Development, Investigation, and Comparative Study of the Effects of Various Metal Oxides on Optical Electrochemical Properties Using a Doped PANI Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Preparation of Hybrid Materials
2.3. Physicochemical Characterization
2.4. Electrochemical Properties
3. Results and Discussions
3.1. X-ray Photoelectron Spectroscopy (XPS) Spectra
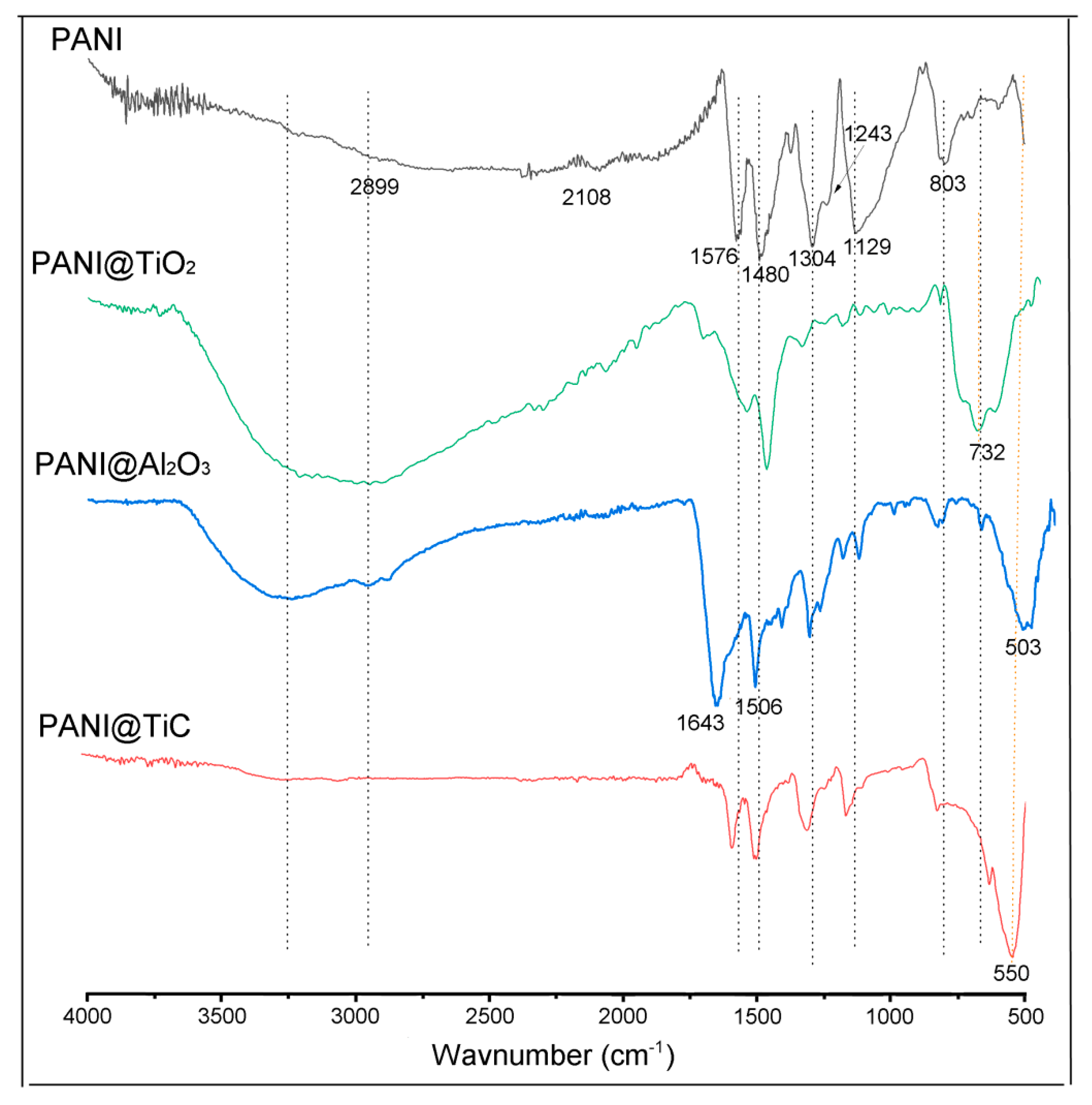
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Spectra
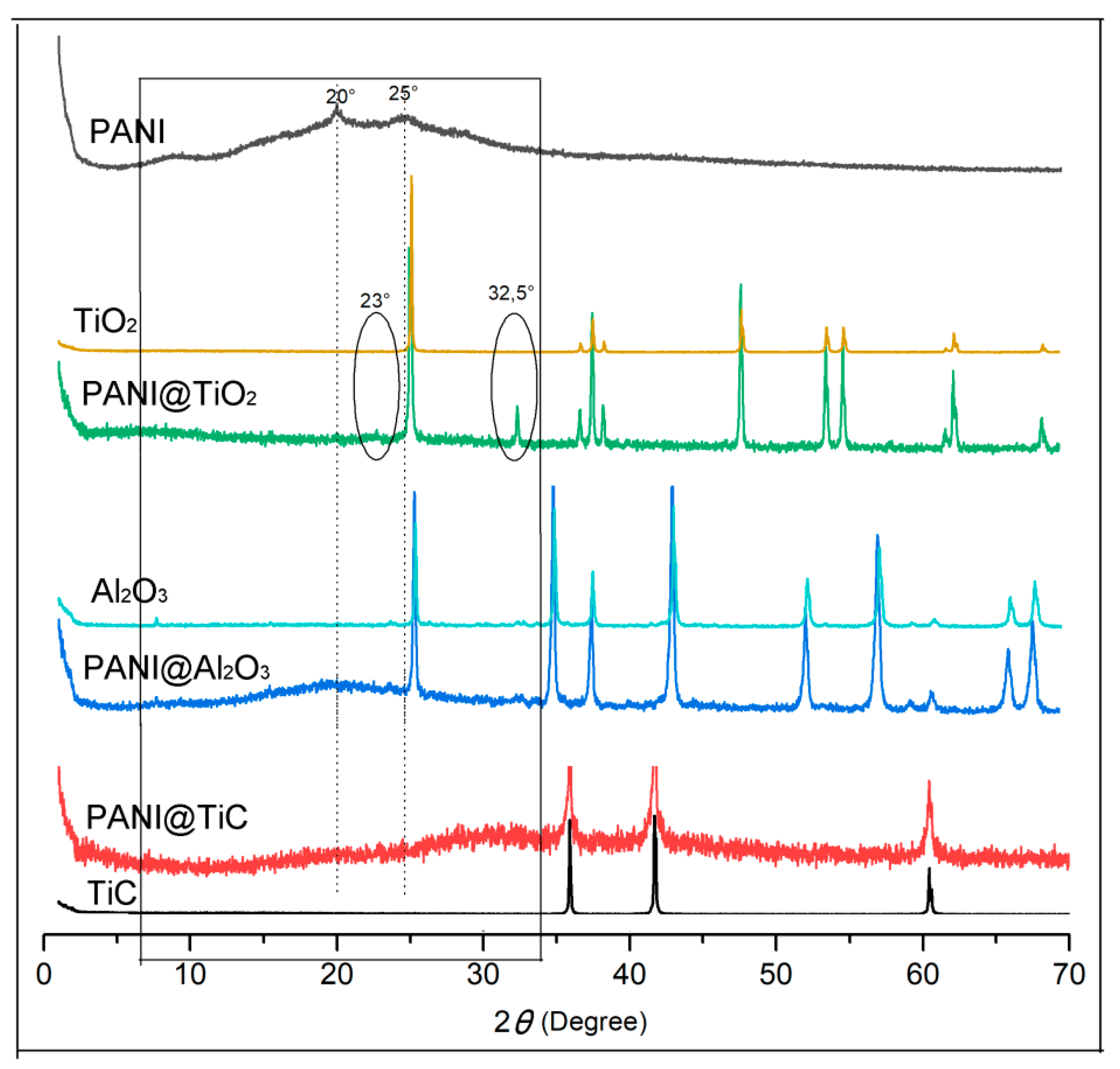
3.3. X-ray Diffraction (XRD) Studies
3.4. UV/Visible (UV/Vis) Spectroscopy
3.5. Thermogravimetric Analysis (TGA)
3.6. Transmission Electron Microscopy (TEM) Analysis
3.7. Electrochemical Properties
3.8. Conductivity Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anbarasan, R.; Ponprapakaran, K.; Subramani, R.H.; Baskaran, R.; Tung, K.-L. Synthesis, characterization and catalytic activity of copolymer/metal oxide nanocomposites. Polym. Bull. 2018, 76, 4117–4138. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Castro, M.C.R.; Mesquita, I.; Andrade, L.; Mendes, A.; Raposo, M.M.M. Synthesis and characterization of novel thieno [3,2-b] thiophene based metal-free organic dyes with different heteroaromatic donor moieties as sensitizers for dye-sensitized solar cells. Dye. Pigment. 2017, 136, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Radja, I.; Djelad, H.; Morallon, E.; Benyoucef, A. Characterization and electrochemical properties of conducting nanocomposites synthesized from p-anisidine and aniline with titanium carbide by chemical oxidative method. Synth. Met. 2015, 202, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.-N.; Xu, F.; Sun, L.-X.; Zeng, J.-L.; Liu, Y.-Y. Thermal stability and glass transition behavior of PANI/α-Al2O3 composites. J. Therm. Anal. Calorim. 2008, 94, 553–557. [Google Scholar] [CrossRef]
- Lazarev, A.N. Vibrational Spectra and Structure of Silicates; Springer: New York, NY, USA, 1995. [Google Scholar]
- Rahman, S.U.; Röse, P.; Surati, M.; Shah, A.U.H.A.; Krewer, U.; Bilal, S. 3D Polyaniline Nanofibers Anchored on Carbon Paper for High-Performance and Light-Weight Supercapacitors. Polymers 2020, 12, 2705. [Google Scholar] [CrossRef]
- Rahman, S.U.; Röse, P.; Shah, A.U.H.A.; Krewer, U.; Bilal, S.; Farooq, S. Exploring the Functional Properties of Sodium Phytate Doped Polyaniline Nanofibers Modified FTO Electrodes for High-Performance Binder Free Symmetric Supercapacitors. Polymers 2021, 13, 2329. [Google Scholar] [CrossRef]
- Cai, G.; Tu, J.; Zhou, D.; Zhang, J.; Xiong, Q.; Zhao, X.; Wang, X.; Gu, C. Multicolor Electrochromic Film Based on TiO2@Polyaniline Core/Shell Nanorod Array. J. Phys. Chem. C 2013, 117, 15967–15975. [Google Scholar] [CrossRef]
- Rahman, S.U.; Röse, P.; Shah, A.U.H.A.; Krewer, U.; Bilal, S. An Amazingly Simple, Fast and Green Synthesis Route to Polyaniline Nanofibers for Efficient Energy Storage. Polymers 2020, 12, 2212. [Google Scholar] [CrossRef]
- Farooq, S.; Tahir, A.; Krewer, U.; Shah, A.U.H.A.; Bilal, S. Efficient photocatalysis through conductive polymer coated FTO counter electrode in platinum free dye sensitized solar cells. Electrochimica Acta 2019, 320, 134544. [Google Scholar] [CrossRef]
- Ullah, R.; Yaseen, S.; Shah, A.-U.-H.A.; Bilal, S.; Kamran, M.; Rahim, M. Anticorrosive polyaniline synthesized using coconut oil as the dispersion medium. Mater. Chem. Phys. 2021, 273, 125071. [Google Scholar] [CrossRef]
- Zia, T.U.H.; Shah, A.U.H.A. Understanding the Adsorption of 1 NLB Antibody on Polyaniline Nanotubes as a Function of Zeta Potential and Surface Charge Density for Detection of Hepatitis C Core Antigen: A Label-Free Impedimetric Immunosensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127076. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, M. Nanostructures of polyaniline composites containing nano-magnet. Synth. Met. 2003, 132, 205–212. [Google Scholar] [CrossRef]
- He, Y. A novel emulsion route to sub-micrometer polyaniline/nano-ZnO composite fibers. Appl. Surf. Sci. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Z.; Li, Y.; Sun, L.; Zhang, T. Synthesis, characterization and thermal analysis of polyaniline/ZrO2 composites. Thermochim. Acta 2006, 441, 191–194. [Google Scholar] [CrossRef]
- Cheng, Q.; Fang, Z.; Yi, X.; An, X.; Tang, B.; Xu, Y. “Ex Situ” Concept for toughening the RTmable BMI matrix composites, part I: Improving the interlaminar fracture toughness. J. Appl. Polym. Sci. 2008, 109, 1625–1634. [Google Scholar] [CrossRef]
- Benykhlef, S.; Bekhoukh, A.; Berenguer, R.; Benyoucef, A.; Morallon, E. PANI-derived polymer/Al2O3 nanocomposites: Synthesis, characterization, and electrochemical studies. Colloid Polym. Sci. 2016, 294, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, S.; Ohashi, F.; Ohnishi, Y.; Nonami, T. Synthesis of polyaniline–montmorillonite nanocomposites by the mechanochemical intercalation method. Synth. Met. 2004, 145, 265–270. [Google Scholar] [CrossRef]
- Dakshayini, B.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Rehman, M.N.U.; Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Maqbool, A.; Riaz, M.; Manzoor, S.; Ashiq, M.N.; Iqbal, F. Facile synthesis and characterization of conducting polymer-metal oxide based core-shell PANI-Pr2O–NiO–Co3O4 nanocomposite: As electrode material for supercapacitor. Ceram. Int. 2021, 47, 18497–18509. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Rabbani, G.; Mollah, M.Y.A.; Susan, A.B.H.; Islam, M. In situ electrodeposition of conducting polymer/metal oxide composites on iron electrode for energy storage applications. Mater. Today: Proc. 2020, 29, 1192–1198. [Google Scholar] [CrossRef]
- Lokhande, V.; Lokhande, C.; Kim, J.H.; Ji, T. Supercapacitive composite metal oxide electrodes formed with carbon, metal oxides and conducting polymers. J. Alloy. Compd. 2016, 682, 381–403. [Google Scholar] [CrossRef]
- Benabid, F.Z.; Zouai, F.; Benachour, D. Dielectric properties of some polymers/ metal oxide nanoparticles nanocomposites using fast technique. Rev. Roum. Chim. 2020, 65, 1017–1025. [Google Scholar] [CrossRef]
- Tudorache, F.; Grigoraş, M. Study of polyaniline-iron oxides composites using for gas detection. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 43–47. [Google Scholar]
- Zenasni, M.; Quintero-Jaime, A.; Benyoucef, A.; Benghalem, A. Synthesis and characterization of polymer/V2O5 composites based on poly(2-aminodiphenylamine). Polym. Compos. 2021, 42, 1064–1074. [Google Scholar] [CrossRef]
- Maaza, L.; Djafri, F.; Belmokhtar, A.; Benyoucef, A. Evaluation of the influence of Al2O3 nanoparticles on the thermal stability and optical and electrochemical properties of PANI-derived matrix reinforced conducting polymer composites. J. Phys. Chem. Solids 2021, 152, 109970. [Google Scholar] [CrossRef]
- Kouidri, F.Z.; Moulefera, I.; Bahoussi, S.; Belmokhtar, A.; Benyoucef, A. Development of hybrid materials based on carbon black reinforced poly(2-methoxyaniline): Preparation, characterization and tailoring optical, thermal and electrochemical properties. Colloid Polym. Sci. 2021, 299, 1–9. [Google Scholar] [CrossRef]
- Boutaleb, N.; Chouli, F.; Benyoucef, A.; Zeggai, F.Z.; Bachari, K. A comparative study on surfactant c etyltrimethylammoniumbromide modified clay-based poly(p-anisidine) nanocomposites: Synthesis, characterization, optical and electrochemical properties. Polym. Compos. 2021, 42, 1648–1658. [Google Scholar] [CrossRef]
- Babazadeh, M.; Zalloi, F.; Olad, A. Fabrication of Conductive Polyaniline Nanocomposites Based on Silica Nanoparticles via In-Situ Chemical Oxidative Polymerization Technique. Synth. React. Inorg. Met. Chem. 2014, 45, 86–91. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Zheng, J. Synthesis and electrochemical properties of PANI–TiC nanocomposite and its electrocatalytic behavior. Mater. Lett. 2013, 93, 99–102. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Patil, H.K.; Bodkhe, G.A.; Yasuzawa, M.; Koinkar, P.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA-modified PANI/SWNTs nanocomposite for differential pulse voltammetry based determination of Cu(II) ions. Sens. Actuators B Chem. 2018, 260, 331–338. [Google Scholar] [CrossRef]
- Pawar, S.G.; Patil, S.L.; Chougule, M.A.; Mane, A.T.; Jundale, D.M.; Patil, V.B. Synthesis and Characterization of Polyaniline:TiO2Nanocomposites. Int. J. Polym. Mater. 2010, 59, 777–785. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, X. Formation of Polyaniline Nanofibers: A Morphological Study. J. Phys. Chem. B 2008, 112, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, P.; Jayakannan, M. New Renewable Resource Amphiphilic Molecular Design for Size-Controlled and Highly Ordered Polyaniline Nanofibers. Langmuir 2006, 22, 5952–5957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, P.; Su, Z. Preparation of PANI–TiO2 nanocomposites and their solid-phase photocatalytic degradation. Polym. Degrad. Stab. 2006, 91, 2213–2219. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative study of polyaniline (PANI), poly(3,4-ethylenedioxythiophene) (PEDOT) and PANI-PEDOT films electrochemically deposited on transparent indium thin oxide based electrodes. Polymers 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Wei, Y.; Jang, G.-W.; Hsueh, K.F.; Scherr, E.M.; MacDiarmid, A.G.; Epstein, A.J. Thermal transitions and mechanical properties of films of chemically prepared polyaniline. Polymers 1992, 33, 314–322. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI–PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef]
- Begum, B.; Bilal, S.; Shah, A.U.H.A.; Röse, P. Physical, Chemical, and Electrochemical Properties of Redox-Responsive Polybenzopyrrole as Electrode Material for Faradaic Energy Storage. Polymers 2021, 13, 2883. [Google Scholar] [CrossRef]
- Samain, L.; Jaworski, A.; Edén, M.; Ladd, D.M.; Seo, D.-K.; Garcia-Garcia, F.J.; Häussermann, U. Structural analysis of highly porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Chouli, F.; Radja, I.; Morallon, E.; Benyoucef, A. A novel conducting nanocomposite obtained by p-anisidine and aniline with titanium(IV) oxide nanoparticles: Synthesis, Characterization, and Electrochemical properties. Polym. Compos. 2015, 38, E254–E260. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, M.A.; Gicevicius, M.; Ramanaviciene, A.; Shirsat, M.; Viter, R.; Ramanavicius, A. Hybrid electrochemical/electrochromic Cu(II) ion sensor prototype based on PANI/ITO-electrode. Sens. Actuators B Chem. 2017, 248, 527–535. [Google Scholar] [CrossRef]
- Basnayaka, P.A.; Ram, M.K.; Stefanakos, E.K.; Kumar, A. Supercapacitors based on graphene–polyaniline derivative nanocomposite electrode materials. Electrochim. Acta 2013, 92, 376–382. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Jing, X.; Li, Y.; Zhu, J.; Zhang, M. Synthesis and characterization of phosphorized polyaniline doped with phytic acid and its anticorrosion properties for Mg-Li alloy. J. Macromol. Sci. Part A 2018, 55, 24–35. [Google Scholar] [CrossRef]






| Materials | Binding Energy (eV) | ||||
|---|---|---|---|---|---|
| N1s | [=N–]/[N–H] | [N+]/[=N– + N–H] | |||
| PANI@TiC | 398.51 | 399.71 | 400.83 | 0.56 | 0.05 |
| PANI@ TiO2 | 398.39 | 399.53 | 400.33 | 0.29 | 0.51 |
| PANI@Al2O3 | 398.22 | 399.48 | // | 0.42 | // |
| PANI | 398.24 | 399.52 | 400.99 | 0.22 | 0.60 |
| Assignments | =N− | −NH−/−NC− | −N+−/=N+− | ||
| Materials | Absorption Band (nm) | Redox Peaks (V) | Eg (eV) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| π–π* | n–π* | Eox1 | Ered1 | Ep1 | Eox2 | Ered2 | Ep2 | ||
| PANI | 314 | 591 | 0.40 | 0.28 | 0.12 | 0.93 | 0.84 | 0.09 | 3.22 |
| PANI@Al2O3 | 380 | 606 | 0.48 | 0.32 | 0.16 | 0.86 | 0.74 | 0.12 | 2.64 |
| PANI@TiC | 394 | 603 | 0.44 | 0.25 | 0.19 | 0.75 | 0.59 | 0.25 | 3.20 |
| PANI@TiO2 | 316 | 616 | 0.38 | 0.24 | 0.06 | 0.69 | 0.56 | 0.13 | 2.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekhoukh, A.; Moulefera, I.; Sabantina, L.; Benyoucef, A. Development, Investigation, and Comparative Study of the Effects of Various Metal Oxides on Optical Electrochemical Properties Using a Doped PANI Matrix. Polymers 2021, 13, 3344. https://doi.org/10.3390/polym13193344
Bekhoukh A, Moulefera I, Sabantina L, Benyoucef A. Development, Investigation, and Comparative Study of the Effects of Various Metal Oxides on Optical Electrochemical Properties Using a Doped PANI Matrix. Polymers. 2021; 13(19):3344. https://doi.org/10.3390/polym13193344
Chicago/Turabian StyleBekhoukh, Amina, Imane Moulefera, Lilia Sabantina, and Abdelghani Benyoucef. 2021. "Development, Investigation, and Comparative Study of the Effects of Various Metal Oxides on Optical Electrochemical Properties Using a Doped PANI Matrix" Polymers 13, no. 19: 3344. https://doi.org/10.3390/polym13193344








