Densification: A Route towards Enhanced Thermal Conductivity of Epoxy Composites
Abstract
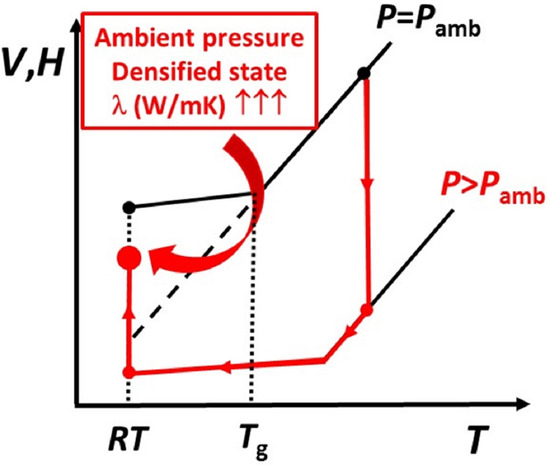
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Thermal Conductivity
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Density Measurement
3. Results and Discussion
3.1. Effect of Pressure on Thermal Conductivity
3.2. Effect of Densification on Enthalpy
3.3. Reversibility of Densification
3.4. Comparison with Literature Values
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vadivelu, M.A.; Kumar, C.R.; Joshi, G.M. Polymer composites for thermal management: A review. Compos. Interfaces 2016, 23, 847–872. [Google Scholar] [CrossRef]
- Xiao, M.; Du, B.X. Review of high thermal conductivity polymer dielectrics for electrical insulation. High Volt. 2016, 1, 34–42. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, J.U.; Lee, H.; Kim, S.Y.; Kim, S.H.; Kim, J.; Jung, Y.C.; Yang, B.J. Thermal management in polymer composites: A review of physical and structural parameters. Adv. Eng. Mater. 2018, 20, 1800204. [Google Scholar] [CrossRef]
- Adnan, M.M.; Tveten, E.G.; Glaum, J.; Ese, M.H.G.; Hvidsten, S.; Glomm, W.; Einarsrud, M.A. Epoxy-Based nanocomposites for high-voltage insulation: A review. Adv. Electr. Mater. 2019, 5, 1800505. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Moradi, S. Thermal conductivity of epoxy-BN composites: A review. Materials 2020, 13, 3634. [Google Scholar] [CrossRef]
- Ma, H.Q.; Gao, B.; Wang, M.Y.; Yuan, Z.Y.; Shen, J.B.; Zhao, J.Q.; Feng, Y.K. Strategies for enhancing thermal conductivity of polymer-based thermal interface materials: A review. J. Mater. Sci. 2020, 56, 1064–1086. [Google Scholar] [CrossRef]
- Bahru, R.; Zamri, M.F.M.A.; Shamsuddin, A.; Shaari, N.; Mohamed, M.A. A review of thermal interface material fabrication method toward enhancing heat dissipation. Int. J. Energy Res. 2020. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, P.; Lv, P.; Xu, T.; Zheng, J.; Ma, C.; Yu, K.; Feng, W.; Wei, W.; Chen, L. Densely packed polymer/boron nitride composite for superior anisotropic thermal conductivity. Polym. Compos. 2018, 39, E1653–E1658. [Google Scholar] [CrossRef]
- Lewis, J.S.; Barani, Z.; Sanchez Magana, A.; Kargar, F.; Balandin, A.A. Thermal and electrical conductivity control in hybrid composites with graphene and boron nitride fillers. Mater. Res. Express 2019, 6, 085325. [Google Scholar] [CrossRef] [Green Version]
- Isarn, I.; Ferrando, F.; Serra, A.; Urbina, C. Novel BN-epoxy/anhydride composites with enhanced thermal conductivity. Polym. Adv. Technol. 2020. [Google Scholar] [CrossRef]
- Chung, S.; Lin, J. Thermal conductivity of epoxy resin composites filled with combustion synthesized h-BN particles. Molecules 2016, 21, 670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, J.; Ren, L.; Yao, Y.; Wang, M.; Zeng, X.; Sun, R.; Xua, J.; Wong, C. Nacre-inspired polymer composites with high thermal conductivity and enhanced mechanical strength. Compos. Part A 2019, 121, 92–99. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Liu, W.; Liu, Y. Functionalization of boron nitride nanoparticles and their utilization in epoxy composites with enhanced thermal conductivity. Phys. Stat. Sol. 2013, 211, 677–684. [Google Scholar] [CrossRef]
- Jang, I.; Shin, K.; Yang, I.; Kim, H.; Kim, J.; Kim, W.; Jeon, S.; Kim, J. Enhancement of thermal conductivity of BN/epoxy composite through surface modification with silane coupling agents. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 64–72. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Yao, Y.; Zeng, X.; Pan, G.; Huang, Y.; Hu, J.; Sun, R.; Xu, J.; Wong, C. Boron nitride microsphere/epoxy composites with enhanced thermal conductivity. High Volt. 2017, 2, 147–153. [Google Scholar] [CrossRef]
- Mun, S.Y.; Lim, H.M.; Lee, S. Thermal and electrical properties of epoxy composite with expanded graphite-ceramic core-shell hybrids. Mater. Res. Bull. 2018, 97, 19–23. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic effects of boron nitride (BN) nanosheets and silver (Ag) nanoparticles on thermal conductivity and electrical properties of epoxy nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Chung, D.D.L. Increasing the thermal conductivity of boron nitride and aluminum nitride particle epoxy-matrix composites by particle surface treatments. Compos. Interfaces 2000, 7, 243–256. [Google Scholar] [CrossRef]
- Hong, J.; Yoon, S.; Hwang, T.; Lee, Y.; Won, S.; Nam, J. Interphase control of boron nitride/epoxy composites for high thermal conductivity. Korea-Aust. Rheol. J. 2010, 22, 259–264. [Google Scholar]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. The adsorption of cationic surfactants on BN surface: Its effects on the thermal conductivity and mechanical properties of BN-epoxy composite. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 203–210. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Effective surface treatments for enhancing the thermal conductivity of BN-filled epoxy composite. J. Appl. Polym. Sci. 2011, 119, 3234–3243. [Google Scholar] [CrossRef]
- Hong, J.; Yoon, S.; Hwang, T.; Oh, J.; Hong, S.; Lee, Y.; Nam, J. High thermal conductivity epoxy composites with bimodal distribution of aluminum nitride and boron nitride fillers. Thermochim. Acta 2012, 537, 70–75. [Google Scholar] [CrossRef]
- Xia, C.; Garcia, A.C.; Shi, S.Q.; Qiu, Y.; Warner, N.; Wu, Y.; Cai, L.; Rizvi, H.R.; D’Souza, N.A.; Nie, X. Hybrid boron nitride-natural fiber composites for enhanced thermal conductivity. Sci. Rep. 2016, 6, 34726. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, Y.; Zeng, X.; Li, Q.; Ren, L.; Sun, R.; Xu, J.; Wong, C. Polymer composite with enhanced thermal conductivity and mechanical strength through orientation manipulating of BN. Compos. Sci. Technol. 2018, 160, 127–137. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Luo, X.; Zheng, N.; Ma, T.; Tan, J.; Li, C.; Zhang, Q.; Gu, J. Self-healing, recoverable epoxy elastomers and their composites with desirable thermal conductivities by incorporating BN fillers via in-situ polymerization. Compos. Sci. Technol. 2018, 164, 59–64. [Google Scholar] [CrossRef]
- Tang, L.; He, M.; Na, X.; Guan, X.; Zhang, R.; Zhang, J.; Gu, J. Functionalized glass fibers cloth/spherical BN fillers/epoxy laminated composites with excellent thermal conductivities and electrical insulation properties. Compos. Commun. 2019, 16, 5–10. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, X. Novel functionalized BN nanosheets/epoxy composites with advanced thermal conductivity and mechanical properties. ACS Appl. Mater. Interfaces 2020, 12, 6503–6515. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Shi, X.-T.; Tang, L.; Liu, Z.; Zhang, J.-L.; Guo, Y.-Q.; Gu, J.-W. Thermally conductive and insulating epoxy composites by synchronously incorporating Si-sol functionalized glass fibers and boron nitride fillers. Chin. J. Polym. Sci. 2020, 38, 730–739. [Google Scholar] [CrossRef]
- Hutchinson, J.M. Physical aging of polymers. Prog. Polym. Sci. 1995, 20, 703–760. [Google Scholar] [CrossRef]
- Hutchinson, J.M. Relaxation processes and physical aging. In The Physics of Glassy Polymers, 2nd ed.; Haward, R.N., Young, R.J., Eds.; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Senapati, H.; Parthasarathy, G.; Lakshmikumar, S.T.; Rao, K.J. Effect of pressure on the fast-ion conduction in AgI-Ag2O-AgMoO3 glasses. Phil. Mag. B 1983, 47, 291–297. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Ingram, M.D.; Robertson, A.H.J. The effects of pressure and densification on ionic conductivities in silver iodomolybdate glasses. Phil. Mag. B 1992, 66, 449–461. [Google Scholar] [CrossRef]
- Carbotherm BN Thermal Fillers. Available online: https://www.bn.saint-gobain.com/sites/imdf.bn.com/files/carbotherm-bn-thermal-fillers-ds_0.pdf (accessed on 10 December 2020).
- Moradi, S.; Calventus, Y.; Román, F.; Hutchinson, J.M. Achieving high thermal conductivity in epoxy composites: Effect of boron nitride particle size and matrix-filler interface. Polymers 2019, 11, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, S.; Calventus, Y.; Román, F.; Ruiz, P.; Hutchinson, J.M. Epoxy composites filled with boron nitride: Cure kinetics and the effect of particle shape on the thermal conductivity. J. Therm. Anal. Calorim. 2020, 142, 595–605. [Google Scholar] [CrossRef]
- Hammerschmidt, U.; Meier, V. New Transient Hot-Bridge sensor to measure thermal conductivity, thermal diffusivity, and volumetric specific heat. Int. J. Thermophys. 2006, 27, 840–865. [Google Scholar] [CrossRef]
- Moradi, S.; Román, F.; Calventus, Y.; Hutchinson, J.M. Remarkable thermal conductivity of epoxy composites filled with boron nitride and cured under pressure. J. Appl. Polym. Sci. 2021. submitted. [Google Scholar]






| Sample | Pressure (MPa) | Thermal Conductivity (W/mK) | Density (g/cm3) |
|---|---|---|---|
| S1 | ambient | 3.34 | 1.55 |
| S2 | 0.175 | 4.77 | 1.56 |
| S3 | 1.4 | 6.47 | 1.63 |
| S4 | 2.0 | 7.67 | 1.71 |
| S5 | 2.0 | 6.86 | 1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradi, S.; Román, F.; Calventus, Y.; Hutchinson, J.M. Densification: A Route towards Enhanced Thermal Conductivity of Epoxy Composites. Polymers 2021, 13, 286. https://doi.org/10.3390/polym13020286
Moradi S, Román F, Calventus Y, Hutchinson JM. Densification: A Route towards Enhanced Thermal Conductivity of Epoxy Composites. Polymers. 2021; 13(2):286. https://doi.org/10.3390/polym13020286
Chicago/Turabian StyleMoradi, Sasan, Frida Román, Yolanda Calventus, and John M. Hutchinson. 2021. "Densification: A Route towards Enhanced Thermal Conductivity of Epoxy Composites" Polymers 13, no. 2: 286. https://doi.org/10.3390/polym13020286
APA StyleMoradi, S., Román, F., Calventus, Y., & Hutchinson, J. M. (2021). Densification: A Route towards Enhanced Thermal Conductivity of Epoxy Composites. Polymers, 13(2), 286. https://doi.org/10.3390/polym13020286