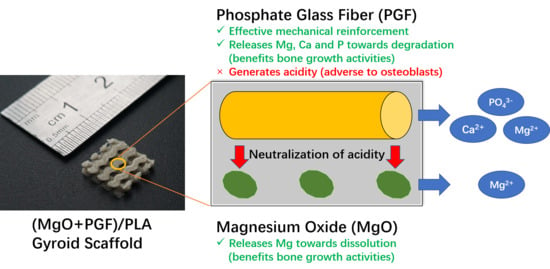
Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Feedstocks for FDM Additive Manufacturing
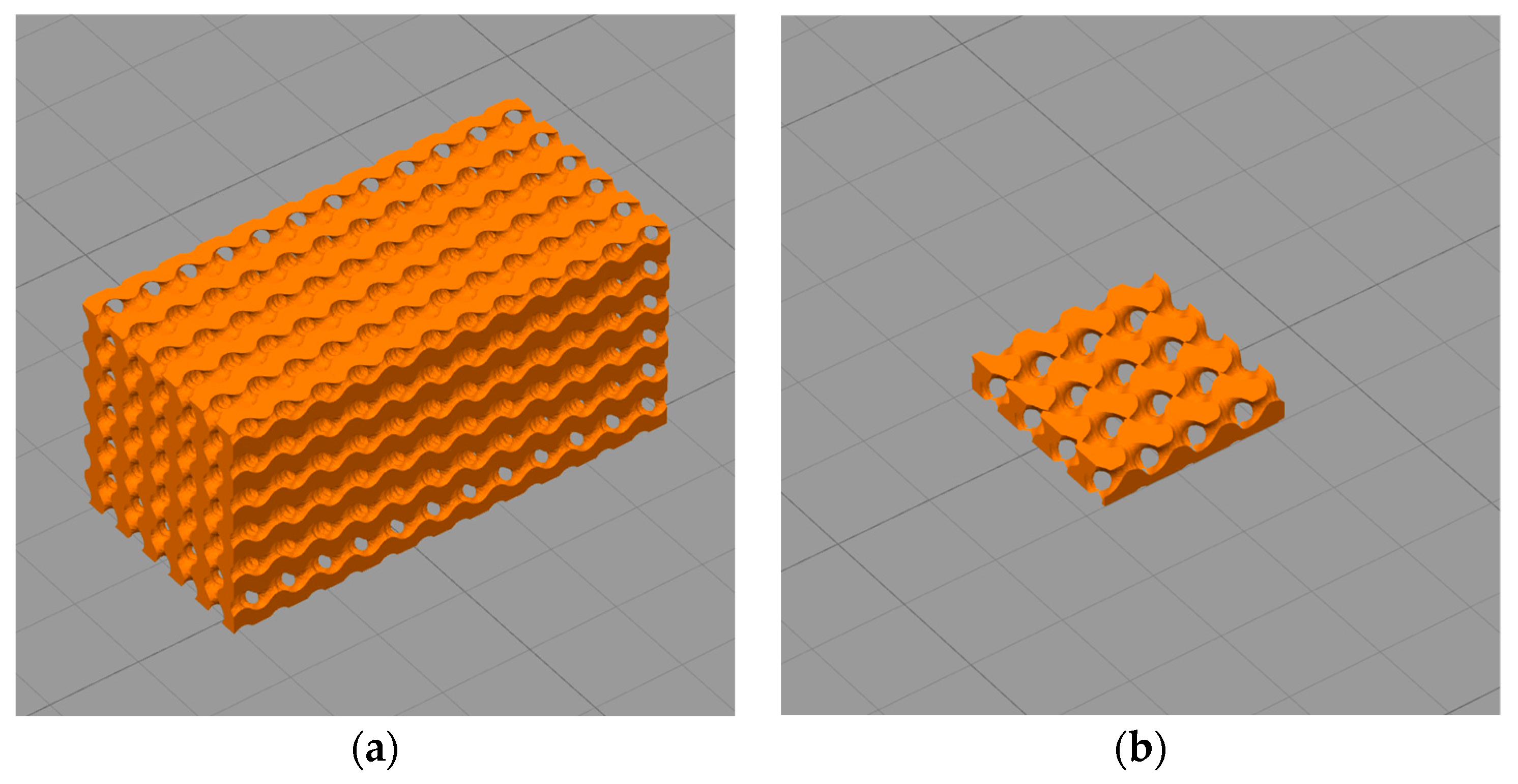
2.3. Design of Gyroid Porous Structure
2.4. Additive Manufacturing via Fused Deposition Modelling (FDM)
2.5. Compressive Properties of the Scaffold
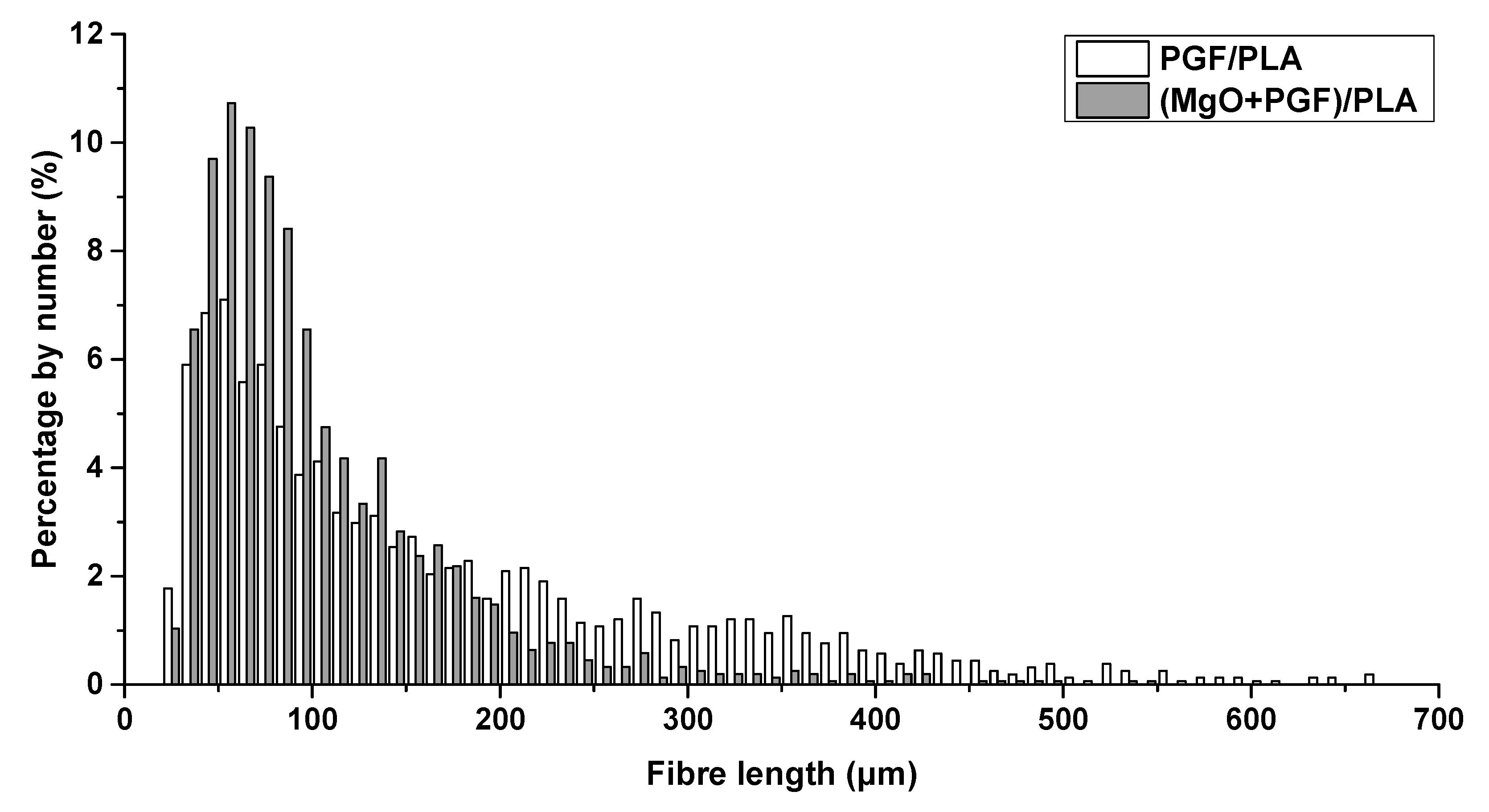
2.6. Fiber Length Measurement
2.7. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.8. In-Vitro Degradation in Tris-HCl Buffer
2.9. Concentration of Mg2+ and Ca2+ in Degradation Media
2.10. Statistical Analysis
3. Results
3.1. Compressive Properties of Scaffolds
3.2. Fiber Length Measurements
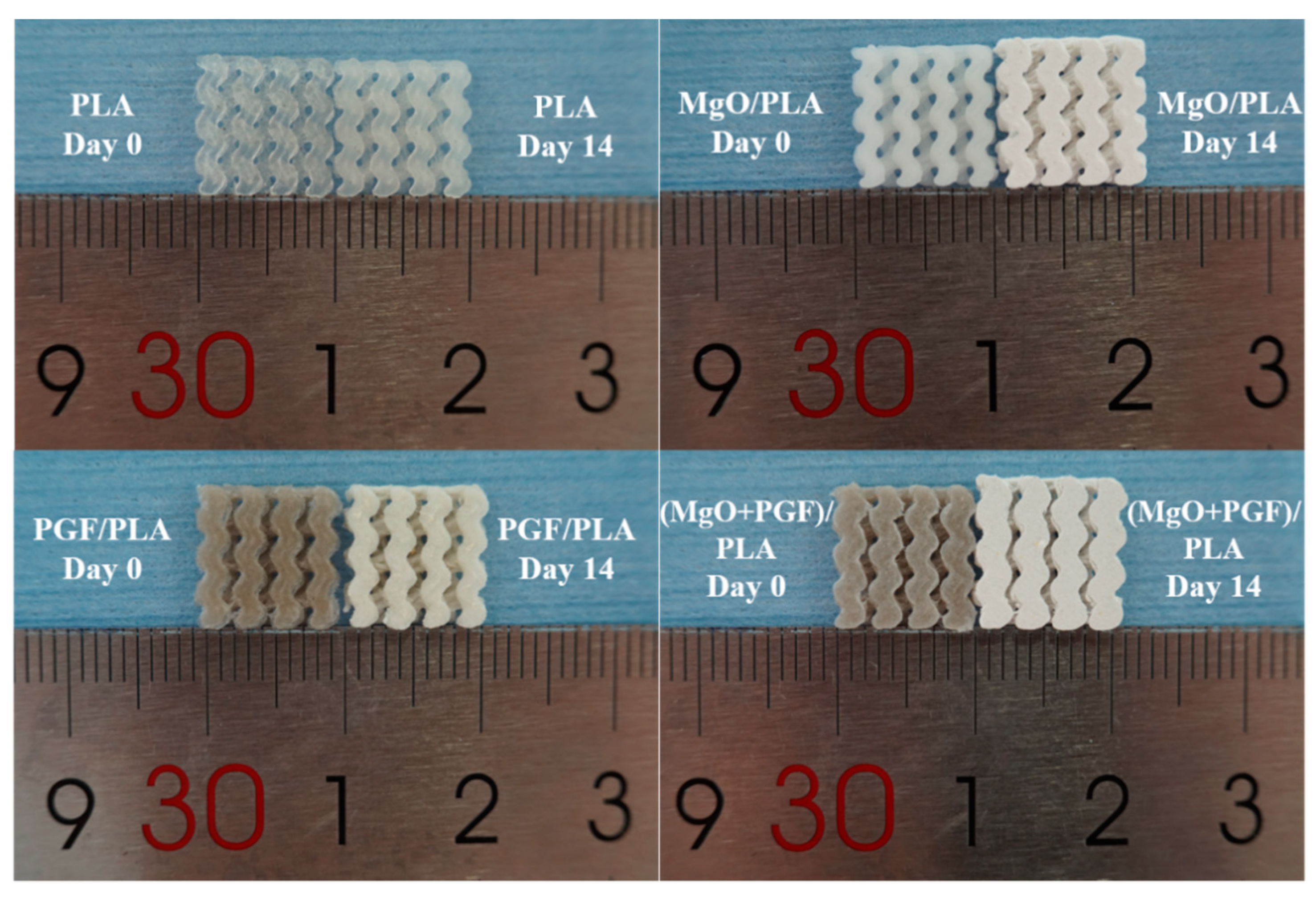
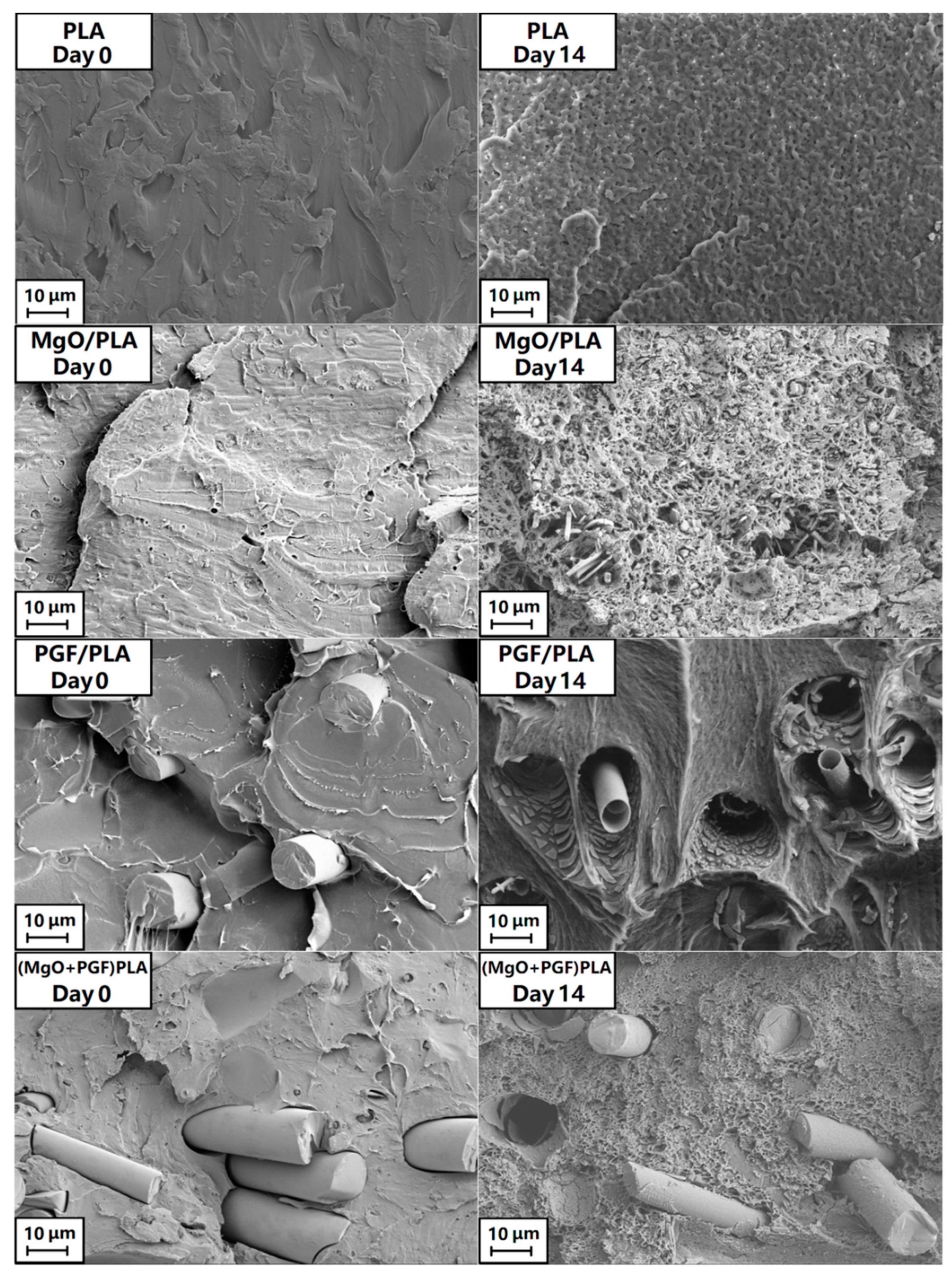
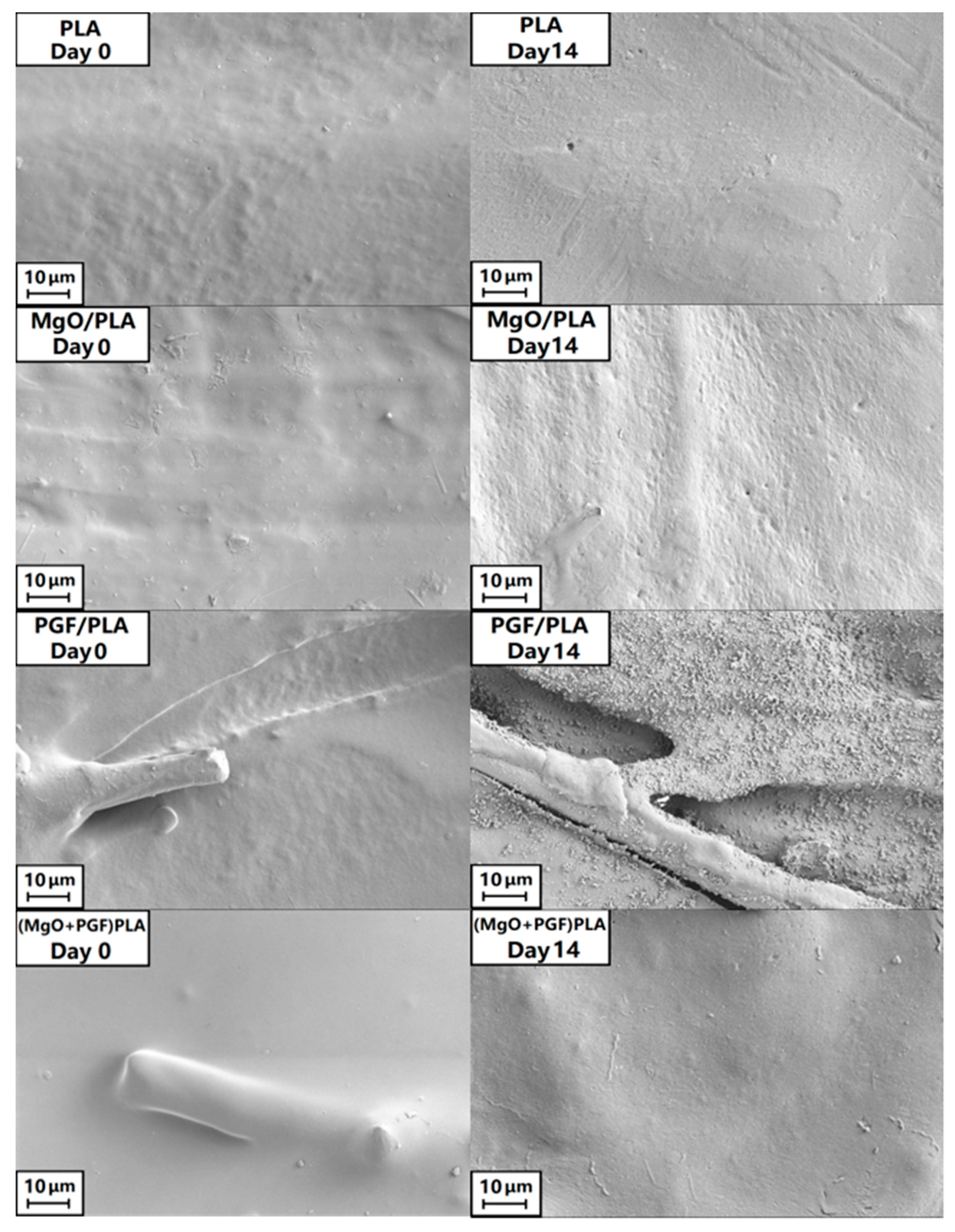
3.3. Change of Size, Internal Structure, and Surface Morphologies of Scaffolds
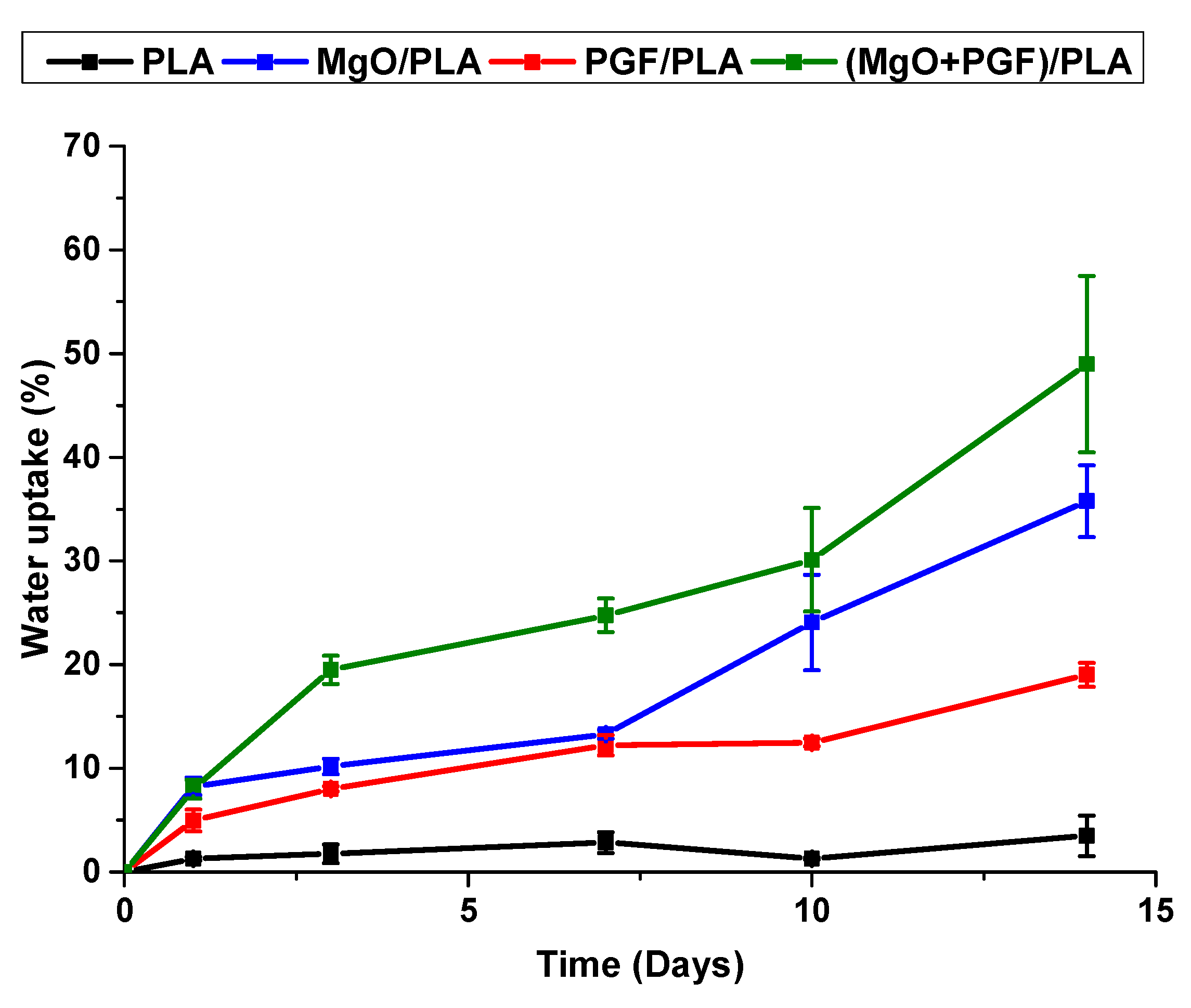
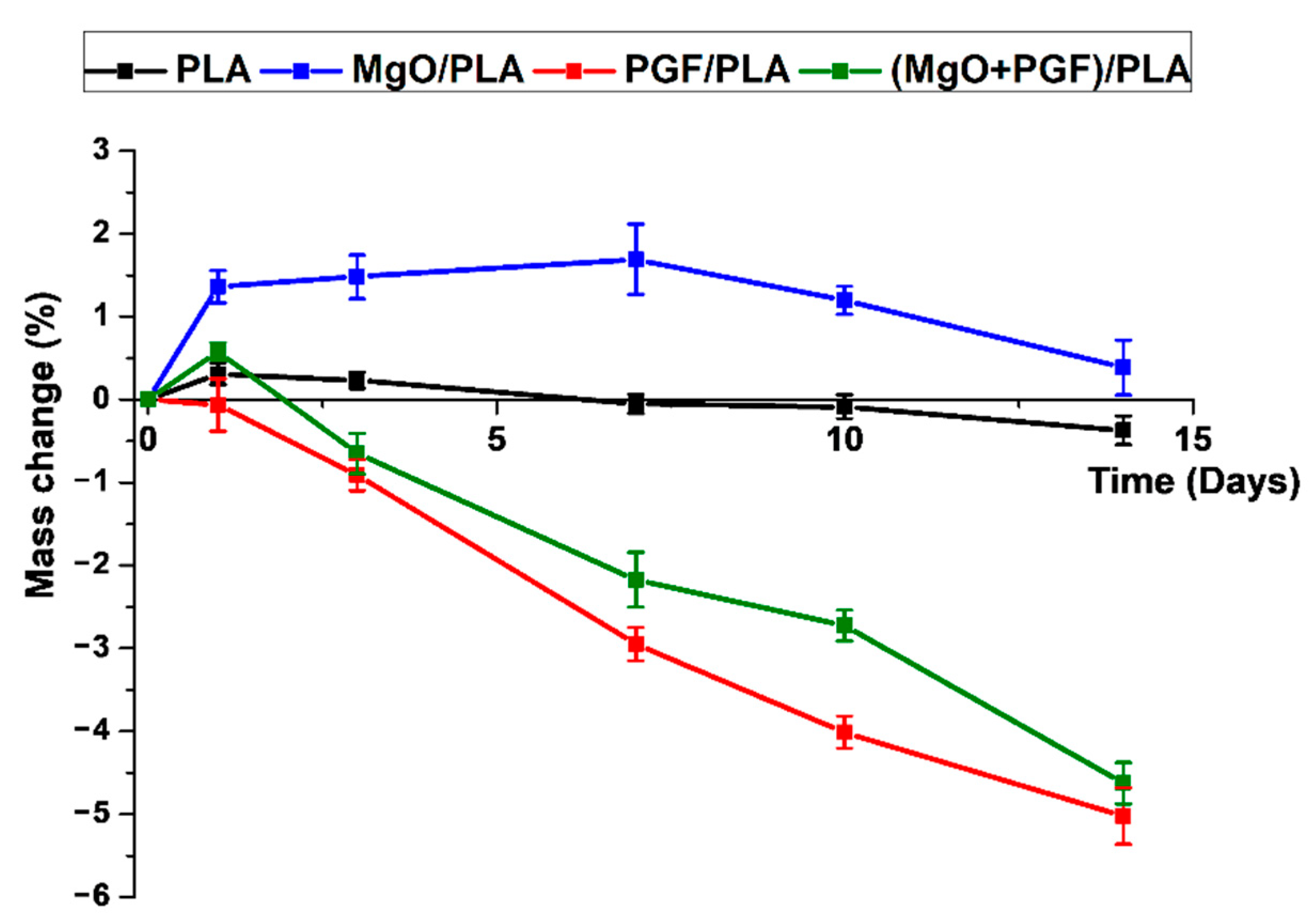
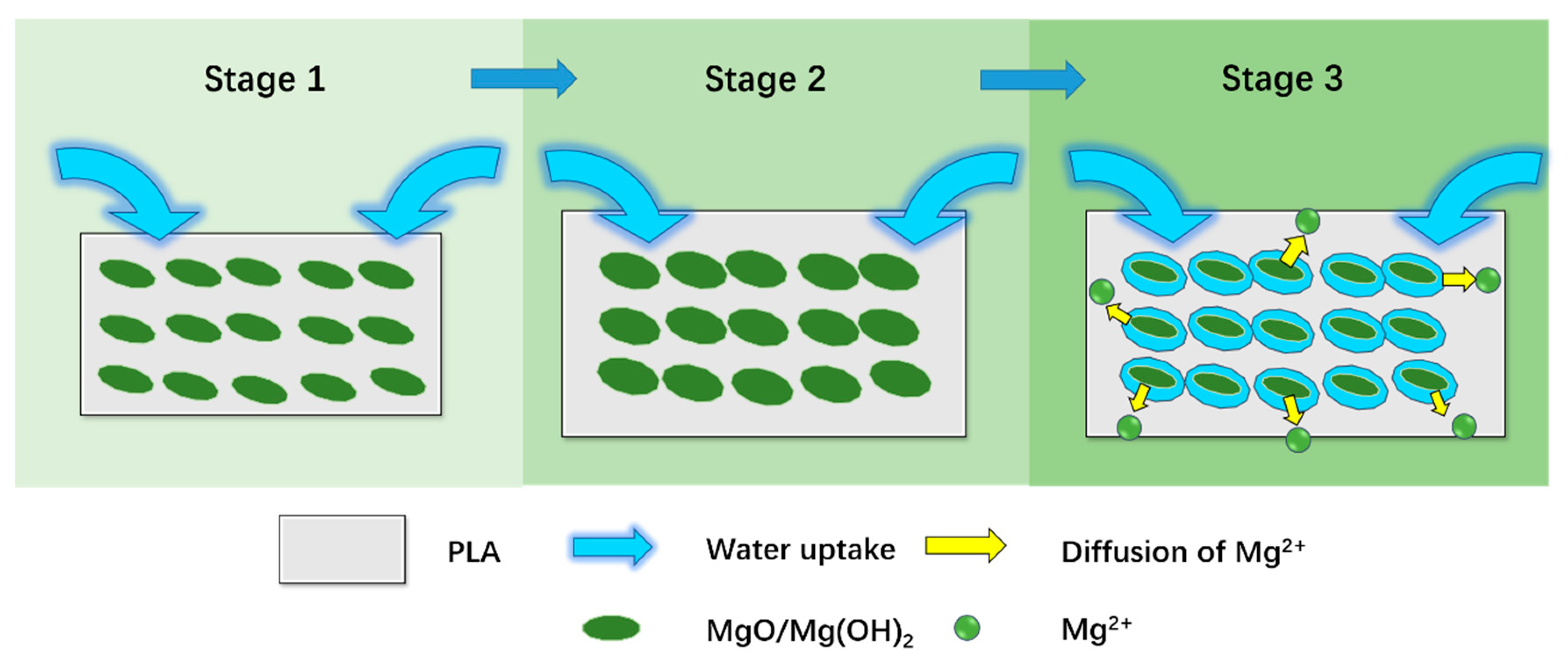
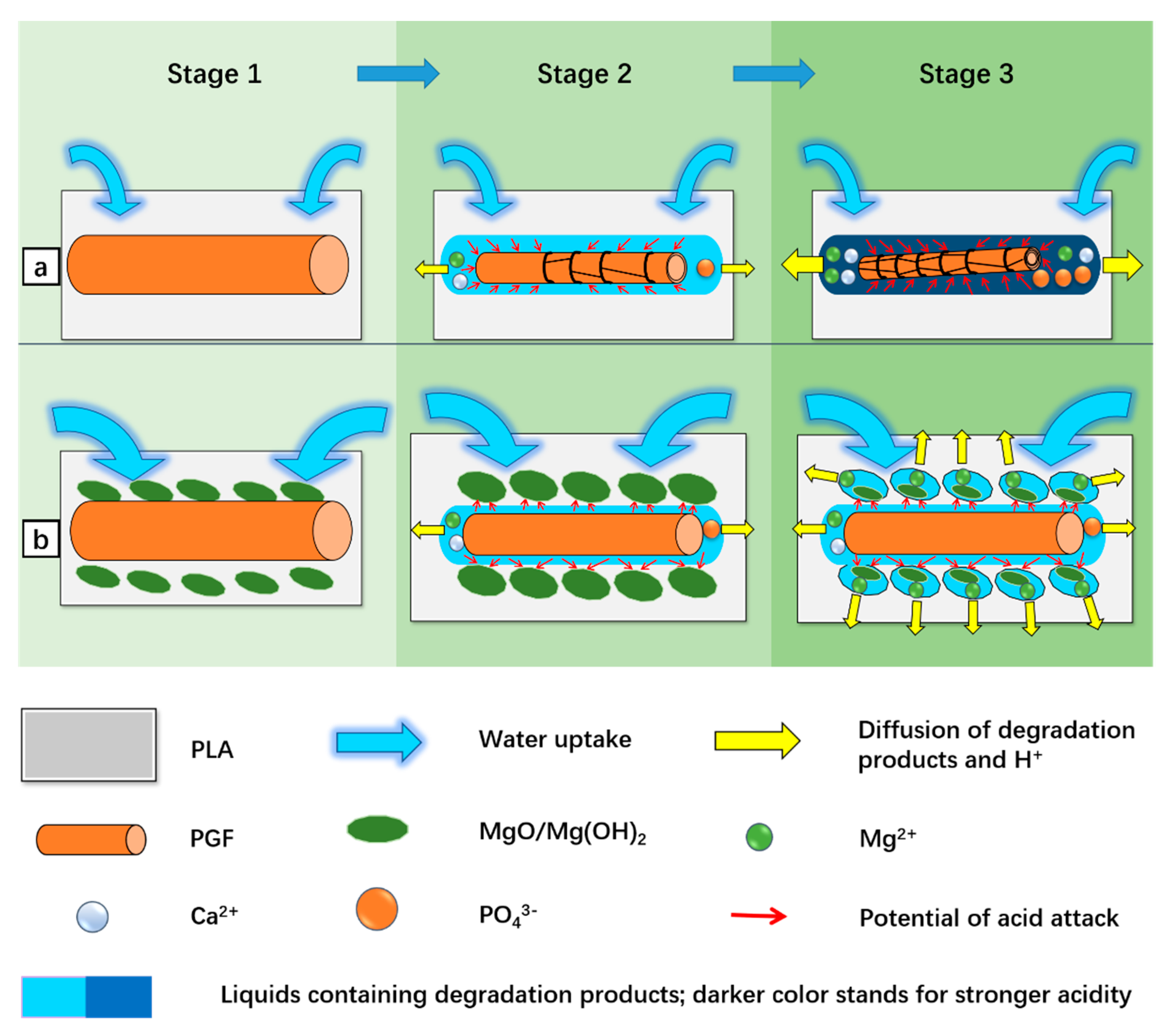
3.4. Water Uptake and Mass Change of Scaffolds
3.5. pH of Degradation Media (Tris-HCl Buffer)
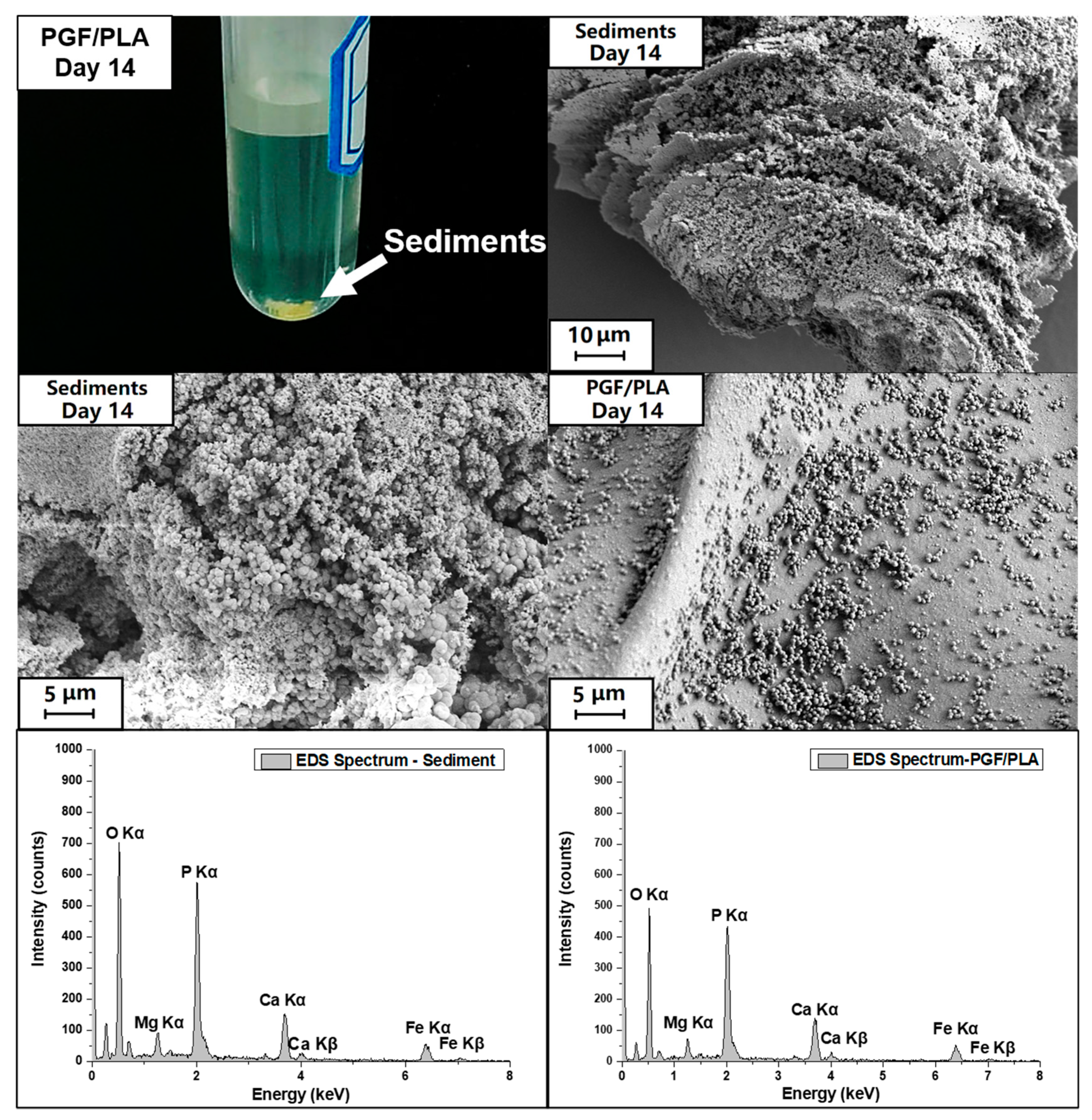
3.6. Ion Release after Degradation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, D.; Zhang, X. (Eds.) VI—Regenerative medicine. In Definitions of Biomaterials for the Twenty-First Century; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–153. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Zhang, L.; Zhang, S.; Hsi Fuh, J.Y.; Lu, W.F. Triply Periodic Minimal Surfaces Sheet Scaffolds for Tissue Engineering Applications: An Optimization Approach toward Biomimetic Scaffold Design. ACS Appl. Bio Mater. 2018, 1, 259–269. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Robb, R.A. Schwarz meets Schwann: Design and fabrication of biomorphic and durataxic tissue engineering scaffolds. Med. Image Anal. 2006, 10, 693–712. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- Abbasi, N.; HamLet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Callens, S.J.P.; Uyttendaele, R.J.C.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Substrate curvature as a cue to guide spatiotemporal cell and tissue organization. Biomaterials 2020, 232, 119739. [Google Scholar] [CrossRef]
- Spece, H.; Yu, T.; Law, A.W.; Marcolongo, M.; Kurtz, S.M. 3D printed porous PEEK created via fused filament fabrication for osteoconductive orthopaedic surfaces. J. Mech. Behav. Biomed. Mater. 2020, 109, 103850. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Henkel, J.; Hutmacher, D.W. Design and fabrication of scaffold-based tissue engineering. BioNanoMaterials 2013, 14, 171–193. [Google Scholar] [CrossRef] [Green Version]
- Colquhoun, R.; Tanner, K.E. Mechanical behaviour of degradable phosphate glass fibres and composites-a review. Biomed. Mater. 2015, 11, 014105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, J.; Huang, S.; Rudd, C.; Liu, X. The Structure, Degradation and Fibre Drawing Properties of Phosphate based Glasses Fibre: The Effects of Fe2O3 and B2O3 Addition. Ceram. Silik. 2018, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Aparicio, C.; Charles-Harris, M.; Ginebra, M.P.; Engel, E.; Planell, J.A. Development of a Biodegradable Composite Scaffold for Bone Tissue Engineering: Physicochemical, Topographical, Mechanical, Degradation, and Biological Properties. In Ordered Polymeric Nanostructures at Surfaces. Advances in Polymer Science; Vancso, G.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 209–231. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Govindan, R.; Gu, F.L.; Karthi, S.; Girija, E.K. Effect of phosphate glass reinforcement on the mechanical and biological properties of freeze-dried gelatin composite scaffolds for bone tissue engineering applications. Mater. Today Commun. 2020, 22. [Google Scholar] [CrossRef]
- Charles-Harris, M.; Koch, M.A.; Navarro, M.; Lacroix, D.; Engel, E.; Planell, J.A. A PLA/calcium phosphate degradable composite material for bone tissue engineering: An in vitro study. J. Mater. Sci. Mater. Med. 2008, 19, 1503–1513. [Google Scholar] [CrossRef]
- He, L.; Zhong, J.; Zhu, C.; Liu, X. Mechanical properties and in vitro degradation behavior of additively manufactured phosphate glass particles/fibers reinforced polylactide. J. Appl. Polym. Sci. 2019, 136, 48171. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef]
- Ahmed, I.; Cronin, P.S.; Abou Neel, E.A.; Parsons, A.J.; Knowles, J.C.; Rudd, C.D. Retention of mechanical properties and cytocompatibility of a phosphate-based glass fiber/polylactic acid composite. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 18–27. [Google Scholar] [CrossRef]
- Georgiou, G.; Mathieu, L.; Pioletti, D.P.; Bourban, P.E.; Manson, J.A.; Knowles, J.C.; Nazhat, S.N. Polylactic acid-phosphate glass composite foams as scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 322–331. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, W.; Wen, C.; Pan, H.; Wang, T.; Darvell, B.W.; Lu, W.W.; Huang, W. Bone regeneration: Importance of local pH—strontium-doped borosilicate scaffold. J. Mater. Chem. 2012, 22, 8662–8670. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, Q.; Li, Y.; Xu, C.; Tang, X.; Ci, J.; Sun, S.; Xu, B.; Li, Y. Acidic pH environment induces autophagy in osteoblasts. Sci. Rep. 2017, 7, 46161. [Google Scholar] [CrossRef]
- Arnett, T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008, 138, 415S–418S. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chang, J. pH-compensation effect of bioactive inorganic fillers on the degradation of PLGA. Compos. Sci. Technol. 2005, 65, 2226–2232. [Google Scholar] [CrossRef]
- Kobayashi, H.Y.L.S.; Brauer, D.S.; Rüssel, C. Mechanical properties of a degradable phosphate glass fibre reinforced polymer composite for internal fracture fixation. Mater. Sci. Eng. C 2010, 30, 1003–1007. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Yang, H.; Ying, J.; Qiao, F.; Peng, M. Preparation and mechanical properties of carbon fiber reinforced hydroxyapatite/polylactide biocomposites. J. Mater. Sci. Mater. Med. 2009, 20, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-L.; Fang, H.-W.; Tseng, T.; Lee, W.-H. Effects of hydroxyapatite dosage on mechanical and biological behaviors of polylactic acid composite materials. Mater. Lett. 2007, 61, 3009–3013. [Google Scholar] [CrossRef]
- He, L.; Zhu, C.; Jiahao, W.; Liu, X. Hybrid Composites of Phosphate Glass Fibre/Nano-Hydroxyapatite/Polylactide: Effects of Nano-Hydroxyapatite on Mechanical Properties and Degradation Behaviour. J. Mater. Sci. Chem. Eng. 2018, 6, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Wu, H.; Ni, J.; Zhang, S.; Zhang, X. Development of PLA/Mg composite for orthopedic implant: Tunable degradation and enhanced mineralization. Compos. Sci. Technol. 2017, 147, 8–15. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Gavilan, R.; Lieblich, M.; Benavente, R.; Gonzalez-Carrasco, J.L. In vitro degradation of biodegradable polylactic acid/magnesium composites: Relevance of Mg particle shape. Acta Biomater. 2016, 32, 348–357. [Google Scholar] [CrossRef]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The effect of hydrogen gas evolution of magnesium implant on the postimplantation mortality of rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Lee, K.B.; Kim, S.Y.; Bode, K.; Jang, Y.S.; Kwon, T.Y.; Jeon, M.H.; Lee, M.H. Gas formation and biological effects of biodegradable magnesium in a preclinical and clinical observation. Sci. Technol. Adv. Mater. 2018, 19, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Amaral, L.F.; Oliveira, I.R.; Salomão, R.; Frollini, E.; Pandolfelli, V.C. Temperature and common-ion effect on magnesium oxide (MgO) hydration. Ceram. Int. 2010, 36, 1047–1054. [Google Scholar] [CrossRef]
- Shuai, C.; Zan, J.; Qi, F.; Wang, G.; Liu, Z.; Yang, Y.; Peng, S. nMgO-incorporated PLLA bone scaffolds: Enhanced crystallinity and neutralized acidic products. Mater. Des. 2019, 174. [Google Scholar] [CrossRef]
- ASTM International. ASTM D695-15, Standard Test Method for Compressive Properties of Rigid Plastics; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar] [CrossRef]
- Felfel, R.M.; Ahmed, I.; Parsons, A.J.; Walker, G.S.; Rudd, C.D. In vitro degradation, flexural, compressive and shear properties of fully bioresorbable composite rods. J. Mech. Behav. Biomed. Mater. 2011, 4, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Vahid, A.; Ataee, A.; Wen, C. High strength porous PLA gyroid scaffolds manufactured via fused deposition modeling for tissue-engineering applications. Smart Mater. Med. 2021, 2, 15–25. [Google Scholar] [CrossRef]
- Coogan, T.J.; Kazmer, D.O. Prediction of interlayer strength in material extrusion additive manufacturing. Addit. Manuf. 2020, 35. [Google Scholar] [CrossRef]
- Wen, W.; Luo, B.; Qin, X.; Li, C.; Liu, M.; Ding, S.; Zhou, C. Strengthening and toughening of poly(L-lactide) composites by surface modified MgO whiskers. Appl. Surf. Sci. 2015, 332, 215–223. [Google Scholar] [CrossRef]
- Franzén, B.; Klason, C.; Kubát, J.; Kitano, T. Fibre degradation during processing of short fibre reinforced thermoplastics. Composites 1989, 20, 65–76. [Google Scholar] [CrossRef]
- Von Turkovich, R.; Erwin, L. Fiber fracture in reinforced thermoplastic processing. Polym. Eng. Sci. 1983, 23, 743–749. [Google Scholar] [CrossRef]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.; Al-Tabbaa, A. The effect of magnesia on the self-healing performance of Portland cement with increased curing time. In Proceedings of the 14th International Conference on Ageing of Materials & Structures, Delft, The Netherlands, 26–28 May 2014; pp. 635–642. [Google Scholar]
- Shah Mohammadi, M.; Ahmed, I.; Marelli, B.; Rudd, C.; Bureau, M.N.; Nazhat, S.N. Modulation of polycaprolactone composite properties through incorporation of mixed phosphate glass formulations. Acta Biomater. 2010, 6, 3157–3168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsson, M.S.; Nancollas, G.H. The role of brushite and octacalcium phosphate in apatite formation. Crit. Rev. Oral Biol. Med. 1992, 3, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Alani, A.; Knowles, J.C.; Chrzanowski, W.; Ng, Y.-L.; Gulabivala, K. Ion release characteristics, precipitate formation and sealing ability of a phosphate glass–polycaprolactone-based composite for use as a root canal obturation material. Dent. Mater. 2009, 25, 400–410. [Google Scholar] [CrossRef]
- Shah Mohammadi, M.; Chicatun, F.; Stähli, C.; Muja, N.; Bureau, M.N.; Nazhat, S.N. Osteoblastic differentiation under controlled bioactive ion release by silica and titania doped sodium-free calcium phosphate-based glass. Colloids Surf. B Biointerfaces 2014, 121, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, S.; Papaspyrides, C.D. The effect of hygrothermal history on water sorption and interlaminar shear strength of glass/polyester composites with different interfacial strength. Compos. Part A Appl. Sci. Manuf. 2003, 34, 1117–1124. [Google Scholar] [CrossRef]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhao, H.; Zhang, W.; Xu, M. Influence of moisture absorption on the DC conduction and space charge property of MgO/LDPE nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1957–1964. [Google Scholar] [CrossRef]
- Han, D.; Qu, M.; Yue, C.Y.; Lou, Y.; Musso, S.; Robisson, A. Swellable elastomeric HNBR–MgO composite: Magnesium oxide as a novel swelling and reinforcement filler. Compos. Sci. Technol. 2014, 99, 52–58. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, H.; Zhang, S.; Qu, S.; Jiang, Y.; Chen, M. Effects of Magnesium Oxide (MgO) Shapes on In Vitro and In Vivo Degradation Behaviors of PLA/MgO Composites in Long Term. Polymers 2020, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, B.; Bi, H.; Yang, J.; Li, W.; Liang, H.; Liang, Y.; Jia, Z.; Shi, S.; Chen, M. The Degradation Properties of MgO Whiskers/PLLA Composite In Vitro. Int. J. Mol. Sci. 2018, 19, 2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuai, C.; Li, Y.; Feng, P.; Guo, W.; Yang, W.; Peng, S. Positive feedback effects of Mg on the hydrolysis of poly-l-lactic acid (PLLA): Promoted degradation of PLLA scaffolds. Polym. Test. 2018, 68, 27–33. [Google Scholar] [CrossRef]
- Bunker, B.C.; Arnold, G.W.; Wilder, J.A. Phosphate glass dissolution in aqueous solutions. J. Non-Cryst. Solids 1984, 64, 291–316. [Google Scholar] [CrossRef]
- Gao, H.; Tan, T.; Wang, D. Dissolution mechanism and release kinetics of phosphate controlled release glasses in aqueous medium. J. Control Release 2004, 96, 29–36. [Google Scholar] [CrossRef]
- Thissen, P.; Thissen, V.; Wippermann, S.; Chabal, Y.J.; Grundmeier, G.; Schmidt, W.G. pH-dependent structure and energetics of H2O/MgO(100). Surf. Sci. 2012, 606, 902–907. [Google Scholar] [CrossRef]
- Fedoročková, A.; Raschman, P. Effects of pH and acid anions on the dissolution kinetics of MgO. Chem. Eng. J. 2008, 143, 265–272. [Google Scholar] [CrossRef]
- Nakade, O.; Takahashi, K.; Takuma, T.; Aoki, T.; Kaku, T. Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells. J. Bone Min. Metab. 2001, 19, 13–19. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Maradze, D.; Musson, D.; Zheng, Y.; Cornish, J.; Lewis, M.; Liu, Y. High Magnesium Corrosion Rate has an Effect on Osteoclast and Mesenchymal Stem Cell Role During Bone Remodelling. Sci. Rep. 2018, 8, 10003. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Tocados, J.M.; Herencia, C.; Martinez-Moreno, J.M.; Montes de Oca, A.; Rodriguez-Ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S.; Almaden, Y.; Rodriguez, M.; et al. Magnesium Chloride promotes Osteogenesis through Notch signaling activation and expansion of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, T.; Pattanayak, D.K.; Yamaguchi, S.; Takadama, H.; Matsushita, T.; Kawai, T.; Takemoto, M.; Fujibayashi, S.; Nakamura, T. Positively charged bioactive Ti metal prepared by simple chemical and heat treatments. J. R. Soc. Interface 2010, 7 (Suppl. 5), S503–S513. [Google Scholar] [CrossRef] [Green Version]
- Pattanayak, D.K.; Yamaguchi, S.; Matsushita, T.; Nakamura, T.; Kokubo, T. Apatite-forming ability of titanium in terms of pH of the exposed solution. J. R. Soc. Interface 2012, 9, 2145–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Sample Code | PLA (wt.%) | MgO (wt.%) | PGF (wt.%) |
|---|---|---|---|
| PLA | 100 | - | - |
| MgO/PLA | 98 | 2 | - |
| PGF/PLA | 82 | - | 18 |
| (MgO + PGF)/PLA | 80 | 2 | 18 |
| Parameters | Value/Setting |
|---|---|
| Nozzle diameter | 0.4 mm |
| Layer height | 0.15 mm |
| Build plate temperature | 60 °C |
| Nozzle temperature | 190 °C |
| Default printing speed | 1800 mm/min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Liu, X.; Rudd, C. Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering. Polymers 2021, 13, 270. https://doi.org/10.3390/polym13020270
He L, Liu X, Rudd C. Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering. Polymers. 2021; 13(2):270. https://doi.org/10.3390/polym13020270
Chicago/Turabian StyleHe, Lizhe, Xiaoling Liu, and Chris Rudd. 2021. "Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering" Polymers 13, no. 2: 270. https://doi.org/10.3390/polym13020270
APA StyleHe, L., Liu, X., & Rudd, C. (2021). Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering. Polymers, 13(2), 270. https://doi.org/10.3390/polym13020270