Experimental Design in Polymer Chemistry—A Guide towards True Optimization of a RAFT Polymerization Using Design of Experiments (DoE)
Abstract
:1. Introduction
- Can we effectively spot the optimal setting (or level in DoE terminology) of each factor without innumerable experiments?
- Can we just assume that an optimal factor level stays steadfast when another factor is varied?
2. Materials and Methods
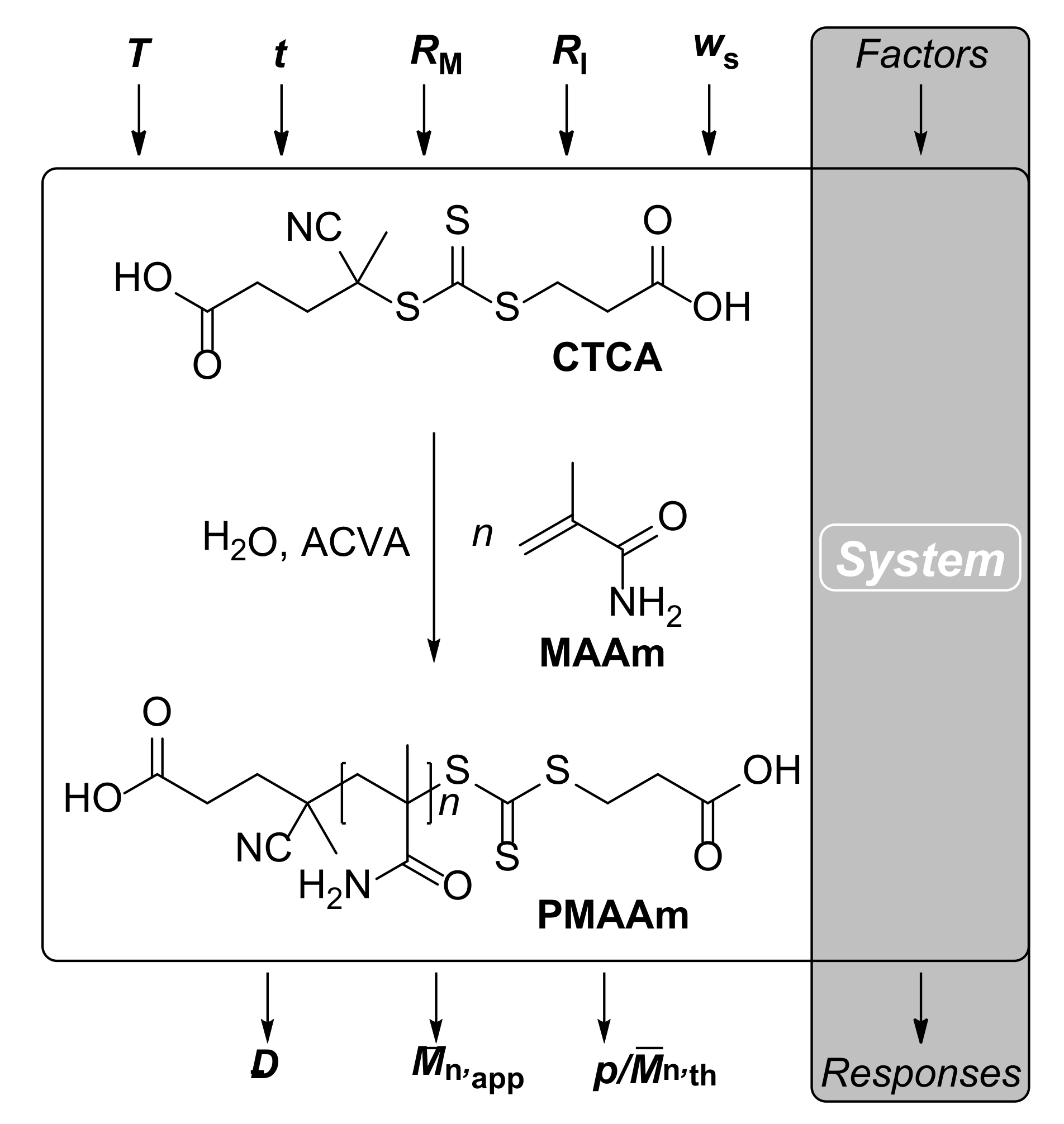
2.1. Materials
2.1.1. RAFT Polymerization of MAAm
2.2. Analytics
2.2.1. Nuclear Magnetic Resonance Spectroscopy
2.2.2. Size Exclusion Chromatography
3. Results and Discussion
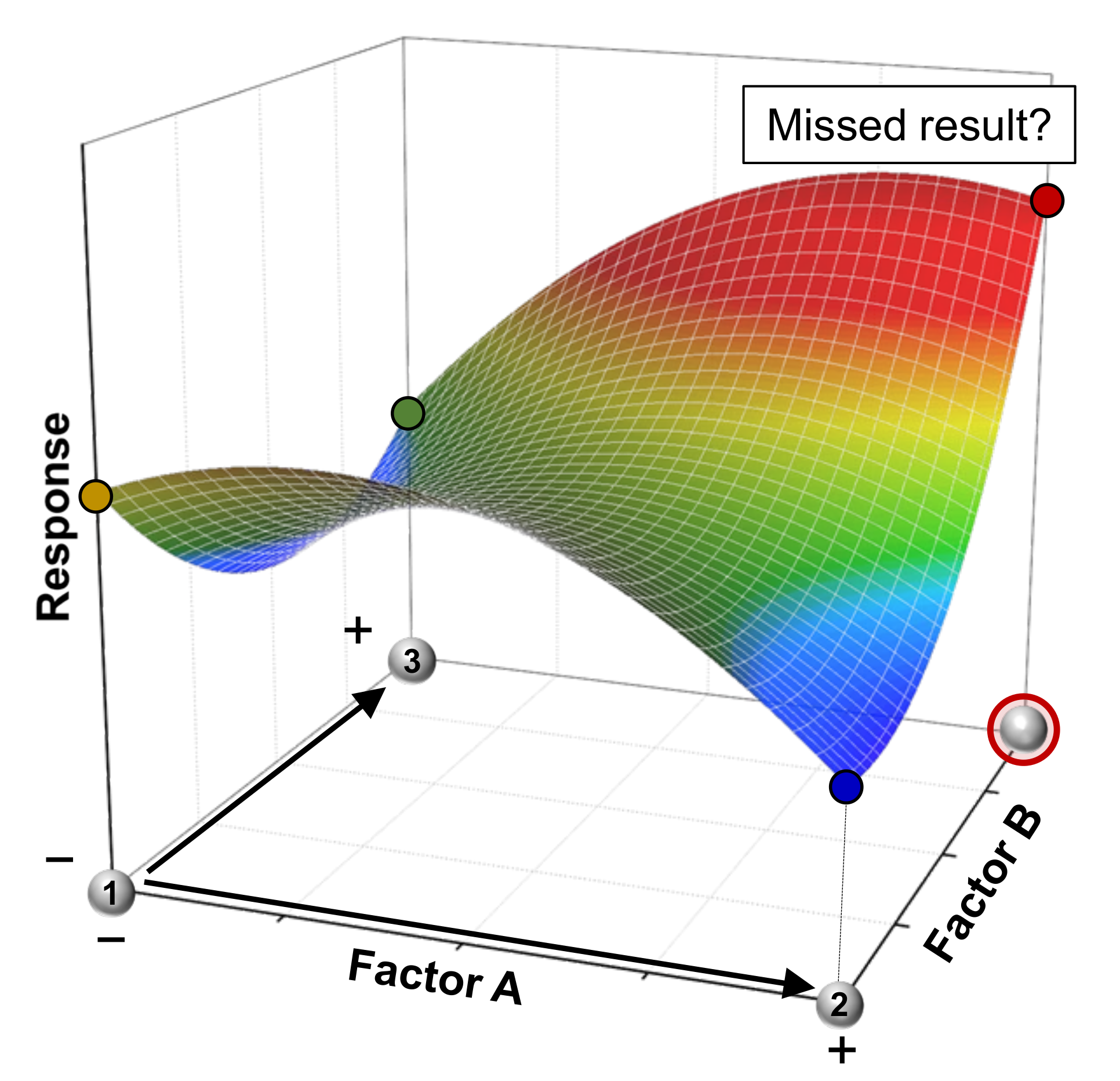
3.1. Screening: Finding the Significant Factors
3.2. Setting Factor Levels and Choosing the Design
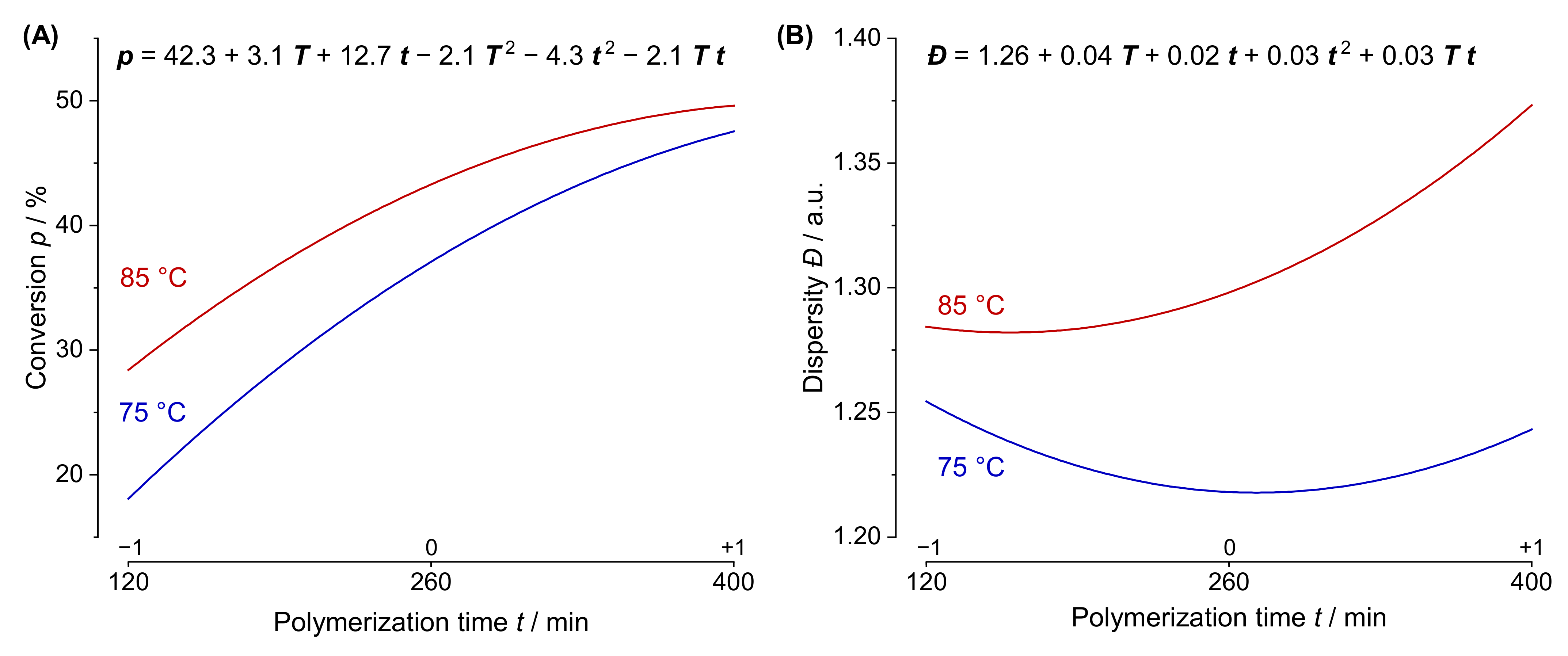
3.3. Response Surface Methodology: Generation and Interpretation of Prediction Models
3.4. True Optimization
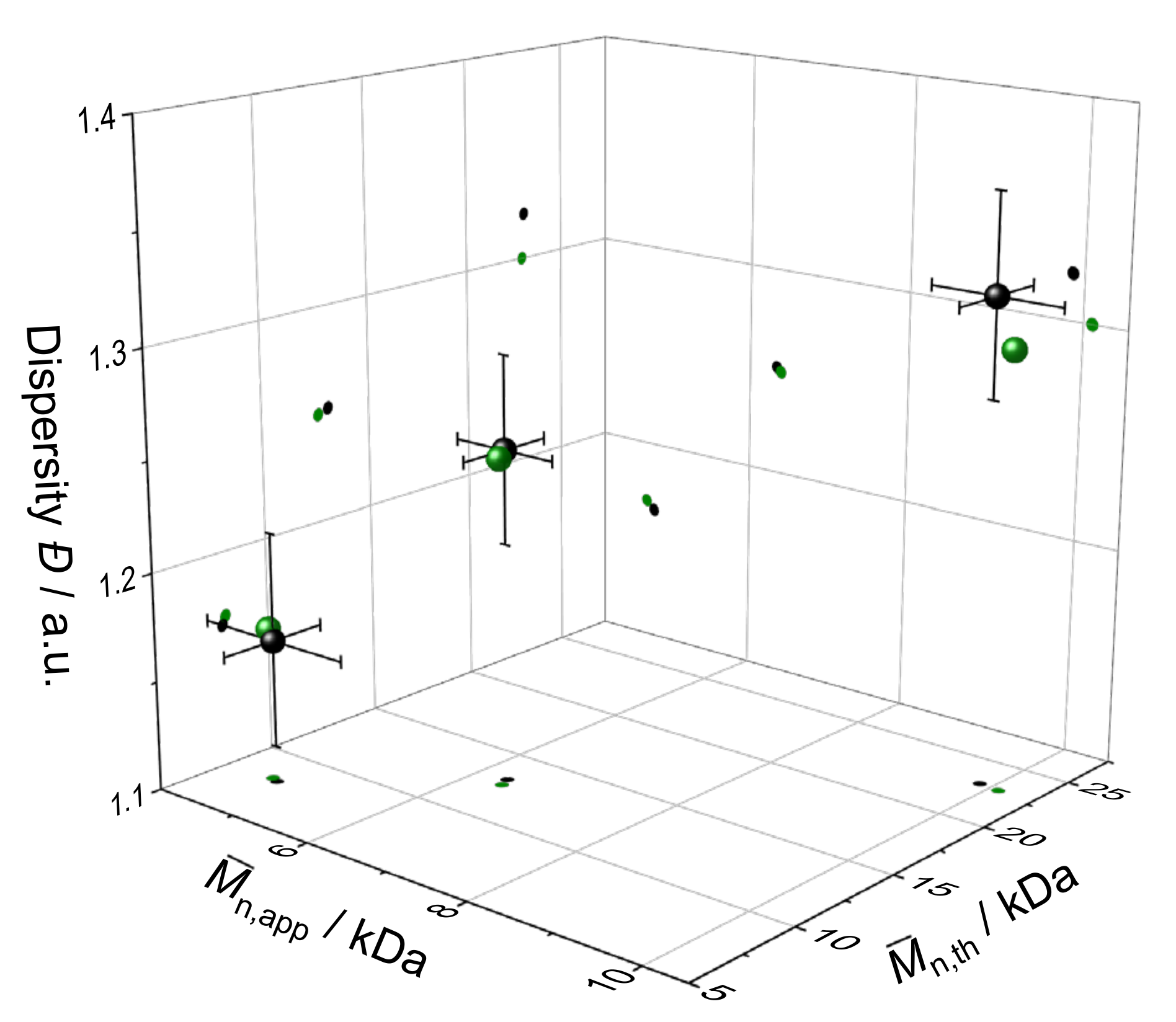
3.5. Model Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente, G.; Coteron, A.; Martinez, M.; Aracil, J. Application of the factorial design of experiments and response surface methodology to optimize biodiesel production. Ind. Crop. Prod. 1998, 8, 29–35. [Google Scholar] [CrossRef]
- Sakkas, V.A.; Islam, M.A.; Stalikas, C.; Albanis, T.A. Photocatalytic degradation using design of experiments: A review and example of the Congo red degradation. J. Hazard. Mater. 2010, 175, 33–44. [Google Scholar] [CrossRef]
- Selekman, J.A.; Qiu, J.; Tran, K.; Stevens, J.; Rosso, V.; Simmons, E.; Xiao, Y.; Janey, J. High-throughput automation in chemical process development. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Ilzarbe, L.; Viles, E.; Álvarez, M.J.; Tanco, M. Practical applications of design of experiments in the field of engineering: A bibliographical review. Qual. Reliab. Eng. Int. 2008, 24, 417–428. [Google Scholar] [CrossRef]
- Grant, C.; da Silva Damas Pinto, A.C.; Lui, H.P.; Woodley, J.M.; Baganz, F. Tools for characterizing the whole-cell bio-oxidation of alkanes at microscale. Biotechnol. Bioeng. 2012, 109, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Shear, T.A.; Lin, F.; Zakharov, L.N.; Johnson, D.W. “Design of Experiments” as a method to optimize dynamic disulfide assemblies: Cages and functionalizable macrocycles. Angew. Chem.—Int. Ed. 2020, 59, 1496–1500. [Google Scholar] [CrossRef]
- Domagalski, N.R.; Mack, B.C.; Tabora, J.E. Analysis of design of experiments with dynamic responses. Org. Process Res. Dev. 2015, 19, 1667–1682. [Google Scholar] [CrossRef]
- Lendrem, D.; Owen, M.; Godbert, S. DOE (design of experiments) in development chemistry: Potential obstacles. Org. Process Res. Dev. 2001, 5, 324–327. [Google Scholar] [CrossRef]
- Weissman, S.A.; Anderson, N.G. Design of Experiments (DoE) and process optimization. A review of recent publications. Org. Process Res. Dev. 2015, 19, 1605–1633. [Google Scholar] [CrossRef]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Tian, X.; Ding, J.; Zhang, B.; Qiu, F.; Zhuang, X.; Chen, Y. Recent advances in RAFT polymerization: Novel initiation mechanisms and optoelectronic applications. Polymers 2018, 10, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrier, S. 50th anniversary perspective: RAFT polymerization—A user guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process—A third update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Semsarilar, M.; Abetz, V. Polymerizations by RAFT: Developments of the technique and its application in the synthesis of tailored (co)polymers. Macromol. Chem. Phys. 2021, 222, 2000311. [Google Scholar] [CrossRef]
- Skandalis, A.; Sentoukas, T.; Giaouzi, D.; Kafetzi, M.; Pispas, S. Latest advances on the synthesis of linear abc-type triblock terpolymers and star-shaped polymers by raft polymerization. Polymers 2021, 13, 1698. [Google Scholar] [CrossRef]
- Eggers, S.; Abetz, V. Surfactant-Free RAFT emulsion polymerization of styrene using thermoresponsive macroRAFT agents: Towards smartwell-defined block copolymers with high molecular weights. Polymers 2017, 9, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebeling, B.; Vana, P. Multiblock copolymers of styrene and butyl acrylate via polytrithiocarbonate-mediated RAFT polymerization. Polymers 2011, 3, 719–739. [Google Scholar] [CrossRef]
- Förster, N.; Schmidt, S.; Vana, P. Tailoring confinement: Nano-carrier synthesis via Z-RAFT star polymerization. Polymers 2015, 7, 695–716. [Google Scholar] [CrossRef] [Green Version]
- Farooq, U.; Upadhyaya, L.; Shakeel, A.; Martinez, G.; Semsarilar, M. pH-responsive nano-structured membranes prepared from oppositely charged block copolymer nanoparticles and iron oxide nanoparticles. J. Memb. Sci. 2020, 611, 118181. [Google Scholar] [CrossRef]
- Oral, I.; Abetz, V. A Highly selective polymer material using Benzo-9-Crown-3 for the extraction of lithium in presence of other interfering alkali metal ions. Macromol. Rapid Commun. 2021, 42, 2000746. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.; Desnos, G.; Semsarilar, M. Nanostructured membranes from soft and hard nanoparticles prepared via RAFT-mediated PISA. Macromol. Chem. Phys. 2018, 219, 1800351. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Thermo-sensitive polymeric micelles based on poly(N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: Applications and recent advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef] [Green Version]
- Eckert, T.; Abetz, V. Polymethacrylamide—An underrated and easily accessible upper critical solution temperature polymer: Green synthesis via photoiniferter reversible addition–fragmentation chain transfer polymerization and analysis of solution behavior in water/ethanol mixtu. J. Polym. Sci. 2020, 58, 3050–3060. [Google Scholar] [CrossRef]
- Xu, J.; Abetz, V. Nonionic UCST–LCST Diblock copolymers with tunable thermoresponsiveness synthesized via PhotoRAFT polymerization. Macromol. Rapid Commun. 2021, 42, 2000648. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, L.; Ying, X.; Jian, Y.; Hong, Y.; Hu, F.; Du, Y. Antitumor drug delivery modulated by a polymeric micelle with an upper critical solution temperature. Angew. Chem. 2015, 127, 3169–3174. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Seuring, J. Polymers with upper critical solution temperature in aqueous solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Destarac, M. Industrial development of reversible-deactivation radical polymerization: Is the induction period over? Polym. Chem. 2018, 9, 4947–4967. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Guan, C.M.; Luo, Z.H. Kinetic modeling of two-step RAFT process for the production of novel fluorosilicone triblock copolymers. Eur. Polym. J. 2010, 46, 2164–2173. [Google Scholar] [CrossRef]
- Lathrop, P.M.; Duan, Z.; Ling, C.; Elabd, Y.A.; Kravaris, C. Modeling and observer-based monitoring of RAFT homopolymerization reactions. Processes 2019, 7, 768. [Google Scholar] [CrossRef] [Green Version]
- Kandelhard, F.; Schuldt, K.; Schymura, J.; Georgopanos, P.; Abetz, V. Model-assisted optimization of RAFT polymerization in micro-scale reactors—A fast screening approach. Macromol. React. Eng. 2021, 15, 2000058. [Google Scholar] [CrossRef]
- Werner, M.; Oliveira, J.C.A.; Meiser, W.; Buback, M.; Mata, R.A. Critical assessment of RAFT equilibrium constants: Theory meets experiment. Macromol. Theory Simul. 2020, 29, 2000022. [Google Scholar] [CrossRef]
- Mastan, E.; Li, X.; Zhu, S. Modeling and theoretical development in controlled radical polymerization. Prog. Polym. Sci. 2015, 45, 71–101. [Google Scholar] [CrossRef]
- Klumperman, B.; Van Den Dungen, E.T.A.; Heuts, J.P.A.; Monteiro, M.J. RAFT-mediated polymerization—A story of incompatible data? Macromol. Rapid Commun. 2010, 31, 1846–1862. [Google Scholar] [CrossRef]
- Destarac, M. On the critical role of RAFT agent design in reversible addition-fragmentation chain transfer (RAFT) polymerization. Polym. Rev. 2011, 51, 163–187. [Google Scholar] [CrossRef]
- Moad, G. A Critical survey of dithiocarbamate Reversible Addition-Fragmentation Chain Transfer (RAFT) agents in radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Favier, A.; Charreyre, M.T. Experimental requirements for an efficient control of free-radical polymerizations via the reversible addition-fragmentation chain transfer (RAFT) process. Macromol. Rapid Commun. 2006, 27, 653–692. [Google Scholar] [CrossRef]
- Santos, C.P.; Rato, T.J.; Reis, M.S. Design of experiments: A comparison study from the non-expert user’s perspective. J. Chemom. 2019, 33, e3087. [Google Scholar] [CrossRef] [Green Version]
- Russell, G.T. The kinetics of free-radical polymerization: Fundamental aspects. Aust. J. Chem. 2002, 55, 399–414. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Postma, A.; Moad, G. Kinetics and mechanism for thermal and photochemical decomposition of 4,4′-azobis(4-cyanopentanoic acid) in aqueous media. Polym. Chem. 2019, 10, 3284–3287. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Lodge, T.P. Polymer Chemistry; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Xu, J.; He, J. Thermal decomposition of dithioesters and its effect on RAFT polymerization. Macromolecules 2006, 39, 3753–3759. [Google Scholar] [CrossRef]
- Bekanova, M.Z.; Neumolotov, N.K.; Jablanović, A.D.; Plutalova, A.V.; Chernikova, E.V.; Kudryavtsev, Y.V. Thermal stability of RAFT-based poly(methyl methacrylate): A kinetic study of the dithiobenzoate and trithiocarbonate end-group effect. Polym. Degrad. Stab. 2019, 164, 18–27. [Google Scholar] [CrossRef]
- Boyer, C.; Liu, J.Q.; Wong, L.J.; Tippett, M.; Bulmus, V.; Davis, T.P. Stability and utility of pyridyl disulfide functionality in RAFT and conventional radical polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]
- Baroutaji, A.; Gilchrist, M.D.; Smyth, D.; Olabi, A.G. Crush analysis and multi-objective optimization design for circular tube under quasi-static lateral loading. Thin-Walled Struct. 2015, 86, 121–131. [Google Scholar] [CrossRef] [Green Version]
- King, C.; Jones, B.; Morgan, J.; Lekivetz, R. Direct construction of globally D-optimal designs for factors at two levels and main effects models. Qual. Reliab. Eng. Int. 2020, 36, 797–816. [Google Scholar] [CrossRef]
- Özdemir, A. D-optimal experimental design for production models in nonstandard experiments. Qual. Reliab. Eng. Int. 2020, 36, 1537–1552. [Google Scholar] [CrossRef]
- Mancenido, M.V.; Pan, R.; Montgomery, D.C.; Anderson-Cook, C.M. Comparing D-optimal designs with common mixture experimental designs for logistic regression. Chemom. Intell. Lab. Syst. 2019, 187, 11–18. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. DOE Simplified, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781498730907. [Google Scholar]
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9788578110796. [Google Scholar]
| T/°C | t/min | RM/a.u. | RI/a.u. | ws/a.u. | |
|---|---|---|---|---|---|
| − | 75 | 120 | 200 | 0.025 | 10.0 |
| 0 | 80 | 260 | 350 | 0.0625 | 15.0 |
| + | 85 | 400 | 500 | 0.1 | 20.0 |
| Goal | pa/% | Đb/a.u. | ||
|---|---|---|---|---|
| 1 | 8.0 | 45.2 | 4.70 | minimize |
| 2 | 13.1 | 57.5 | 6.35 | 1.25 |
| 3 | 23.0 | 58.8 | 9.87 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, T.; Klein, F.C.; Frieler, P.; Thunich, O.; Abetz, V. Experimental Design in Polymer Chemistry—A Guide towards True Optimization of a RAFT Polymerization Using Design of Experiments (DoE). Polymers 2021, 13, 3147. https://doi.org/10.3390/polym13183147
Eckert T, Klein FC, Frieler P, Thunich O, Abetz V. Experimental Design in Polymer Chemistry—A Guide towards True Optimization of a RAFT Polymerization Using Design of Experiments (DoE). Polymers. 2021; 13(18):3147. https://doi.org/10.3390/polym13183147
Chicago/Turabian StyleEckert, Tilman, Florian C. Klein, Piet Frieler, Oliver Thunich, and Volker Abetz. 2021. "Experimental Design in Polymer Chemistry—A Guide towards True Optimization of a RAFT Polymerization Using Design of Experiments (DoE)" Polymers 13, no. 18: 3147. https://doi.org/10.3390/polym13183147







