Gellan Gum Hydrogels Filled Edible Oil Microemulsion for Biomedical Materials: Phase Diagram, Mechanical Behavior, and In Vivo Studies
Abstract
:1. Introduction
2. Materials and Chemicals
2.1. Materials
2.2. Construction of Phase Diagrams
2.3. Preparation of GVCO Hydrogel
2.4. Characterisation of the Sample
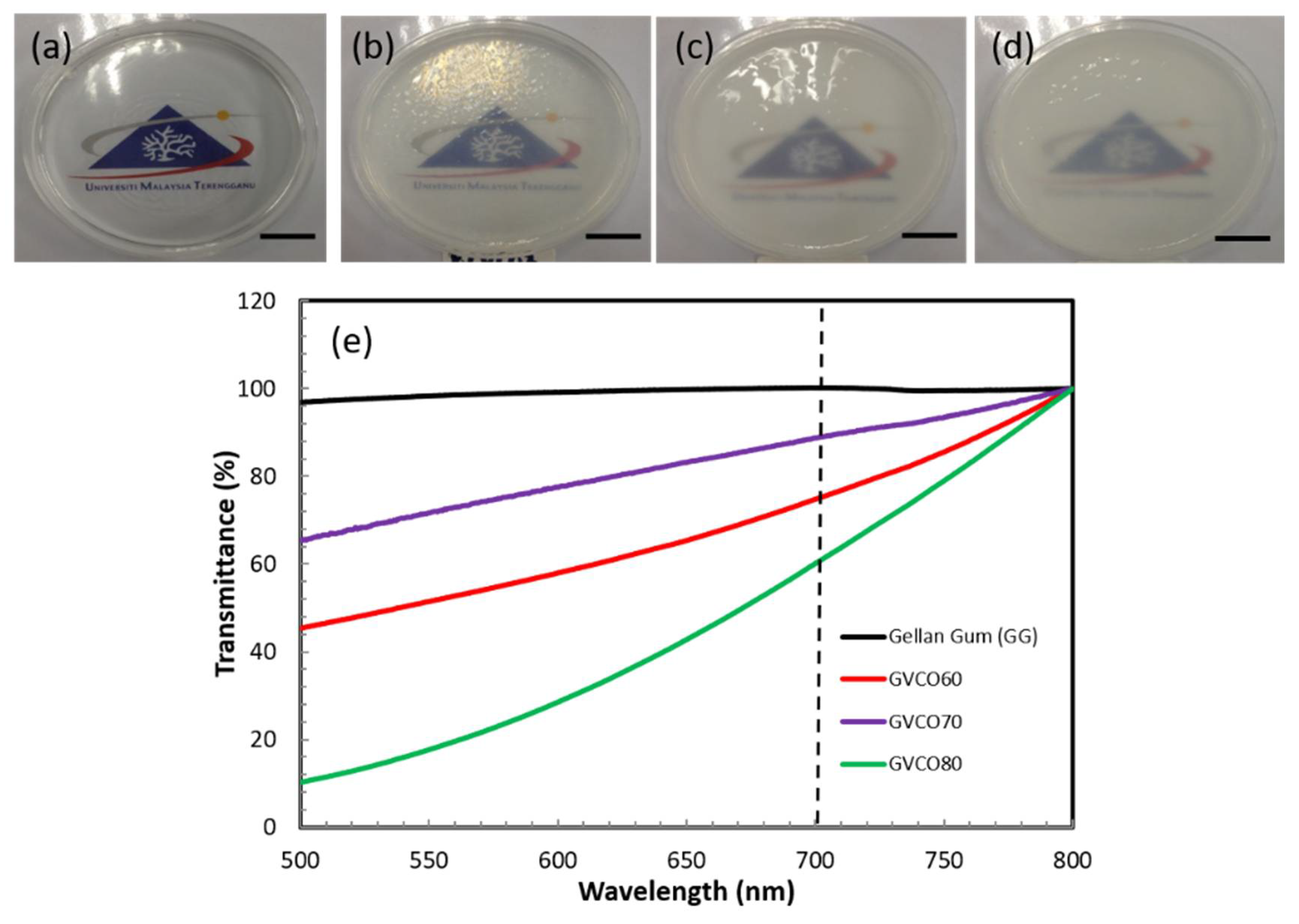
2.4.1. Ultraviolet–Visible Spectroscopy
2.4.2. ATR–FTIR Spectroscopy
2.4.3. Mechanical Properties
2.4.4. Swelling Properties
2.4.5. Water Vapor Transmission Rate (WVTR)
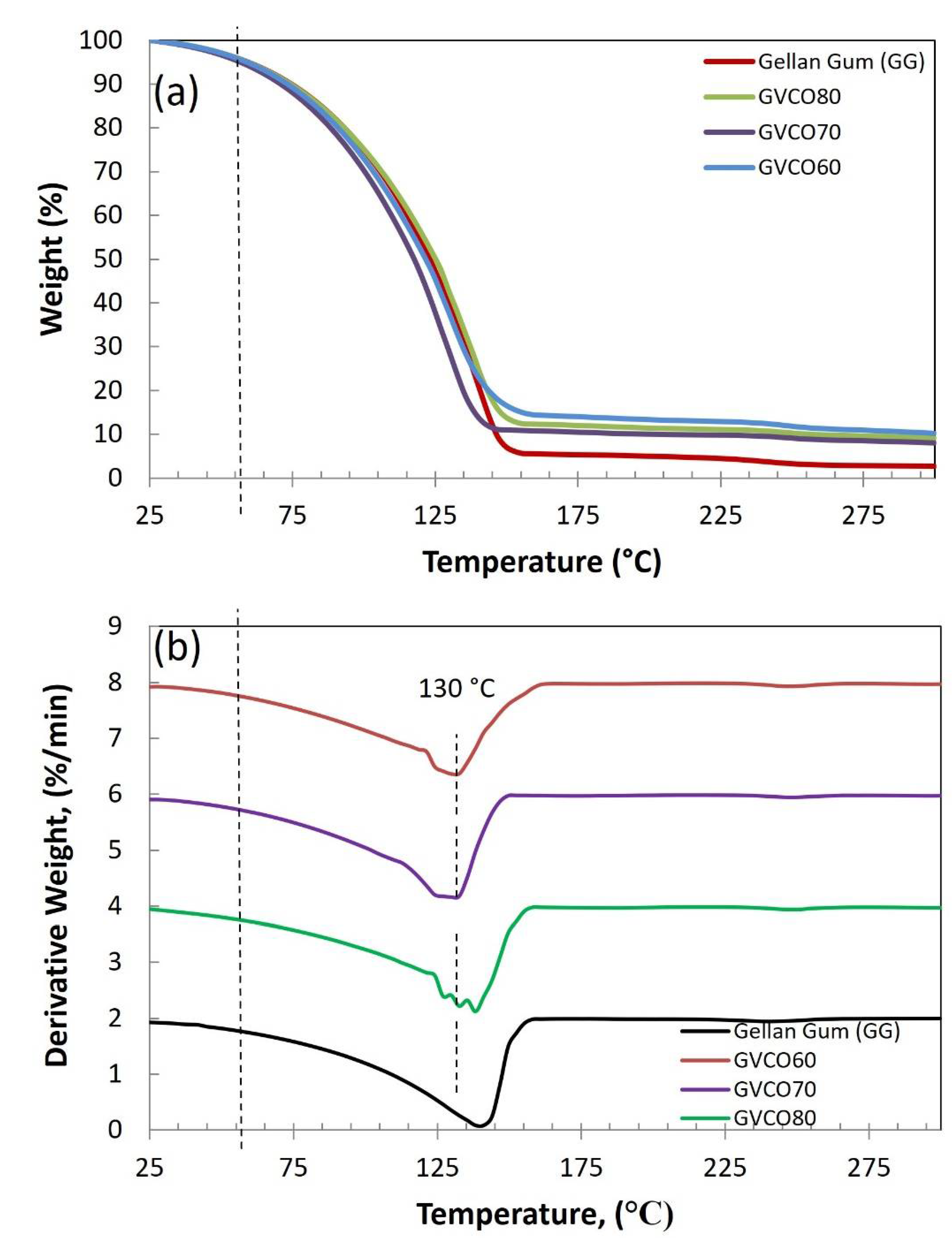
2.4.6. Thermogravimetric Analysis
2.4.7. Scanning Electron Microscopy (SEM)
2.5. Antibacterial Study
2.5.1. Culture Conditions
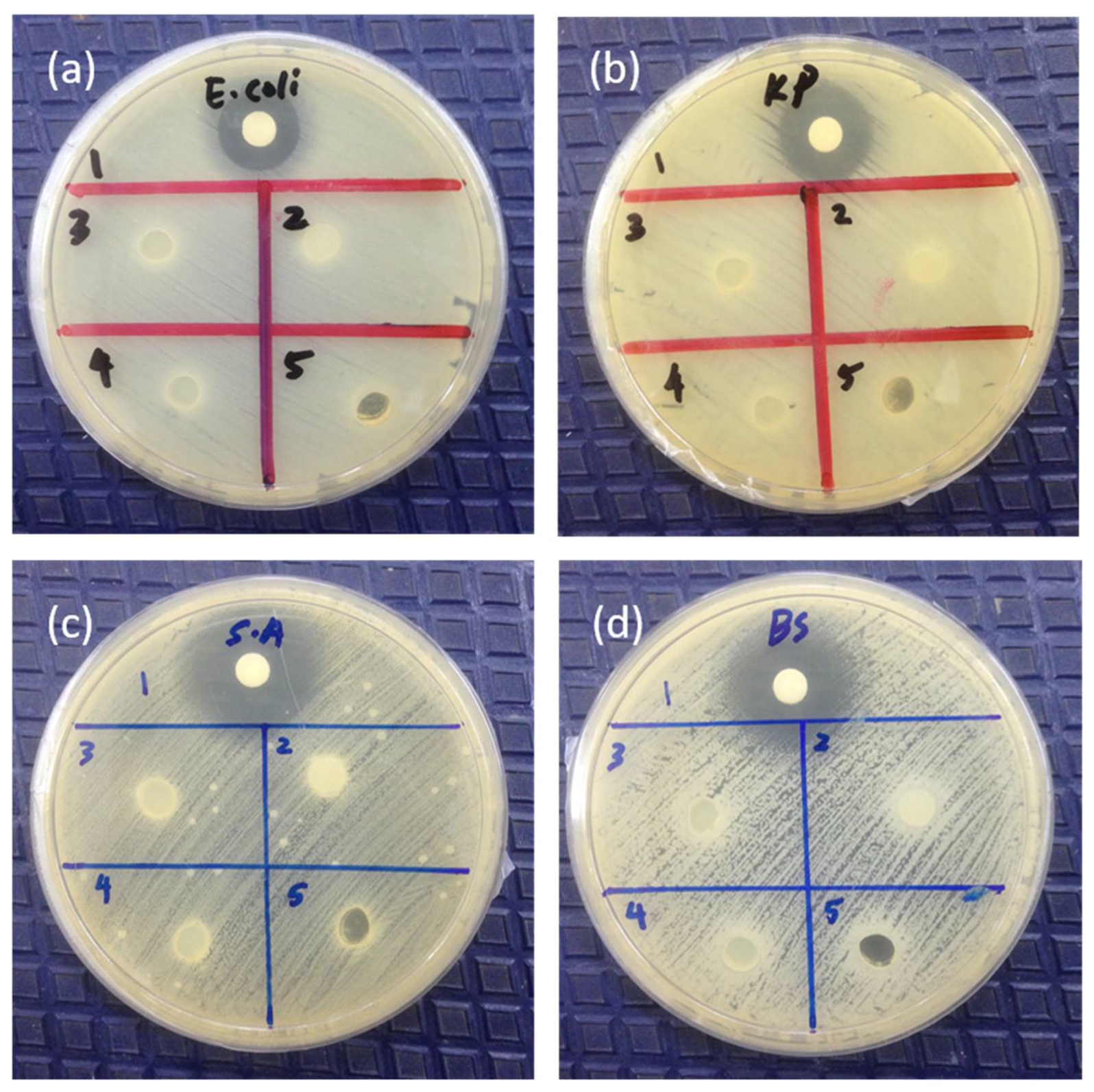
2.5.2. Qualitative Study
2.6. In Vivo Studies
2.6.1. In Vivo Wound Healing Experiments
Animals
Establishment of Wound Skin
Treatment of Hydrogel
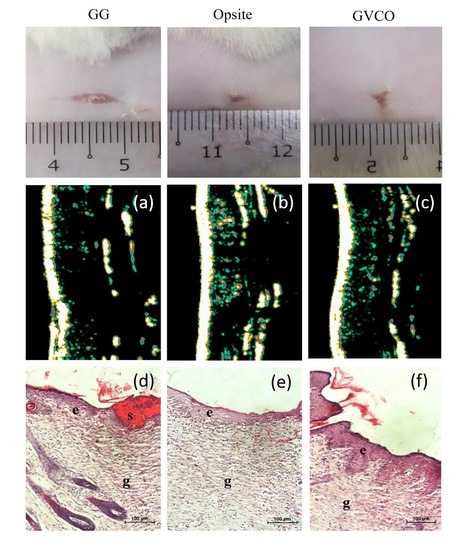
Macroscopic Observation of Wound
2.6.2. Ultrasound Imaging
2.6.3. Histological Examination
Statistical Analysis
3. Results and Discussion
3.1. Formulation of Stabilizing VCO Microemulsion
3.2. Physical Properties of the Hydrogels
3.3. Fourier Transform Infrared Spectroscopy (ATR–FTIR)
3.4. Mechanical Performances
3.5. Swelling Ratio, and Water Vapor Transmission Rate (WVTR)
3.6. Thermal Analysis
3.7. Antibacterial Studies
3.8. In Vivo Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, C.J.; Humphreys, I.; Fletcher, J.; Harding, K.; Chamberlain, G.; Macey, S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int. Wound J. 2016, 13, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Management of diabetic foot ulcers: A review. Fed. Pract. 2016, 33, 16. [Google Scholar] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. Pharm. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Rep. Reg. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Augustine, R.; Hasan, A. Emerging applications of biocompatible phytosynthesized metal/metal oxide nanoparticles in healthcare. J. Drug Deliv. Sci. Technol. 2020, 56, 101516. [Google Scholar] [CrossRef]
- Mohd, S.S.; Abdullah, M.A.A.; Mat Amin, K.A. Gellan gum/clay hydrogels for tissue engineering application: Mechanical, thermal behavior, cell viability, and antibacterial properties. J. Bioact. Compat. Polym. 2016, 31, 648–666. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Sebria, N.J.M.; Amin, K.A.M. Gellan Gum/Ibuprofen Hydrogel for Dressing Application: Mechanical Properties, Release Activity and Biocompatibility Studies. Int. J. Appl. Chem. 2016, 12, 483–498. [Google Scholar]
- Picone, C.S.F.; Cunha, R.L. Influence of pH on formation and properties of gellan gels. Carbohydr. Polym. 2011, 84, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Karvinen, J.; Koivisto, J.T.; Jönkkäri, I.; Kellomäki, M. The production of injectable hydrazone crosslinked gellan gum-hyaluronan-hydrogels with tunable mechanical and physical properties. J. Mech. Behav. Biomed. Mater. 2017, 71, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, J.; Shin, M.E.; Been, S.; Lee, D.H.; Khang, G. Eggshell Membrane/Gellan Gum Composite Hydrogels with Increased Degradability, Biocompatibility, and Anti-Swelling Properties for Effective Regeneration of Retinal Pigment Epithelium. Polymers 2020, 12, 2941. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.G.; Lee, H.; Cho, D.W. 3D printing of organs-on-chips. Bioengineering 2017, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.M.; in het Panhuis, M. Polyelectrolyte complex materials from chitosan and gellan gum. Carbohydr. Polym. 2011, 86, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.V.M.; Stringhetti, F.C.B.; Evangelista, R.C.; Daflon, G.M.P. Development and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 317–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Ferreiro, A.; Barcia, M.G.; Gil-Martínez, M.; Vieites-Prado, A.; Lema, L.; Argibay, B.; Méndez, J.B.; Lamas, M.J.; Otero-Espinar, F.J. In vitro and in vivo ocular safety and eye surface permanence determination by direct and Magnetic Resonance Imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. Eur. J. Pharm. Biopharm. 2015, 94, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.H.; Ismail, N.A.; Amin, K.A.M. Titanium Dioxide Nanotubes Incorporated Gellan Gum Bio-Nanocomposite Film for Wound Healing: Effect of TiO2 Nanotubes Concentration. Int. J. Biol. Macromol. 2020, 153, 1117–1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, Y.; Lin, Y.; Shen, J.B.; Wang, D.A. A novel gellan gel-based microcarrier for anchorage-dependent cell delivery. Acta Biomater. 2008, 4, 1226–1234. [Google Scholar] [CrossRef]
- Vilela, C.A.; Correia, C.; Morais, A.D.S.; Santos, T.C.; Gertrudes, A.C.; Moreira, E.S.; Frias, A.M.; Learmonth, D.A.; Oliveira, P.; Oliveira, J.M.; et al. In vitro and in vivo performance of methacrylated gellan gum hydrogel formulations for cartilage repair. J. Biomed. Mater. Res. Part A 2018, 106, 1987–1996. [Google Scholar] [CrossRef] [Green Version]
- Nevin, K.G.; Rajamohan, T. Effect of Topical Application of Virgin Coconut Oil on Skin Components and Antioxidant Status during Dermal Wound Healing in Young Rats. Skin Pharmacol. Physiol. 2010, 23, 290–297. [Google Scholar] [CrossRef]
- Intahphuak, S.; Khonsung, P.; Panthong, A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm. Biol. 2010, 48, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, H.; Khan, I.U.; Asif, M.; Khan, R.U.; Asghar, S.; Khalid, L.; Khalid, S.H.; Irfan, M.; Rehman, F.; Shahzad, Y.; et al. In vitro and in vivo evaluation of gellan gum hydrogel films: Assessing the co impact of therapeutic oils and ofloxacin on wound healing. Int. J. Biol. Macromol. 2021, 166, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.M.; Barbosa, W.S.; Rangel, W.S.P.; Valle, I.M.M.; Matos, A.P.D.S.; Melgaço, F.G.; Dias, M.L.; Júnior, E.R.; Silva, L.C.P.D.; Abreu, L.C.L.D.; et al. Polymeric membrane based on polyactic acid and babassu oil for wound healing. Mater. Today Commun. 2021, 26, 102173. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Gu, J.; Wang, X.; Zhang, Y.J.; Zheng, W.; Chen, R.; Wang, X.C. Effects of rhamnolipid and Tween-80 on cellulase activities and metabolic functions of the bacterial community during chicken manure composting. Bioresour. Technol. 2019, 288, 121507. [Google Scholar] [CrossRef] [PubMed]
- Amit; Jamwal, R.; Kumari, S.; Dhaulaniya, A.S.; Balanet, B.; Kelly, S.; Cannavanal, A.; Singh, D.K. Utilizing ATR-FTIR spectroscopy combined with multivariate chemometric modelling for the swift detection of mustard oil adulteration in virgin coconut oil. Vib. Spectrosc. 2020, 109, 103066. [Google Scholar] [CrossRef]
- Rohman, A.; Cheman, Y.B.I.N.; Ali, M.D.E. The authentication of virgin coconut oil from grape seed oil and soybean oil using ftir spectroscopy and chemometrics. Int. J. Appl. Pharm. 2019, 11, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.A.; Amin, K.A.M.; Razali, M.H. Novel gellan gum incorporated TiO2 nanotubes film for skin tissue engineering. Mater. Lett. 2018, 228, 116–120. [Google Scholar] [CrossRef]
- Ismail, N.A.; Mohamad, S.F.; Ibrahim, M.A.; Amin, K.A.M. Evaluation of gellan gum film containing virgin coconut oil for transparent dressing materials. Adv. Biomater. 2014, 2014, 351248. [Google Scholar] [CrossRef]
- Amin, K.A.M.; Gilmore, K.J.; Walker, M.J.; Wilson, M.R.; Panhuis, M.I.H. Polyelectrolyte complex materials consisting of antibacterial and cell-supporting layers. Macromol. Biosci. 2012, 12, 374–382. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [Green Version]
- Souza, F.S.D.; Ferreira, I.L.D.M.; Costa, M.A.D.S.; Costa, M.P.M.; Silva, G.M.D. Effect of pH variation and crosslinker absence on the gelling mechanism of high acyl gellan: Morphological, thermal and mechanical approaches. Carbohydr. Polym. 2021, 251, 117002. [Google Scholar] [CrossRef] [PubMed]
- Nitta, Y. Gelation and gel properties of gellan gum and xyloglucan. J. Biol. Macromol. 2005, 5, 47–52. [Google Scholar]
- Wu, P.; Fisher, A.C.; Foo, P.P.; Queen, D.; Gaylor, J.D.S. In vitro assessment of water vapour transmission of synthetic wound dressings. Biomaterials 1995, 16, 171–175. [Google Scholar] [CrossRef]
- Chong, E.W.N.; Jafarzadeh, S.; Paridah, M.T.; Gopakumar, D.A.; Tajarudin, H.A.; Thomas, S.; Abdul Khalil, H.P.S. Enhancement in the Physico-Mechanical Functions of Seaweed Biopolymer Film via Embedding Fillers for Plasticulture Application—A Comparison with Conventional Biodegradable Mulch Film. Polymers 2019, 11, 210. [Google Scholar]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Preparation and characterization of cassava bagasse reinforced thermoplastic cassava starch. Fibers Polym. 2017, 18, 162–171. [Google Scholar] [CrossRef]
- Fangfang, Z.; Xinpeng, B.; Wei, G.; Wang, G.D.; Shi, Z.Z.; Jun, G. Effects of virgin coconut oil on the physicochemical, morphological and antibacterial properties of potato starch-based biodegradable films. Int. J. Food Sci. Technol. 2020, 55, 192–200. [Google Scholar] [CrossRef]
- Hussain, M.S.; Al-Alaq, F.T.; Al-Khafaji, N.S.; Al-Dahmoshi, H.O.M. Antibacterial Effect of Virgin and Refined Coconut Oils on Pathogenic Bacteria: A Review. Indian J. Forensic Med. Toxicol. 2020, 14, 6042–6048. [Google Scholar]
- Nasir, N.A.M.M.; Abllah, Z.; Jalaludin, A.A.; Shahdan, I.A.; Abd Manan, W.N.H.W. Virgin Coconut Oil and Its Antimicrobial Properties against Pathogenic Microorganisms: A Review. In Proceedings of the International Dental Conference of Sumatera Utara 2017 (IDCSU 2017); Atlantis Press: Berlin/Heidelberg, Germany, 2018; pp. 192–199. [Google Scholar]
- Nair, M.K.M.; Vasudevan, P.; Hoagland, T.; Venkitanarayanan, K. Inactivation of Escherichia coli O157: H7 and Listeria monocytogenes in milk by caprylic acid and monocaprylin. Food Microbiol. 2004, 21, 611–616. [Google Scholar] [CrossRef]
- Shilling, M.; Matt, L.; Rubin, E.; Visitacion, M.P.; Haller, N.A.; Grey, S.F.; Woolverton, C.J. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food 2013, 16, 1079–1085. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kima, J.T. Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. Lwt 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.L.; Cheng, B.; Mo, X.M.; Chen, C.; Pan, J.F. Moist-retaining, self-recoverable, bioadhesive, and transparent in situ forming hydrogels to accelerate wound healing. ACS Appl. Mater. Interfaces 2020, 12, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Gani, A.; Benjakul, S. Effect of antioxidants in combination of VCO nanoemulsion on gel properties and storage stability of refrigerated sardine surimi gel. Int. J. Food Sci. Technol. 2020, 55, 2451–2461. [Google Scholar] [CrossRef]
- Soliman, A.M.; Lin, T.S.; Ghafar, N.A.; Das, S. Virgin Coconut Oil and Diabetic Wound Healing: Histopathological and Biochemical Analysis. Eur. J. Anat. 2018, 22, 135–144. [Google Scholar]
- Andriana, N.; Lister, I.N.E.; Fachrial, E.; Ginting, C.N.; Lie, S. Effectiveness Test of Wound Healing Based Virgin Coconut Oil toward Commercial Products on Rabbits. In Proceedings of the 2020 3rd International Conference on Mechanical, Electronics, Computer, and Industrial Technology (MECnIT), Medan, Indonesia, 25–26 June 2020; pp. 104–107. [Google Scholar]
- Yuniati, R.; Subchan, P.; Riawan, W.; Khrisna, M.B.; Restiwijaya, M.; Dyan, N.S.; Nur, M. Topical Ozonated Virgin Coconut Oil Improves Wound Healing and Increases HSP90α, VEGF-A, EGF, BFGF and CD34 in Diabetic Ulcer Mouse Model of Wound Healing. F1000Research 2020, 9, 580. [Google Scholar] [CrossRef]
- Rajagukguk, H.; Syukur, S.; Ibrahim, S.; Syafrizayanti, S. Beneficial Effect of Application of Virgin Coconut Oil (VCO) Product from Padang West Sumatra, Indonesia on Palatoplasty Wound Healing. Am. Sci. Res. J. Eng. Technol. Sci. (ASRJETS) 2017, 34, 231–236. [Google Scholar]
- Ibrahim, A.H.; Li, H.; Al-Rawi, S.S.; Majid, A.S.A.; Al-Habib, O.A.; Xia, X.B.; Majid, A.M.A.; Ji, D. Angiogenic and wound healing potency of fermented virgin coconut oil: In vitro and in vivo studies. Am. J. Transl. Res. 2017, 9, 4936. [Google Scholar]





| Method I | Method II | |||||||
|---|---|---|---|---|---|---|---|---|
| Ratio VCO: Water | VCO (%) | Water (%) | Tween 80 (%) | Total (%) | VCO (%) | Water (%) | TritonX-10 (%) | Total (%) |
| 90:10 | 27.45 | 3.06 | 69.49 | 100 | 12.54 | 1.39 | 86.07 | 100 |
| 80:20 | 35.52 | 8.88 | 55.59 | 100 | 13.41 | 3.35 | 83.24 | 100 |
| 70:30 | 23.98 | 10.29 | 65.73 | 100 | 11.46 | 4.91 | 83.63 | 100 |
| 60:40 | 21.38 | 14.25 | 64.37 | 100 | 12.23 | 8.15 | 79.62 | 100 |
| 50:50 | 16.43 | 16.43 | 67.14 | 100 | 9.66 | 9.66 | 80.68 | 100 |
| 40:60 | 17.27 | 25.91 | 56.84 | 100 | 7.82 | 11.74 | 80.44 | 100 |
| 30:70 | 9.15 | 21.35 | 69.50 | 100 | 6.57 | 15.34 | 78.09 | 100 |
| 20:80 | 6.96 | 27.86 | 65.18 | 100 | 3.88 | 15.55 | 80.57 | 100 |
| 10:90 | 4.03 | 36.23 | 59.74 | 100 | 2.29 | 16.80 | 80.91 | 100 |
| Stress (σ) (kPa) | Strain (γ) (%) | Modulus (YM) (kPa) | Swelling Degree (%) | WVTR (g m−2 d−1) | |
|---|---|---|---|---|---|
| Control | - | - | - | - | 1547 ± 32 |
| GG | 4 ± 0.2 | 10.7 ± 0.4 | 85 ± 7 | 3 ± 0.6 | 964 ± 47 |
| GVCO60 | 6 ± 0.1 | 7.9 ± 0.2 | 134 ± 6 | 12 ± 4.0 | 391 ± 13 |
| GVCO70 | 7 ± 0.4 | 8.5 ± 0.2 | 175 ± 8 | 9 ± 1.5 | 344 ± 23 |
| GVCO80 | 11 ± 0.3 | 9.3 ± 0.4 | 259 ± 7 | 6 ± 1.5 | 332 ± 11 |
| Sample | To (°C) | Temperature Offset (°C) | Weight Loss (%) |
|---|---|---|---|
| GG | 54 | 157 | 95 |
| GVCO60 | 48 | 165 | 86 |
| GVCO70 | 53 | 155 | 89 |
| GVCO80 | 50 | 157 | 88 |
| Day/Treatment | 2nd | 4th | 7th | 11th | 14th |
|---|---|---|---|---|---|
| Percentage (%) of Wound Closure (Mean ± SD) | |||||
| GG | 9 ± 5 | 25 ± 7 | 35 ± 2 | 81 ± 6 | 91 ± 4 |
| Opsite | 25 ± 3 * | 31 ± 3 | 49 ± 11 | 88 ± 5 | 93 ± 4 |
| GVCO80 | 11 ± 3 | 28 ± 2 | 46 ± 7 | 89 ± 3 | 95 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muktar, M.Z.; Bakar, M.A.A.; Amin, K.A.M.; Che Rose, L.; Wan Ismail, W.I.; Razali, M.H.; Abd Razak, S.I.; in het Panhuis, M. Gellan Gum Hydrogels Filled Edible Oil Microemulsion for Biomedical Materials: Phase Diagram, Mechanical Behavior, and In Vivo Studies. Polymers 2021, 13, 3281. https://doi.org/10.3390/polym13193281
Muktar MZ, Bakar MAA, Amin KAM, Che Rose L, Wan Ismail WI, Razali MH, Abd Razak SI, in het Panhuis M. Gellan Gum Hydrogels Filled Edible Oil Microemulsion for Biomedical Materials: Phase Diagram, Mechanical Behavior, and In Vivo Studies. Polymers. 2021; 13(19):3281. https://doi.org/10.3390/polym13193281
Chicago/Turabian StyleMuktar, Muhammad Zulhelmi, Muhammad Ameerul Amin Bakar, Khairul Anuar Mat Amin, Laili Che Rose, Wan Iryani Wan Ismail, Mohd Hasmizam Razali, Saiful Izwan Abd Razak, and Marc in het Panhuis. 2021. "Gellan Gum Hydrogels Filled Edible Oil Microemulsion for Biomedical Materials: Phase Diagram, Mechanical Behavior, and In Vivo Studies" Polymers 13, no. 19: 3281. https://doi.org/10.3390/polym13193281
APA StyleMuktar, M. Z., Bakar, M. A. A., Amin, K. A. M., Che Rose, L., Wan Ismail, W. I., Razali, M. H., Abd Razak, S. I., & in het Panhuis, M. (2021). Gellan Gum Hydrogels Filled Edible Oil Microemulsion for Biomedical Materials: Phase Diagram, Mechanical Behavior, and In Vivo Studies. Polymers, 13(19), 3281. https://doi.org/10.3390/polym13193281