1. Introduction
The continuous improvement of dental composites is permanently in the focus of manufacturers, dental professionals, and researchers [
1]. The characteristics and multiple properties of these materials are determined by those dimethacrylate monomers that are responsible for the formation of its resin matrix. The most commonly used monomers in dental composites are bisphenol A diglycidyl dimethacrylate (Bis-GMA) and its ethoxylated analog (Bis-EMA) and urethane dimethacrylates (UDMA) along with low molecular weight diluents, usually ethylene glycol derivatives, such as triethylene-glycol dimethacrylate (TEGDMA). The effect of chemical composition and different ratios of dimethacrylates (Bis-GMA, UDMA, TEGDMA) on mechanical properties has been demonstrated already in experimental resin composites by Asmussen and Peutzfeldt [
2]. The challenge is to create a resin matrix with low polymerization shrinkage and better depth of cure or degree of conversion along with improved mechanical properties, aesthetics, and biocompatibility [
3]. As they are photosensitive materials, the successful photocuring process requires an efficient initiator molecule and adequate light energy with compatible wavelength. The frequently used initiator is champhoroquinone (CQ) that can be activated by 400–500 nm (maximum at 470 nm) wavelength blue light. CQ is a solid yellow diketone compound with an unbleachable chromophore group, which leads to an undesirable yellowing effect on the final esthetic appearance of a cured material. Furthermore, CQ needs a reducing agent to generate free radicals to initiate the polymerization of photo-activated resin-based filling materials. The tertiary amine used can also add uneven yellow discoloration to the cured restoration. These disadvantages of CQ motivated the researchers to find alternative initiator molecules. Acylphospine oxide derivates such as diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide (lucirin TPO), phenylbis (2,4,6-trimethylbensoyl) phosphine oxide (BAPO), and the pale yellow liquid 1-phenyl and 1,2 propenedione (PPD) have been suggested as an alternative photoinitiator in dental composites [
1,
4].
The other crucial requirement of successful photopolymerization is the question of applying effective light sources. From this point of view, the absorption spectrum of the applied photoinitiator should correlate with the spectral emission profiles of the light-curing units (LCU) [
5]. Historically, several types of LCU were used in dentistry such as quartz-tungsten-halogen lights, argon-ion lasers, plasma arc lights, and light-emitting diodes (LEDs). LEDs proved to be the most successful thanks to their long life service and, in addition, they are compact, portable, and energy-efficient [
6]. As a result of the free radical polymerization of dimethacrylate based dental composite matrix, a three-dimensional connective crosslinked polymer network is formed. The extent of polymerization is quantified by comparing the amount of remaining double bonds in the polymer structure to the initial amount. This ratio, expressed in %, is termed degree of conversion (DC). Generally, DC values vary in a wide range of resin-based dental composite types, from about 35–77% [
7]. Achievement of maximum DC of resin materials requires the presence of optimal circumstances. There are various factors, which affect the polymerization process of resin-based composites, such as the composition of the reaction mixture, curing mode, light-curing time, increment thickness, light-curing units used, post-irradiation time, cavity diameter and its location, distance of the light-curing tip from the surface, the substrate used, type of filler, and temperature [
1]. Increased filler-matrix ratio leads to a reduced degree of conversion, as filler particles can inhibit the polymerization, and could have an effect on curing-light permeability, too. Monomer composition, initiator concentration, and co-initiator/inhibitor system, also affect the depth of cure and hence the degree of conversion of the resin-based composites [
1,
8,
9,
10,
11]. As a solution for the decreasing effect of the inorganic fillers on the curing-light permeability of the resin matrix, the rising temperature may be one alternative. The temperature during polymerization can significantly affect the polymerization efficiency, as an increase from room temperature (22 °C) to mouth temperature (35 °C) results in increased DC (6–10%) as reported by several researchers [
9,
10,
11].
The application of noble nano metals in dental composite is well-known in the literature. The purpose of these investigations is to create resin based esthetic filling materials to protect the formation of secondary caries along the borderline of the cavity, and adjacent tooth structure. The experimental materials release bactericidal noble metals gold and silver ions to effectively prevent the survival of cariogenic bacteria [
12,
13].
Another application of noble metal nanoparticles (NPs) enables the necessary increase in temperature and efficiently releases heat under light irradiation. The heat then diffuses away from the NPs and leads to an elevated temperature of the surrounding medium. This opens up a new set of applications in nanotechnology and gives rise to a new promising field of plasmonic heating [
14].
Gold nanoparticles are highly customizable in size, shape, and surface as well as being biocompatible and chemically stable under various conditions. In addition, they have controllable optical-electronic properties suitable for medical photothermal therapeutic application and biological sensing [
15].
Based on this idea, we have selected spherical gold nanoparticles (AuNPs) for this purpose. However, the resonant excitation of gold nanoparticles requires different wavelengths of light than those used in the dental photocuring system (blue light). Consequently, we have constructed a green-emitting LED curing unit, and our device required a photoinitiator sensitive to the wavelength of 532 nm of green light [
16]. For this reason, the well-known and widely applied Irgacure 784 photoinitiator was chosen for these studies [
17].
The aim of our work was to investigate the thermoplasmonic effect of the green LED excited spherical gold nanoparticles on the physical properties of the dimethacrylate-based dental resin.
4. Discussion
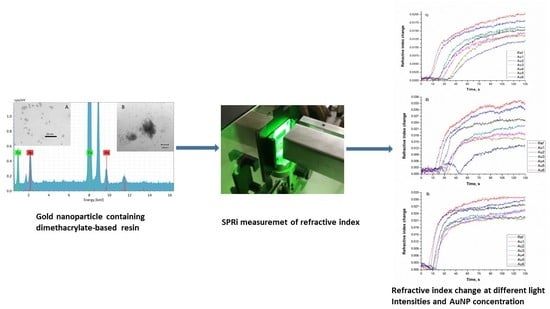
This study aimed to analyze the effect of the thermo-plasmonic effect of AuNPs in an experimental dimethacrylate-based resin. It is well known that AuNPs display surface plasmon resonance (SPR) as a result of irradiating them at a targeted light frequency. SPR yielded a heat to increase the temperature of the surrounding environment and possibly enable the polymerization process. In this study, the AuNP-induced photopolymerization efficiency was investigated by SPRi, transmittance, diametral tensile strength, and degree of conversion. The SPRi measurements were used as a filter to find the optimal AuNPs concentration.
Generally, during a photopolymerization process, inefficient light transmission is a result of surface reflection, photoinitiator, and pigment/dye absorption, scattering by filler particles, and interfacial filler/resin refraction. As the polymerization reaction proceeds, the optical properties change, and the refractive index rises due to a rapid increase in cross-link density and viscosity. Consequently, the polymerization reaction can be monitored with the help of the change of the refractive index in time, thus it seemed to be a suitable method for examining the polymerization kinetics [
21,
22]. In our investigation, increase in the refractive index was detected at undoped reference resin (
Table 1). The measured indices (1.466–1.494) are similar to those reported in the literature [
23,
24]. The changes of the refractive index in sample Ref, as a function of light intensity, corroborate well with our earlier work, in which we observed a significant light intensity dependence of the conversion of Irgacure 784-dimethacrylate resin [
16]. The higher intensity of light was used, the higher refractive index change was detected.
It is visible that the addition of AuNPs to the reaction mixture influences the rate of the photo-polymerization, thanks to their thermoplasmonic effect, i.e., combined effect of temperature and plasmon field.
Table 3 summarizes the change of the refractive index at different light intensities. Au1, Au2, samples showed the highest refractive index change at 1.4 mW/cm
2, the lower value was found at 2.0 mW/cm
2, and the lowest is at 1.0 mW/cm
2 light intensities. We have not observed a significant difference between the refractive index changes. Another important consequence of this measurement is that the Au1, Au2, samples with 0.0208 wt%, 0.0416 wt% AuNPs concentration, respectively, showed the best properties among the samples investigated. Others have found that independently of the composition of the mixture, the refractive index of a photopolymerizable, undoped acrylic formulation varied linearly with the conversion during the reaction. It was also emphasized that the refractive index value of a photopolymerizable medium only depended on the conversion and the temperature, as reported for the Bis-GMA/TEGDMA unloaded resins with a blue light-sensitive photoinitiator system [
22]. Govorov et al. have published a theoretical study in which they estimated the typical time to significantly increase the temperature of the surrounding material (water, ice, and polymer) using a single AuNP and a collection of AuNPs, and described the effect of collective plasmon resonance for the heating enhancement [
25]. They concluded that the light-excited AuNP with light could increase temperature and even melt the surrounding medium. The collective applied AuNPs superstructure can act as an amplifier of the heating effect and also create local areas of high temperature, hot spot (collective plasmon resonance). Because of this, adding AuNPs to the dimethacrylate resin could work also as a hot spot and heat amplifier in our experimental resin to reach the higher conversion. When Au NPs are considered in one medium, this particle is reactive and generates heat, electrons, electric fields and scatters light, on the one hand, it is considered as a solid particle acting as an obstacle in the direction of the exciting light, on the other hand. At higher concentrations, the high number of metal particles and the additional agglomeration of the particles (creating big clusters/obstacles in the direction of the light) can work as an “optical inhibitor” of the photo-polymerization. Thus, if the AuNPs concentration is higher as in the case of samples Au4, Au5 and Au6, such optical inhibition is present, yielding longer initiation time and lower refractive index increment. From the other point of view higher refractive index change was observed for Au1 (0.0326), Au2 (0.0304), at 1.4 mW/cm
2 than at 2.0 mW/cm
2 light intensity. It seems that the optimal intensity of light is around 1.4 mW/cm
2. This phenomenon may be accounted for by the fact that at 2.0 mW/cm
2 light intensity extensive formation of primary radicals from the initiator can take place, which leads to a rapid polymerization resulting in the formation of an incomplete network. The incomplete network probably has a lower refraction index and index increment. In addition, as the polymerization of dimethacrylates proceeds, crosslinked network forms and the propagation becomes diffusion-controlled causing a significant decrease of the polymerization rate (Rp) [
3]. Other researchers have tested embedded silver nanoparticles (AgNPs) in epoxy and methacrylate resins. They have detected a marked increase in the temperature in the extent of polymerization. They have also stated that the principle of plasmonic heating of AgNPs under 420 nm light irradiation can be used to perform the polymerization of a dimethacrylate-based resin initiated by benzoyl peroxide in the absence of photoinitiator. The heat released by the AgNPs results in the thermal decomposition of the benzoyl peroxide and initiates the polymerization [
14]. The possible explanation of our results could be that the light excitation of AuNPs in the resin results in a temperature increase with a help of the thermo plasmonic effect, and the elevated temperature kinetically accelerates and increases the rate of the photo-polymerization procedure. At the same time the presence of a direct plasmon field effect on electron transitions, chemical bonds transformations may be also supposed.
When a metal nanoparticle is illuminated, the intercepted light is partly scattered in the surroundings, and the other part is absorbed and finally dissipated into heat. The balance between scattering and absorption is substantially size-dependent. For instance, while small gold spheres smaller than 10 nm in diameter mainly act as invisible nano sources of heat, scattering processes dominate for diameters larger than 50 nm [
26]. Our dodecanethiol-functionalized spherical gold nanoparticle size is 5 nm, can be potentially able to increase the temperature of the surroundings. The transmittance curves (
Figure 2) demonstrate the absorption of the light in the 525–550 nm spectra, as can be seen on the curves of AuNPs in toluene solution, and AuNPs containing the nanocomposite. The peak at around 450 nm represents the Irgacure 784 photoinitiator transmittance. Earlier Trujillo et al. demonstrated the significant influence of temperature rising on the polymerization rate and conversion of dental composites [
11]. When increasing the temperature of dimethacrylate-based dental composite within the potential biologically compatible limit, increasing polymerization rate and degree of conversion was observed.
According to the literature, statistically, dental composites displayed sufficiently brittle behavior for the diametral tensile test (DTS) to be valid for evaluation of the tensile strength of newer dental composites [
27]. The influence of UDMA, Bis-GMA, and TEGDMA on selected mechanical properties was investigated by Asmussen and Peutzfeldt [
2]. With respect to the fact that these monomers have different molecular stereochemistry and influence on mechanical properties may be different, the mixing ratio is determined by the intended mechanical property of the composite. They observed that DTS increased when Bis-GMA or TEGDMA is replaced by UDMA and when Bis-GMA is replaced by TEGDMA. They explained these findings by the degree of conversion of the polymer matrix and referred to their earlier publication in which they realized the dependence of DTS on the degree of conversion (DC) of the methacrylate double bonds. In their discussion, they concluded that the different monomers could behave differently. Flexible monomer molecules or the ability of urethane linkage to form hydrogen bonds in the copolymer presumably results in restricted sliding of the polymer segments relative to each other [
2,
28]. The DTS data of our reference resin follow the measurements of Asmussen and Peutzfeldt. They tested experimental composites with different ratios of common dimethacrylate (TEGDMA, UDMA, Bis-GMA) components. Their measured mechanical parameters were lower, but they tested (silanized glass) filled resins. Barszczewska-Rybarek has published several factors (chemical structure of dimethacrylate molecules and the formed copolymer network, crosslink density, the degree of conversion) that affect the mechanical properties of the forming polymer [
29]. The literature data prove that the DC is the most evident parameter, defining the dimethacrylate polymer network structure. This is also the most often used technique when structure–property relationships are being investigated. DC highly depends on the monomer chemical structure, initiation technique, curing time, sample thickness, initiator systems, and its concentration, irradiation time and source, and filler content. The minimum DC in the case of conventional dental composites is between 50–55%. The lower DC parameters result in unacceptable clinical use. Homopolymers were arranged according to the following order by limiting DC: Bis-GMA < Bis-EMA < UDMA < TEGDMA. Crosslink density is also an important factor from the point of view of mechanical properties. More theoretical models try to describe the relation of different factors (molecular weight, degree of conversion, double bond concentration). The physical crosslinking in dental dimethacrylate polymer networks results from hydrogen bonding. Hydrogen bonding determines the dimethacrylate monomer viscosity. The lower the viscosity of the dimethacrylate mixture, the higher the degree of conversion. Several instrumental methods are available to allow the DC determination in dimethacrylate polymer networks. Infrared spectroscopy: Fourier-Transform Infrared Spectroscopy (FTIR), Attenuated Total Reflection FTIR, (ATR-FTIR), Near-Infrared Spectroscopy (NIR); Raman spectroscopy, Differential Scanning Calorimetry (DSC) and solid-state Nuclear Magnetic Resonance (ssNMR) are particularly readily used.
Our dimethacrylate resin contained UDMA (53,3%), TEGDMA (25,4%), and Bis-GMA (21,4%) and, therefore, this resin is rich in hydrogen bonds and suitable for cross-link formation. Given the length and elasticity of different dimethacrylate monomers, the theoretical and real crosslink density of their copolymer network affects indirectly the mechanical parameters. The high viscosity aromatic rigid Bis-GMA molecule limits the DC. TEGDMA exhibits relatively high DC, because of favorable stereochemistry. The long flexible chain of dimethacryate glycol acts as a diluent. UDMA is considered also a viscosity reducer and increases DC. The flexibility of urethane linkage why adding this molecule to the mixture to provide better toughness. Our DC data follow the literature. Dental composite displays DC data in the range of 50–77% [
30,
31,
32]. The highest DC and DTS values were measured in the case of the Au1 sample at 1.4 mW/cm
2. Therefore, the ideal AuNPs concentration was applied in sample Au1. Related to the reference resin the higher DC and the consequent higher DTS data could be explained with the presence of AuNPs, and their plasmon effect on the polymerization. From the other side, the lower Au2 DTS (78,92 MPa) and DC (60%) related to Au1 DTS (82,86 MPa) and DC (64%) can be explained with the increased optical inhibition effect of higher AuNPs content.
If we relate the data (DTS, DC) at 1,4 mW/cm2 and 2.0 mW/cm2 it is clear that we obtain lower data at higher intensity whereas differences are not significant. In our earlier work, we described this new dimethacrylate resin containing Irgacure 784 photo-initiator. We showed that the cross-link density does not necessarily change if we increase the light intensity, showing that Irgacure 784 could work successfully at narrow intensities which means the intensity of light is probably not a determinant factor for the results.
Isolated and cluster-forming AuNPs could be seen on the TEM image. Nanoparticles represent the high surface area and display a tendency to agglomerate and form clusters. In the literature, numerical modeling of the temperature evolution time and space was found in the system which contains differently arranged AuNPs. This modeling showed the more agglomerated the AuNPs are, the higher the temperature near that area and the longer the time to reach thermal equilibrium. Thus agglomeration is not ideal for steady heat distribution, but cannot inhibit it [
33]. It seems that AuNPs are applicable to develop and improve dental composite resin, but further investigations are needed.
The limitation of our work is that our material does not contain inorganic filler particles. We applied special initiator (Irgacure 784) that has not been applied in dental resin yet. For the photo-activation we used an experimental LED curing unit that emits in the green light spectra. Our functionalized AuNPs are in a diameter of 5 nm that is not the usual size applied in combination with experimental resins in the literature. We applied low light intensities for the initiation, which is not common in dentistry. In vitro tests have not been undertaken in connection with our experimental resin yet.