-b-PCL Triblock Terpolymers. Polymers 2021, 13, 3133. https://doi.org/10.3390/polym13183133
Matxinandiarena E, Múgica A, Zubitur M, Ladelta V, Zapsas G, Cavallo D, Hadjichristidis N, Müller AJ. Crystallization and Morphology of Triple Crystalline Polyethylene-b-poly(ethylene oxide)-b-poly(?-caprolactone) PE-b-PEO-b-PCL Triblock Terpolymers. Polymers. 2021; 13(18):3133. https://doi.org/10.3390/polym13183133
Chicago/Turabian StyleMatxinandiarena, Eider, Agurtzane Múgica, Manuela Zubitur, Viko Ladelta, George Zapsas, Dario Cavallo, Nikos Hadjichristidis, and Alejandro J. Müller. 2021. "Crystallization and Morphology of Triple Crystalline Polyethylene-b-poly(ethylene oxide)-b-poly(?-caprolactone) PE-b-PEO-b-PCL Triblock Terpolymers" Polymers 13, no. 18: 3133. https://doi.org/10.3390/polym13183133
APA StyleMatxinandiarena, E., Múgica, A., Zubitur, M., Ladelta, V., Zapsas, G., Cavallo, D., Hadjichristidis, N., & Müller, A. J. (2021). Crystallization and Morphology of Triple Crystalline Polyethylene-b-poly(ethylene oxide)-b-poly(?-caprolactone) PE-b-PEO-b-PCL Triblock Terpolymers. Polymers, 13(18), 3133. https://doi.org/10.3390/polym13183133