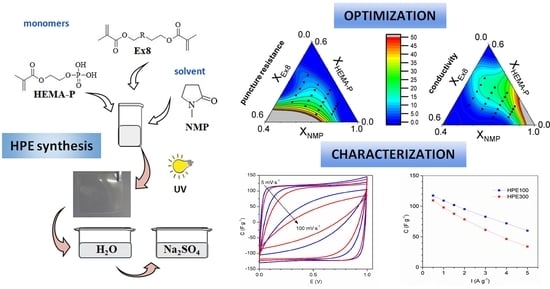
Optimization of the Properties of Photocured Hydrogels for Use in Electrochemical Capacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Photopolymerization Kinetics
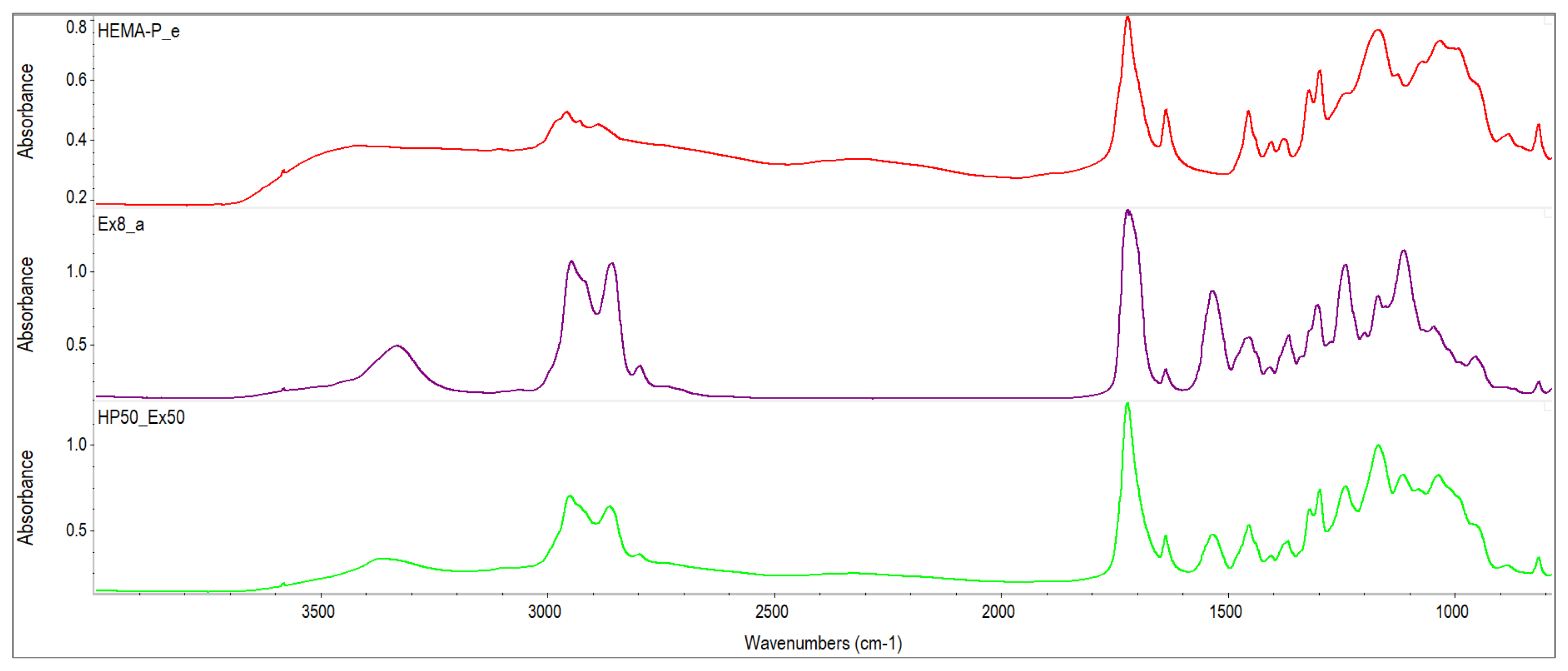
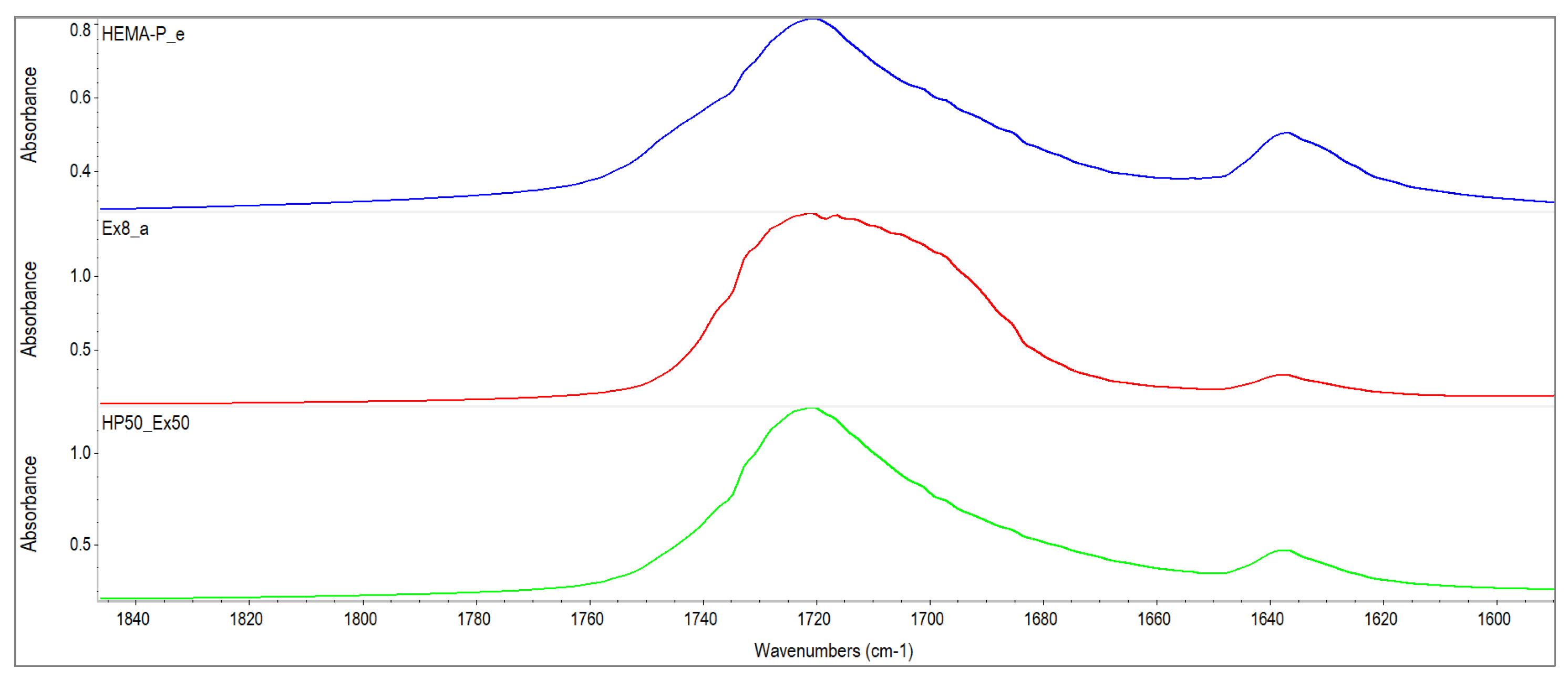
2.2.2. Characterization of Reagents
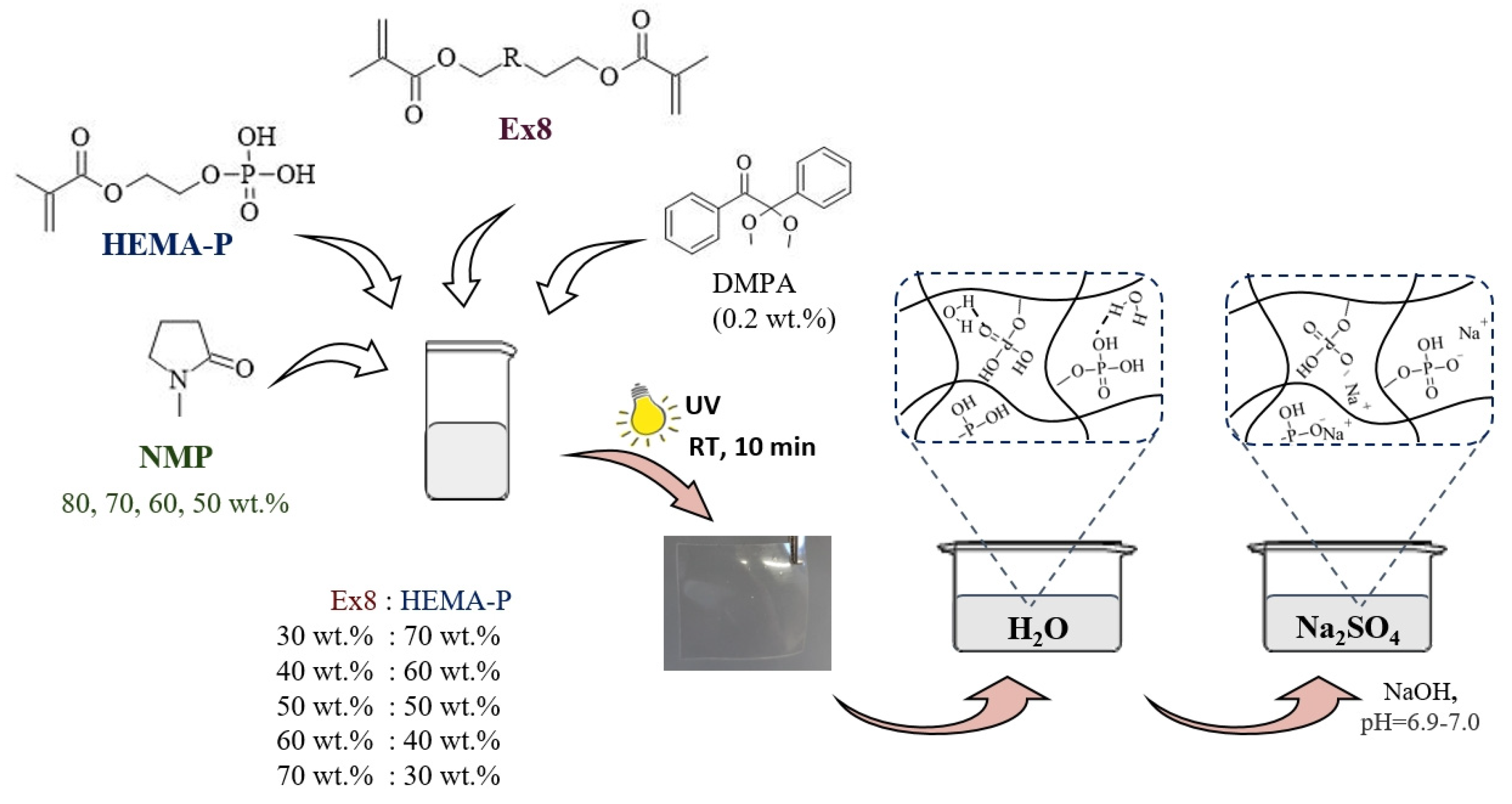
2.2.3. Hydrogel Preparation
2.2.4. Water and Electrolyte Sorption
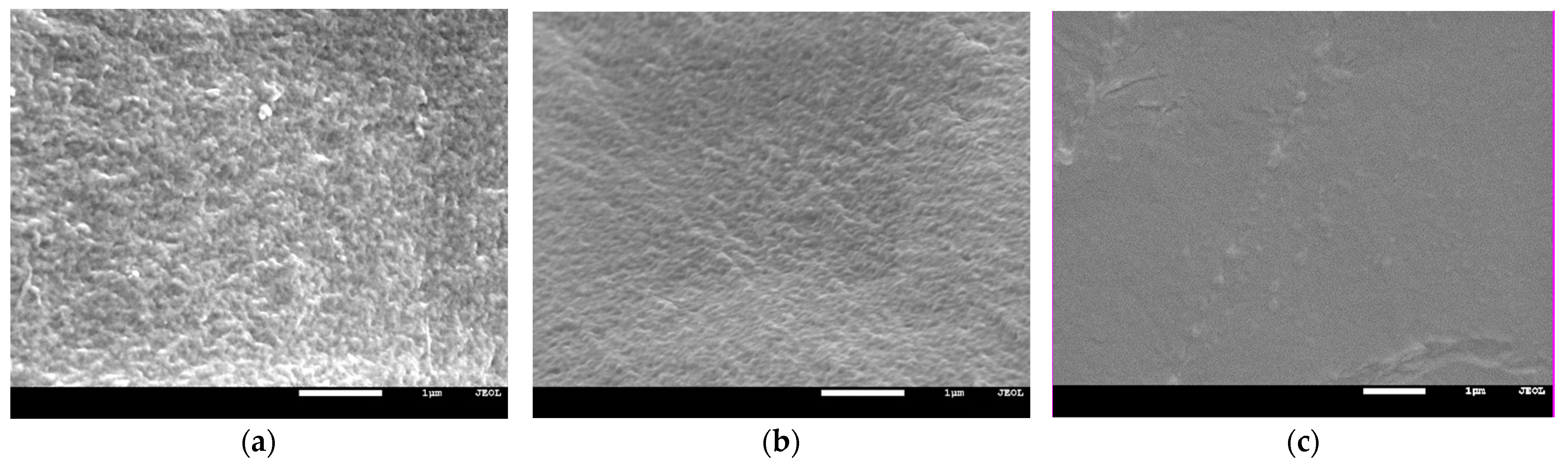
2.2.5. Scanning Electron Microscope (SEM)
2.2.6. Puncture Resistance
2.2.7. Ionic Conductivity
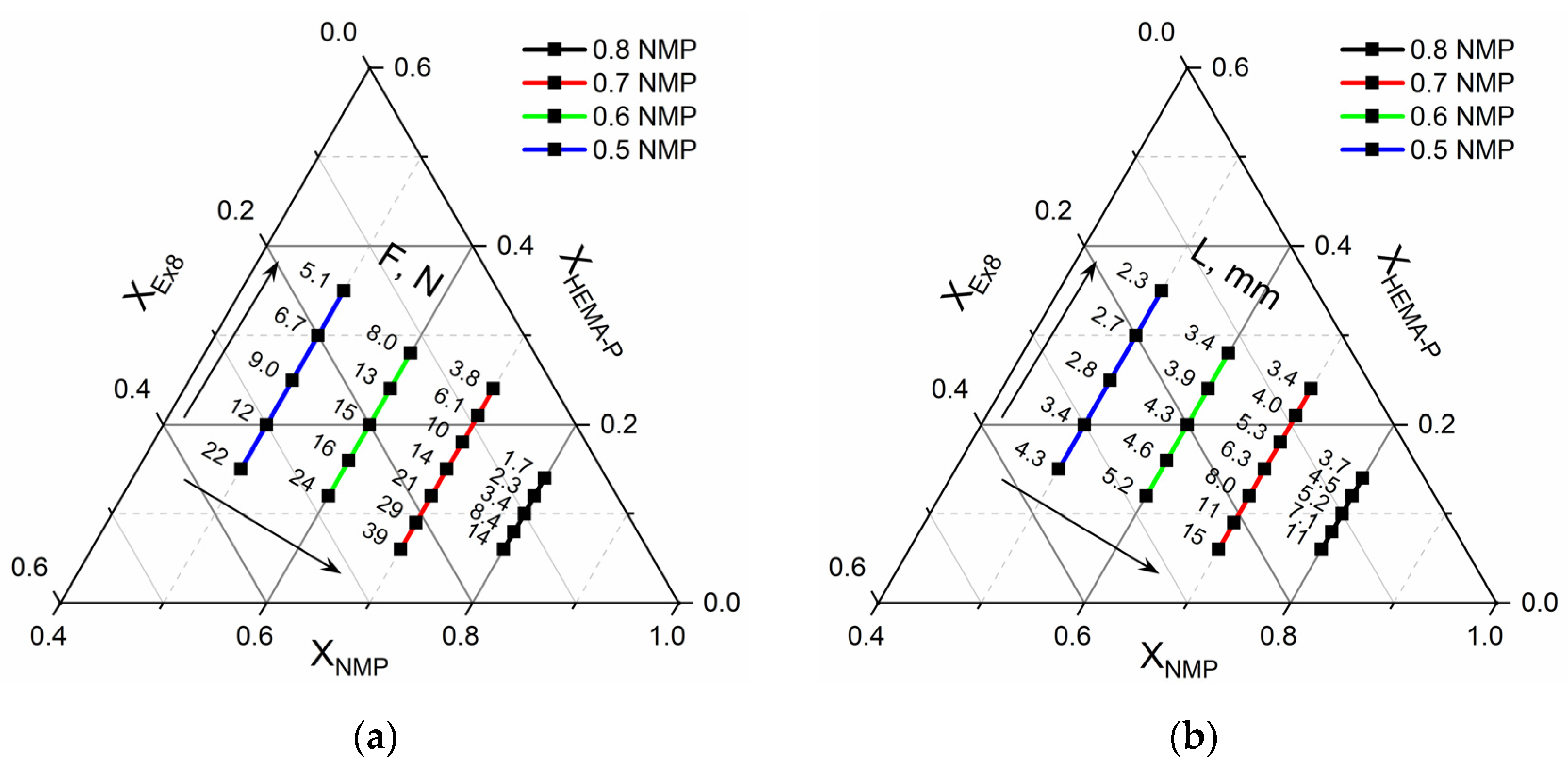
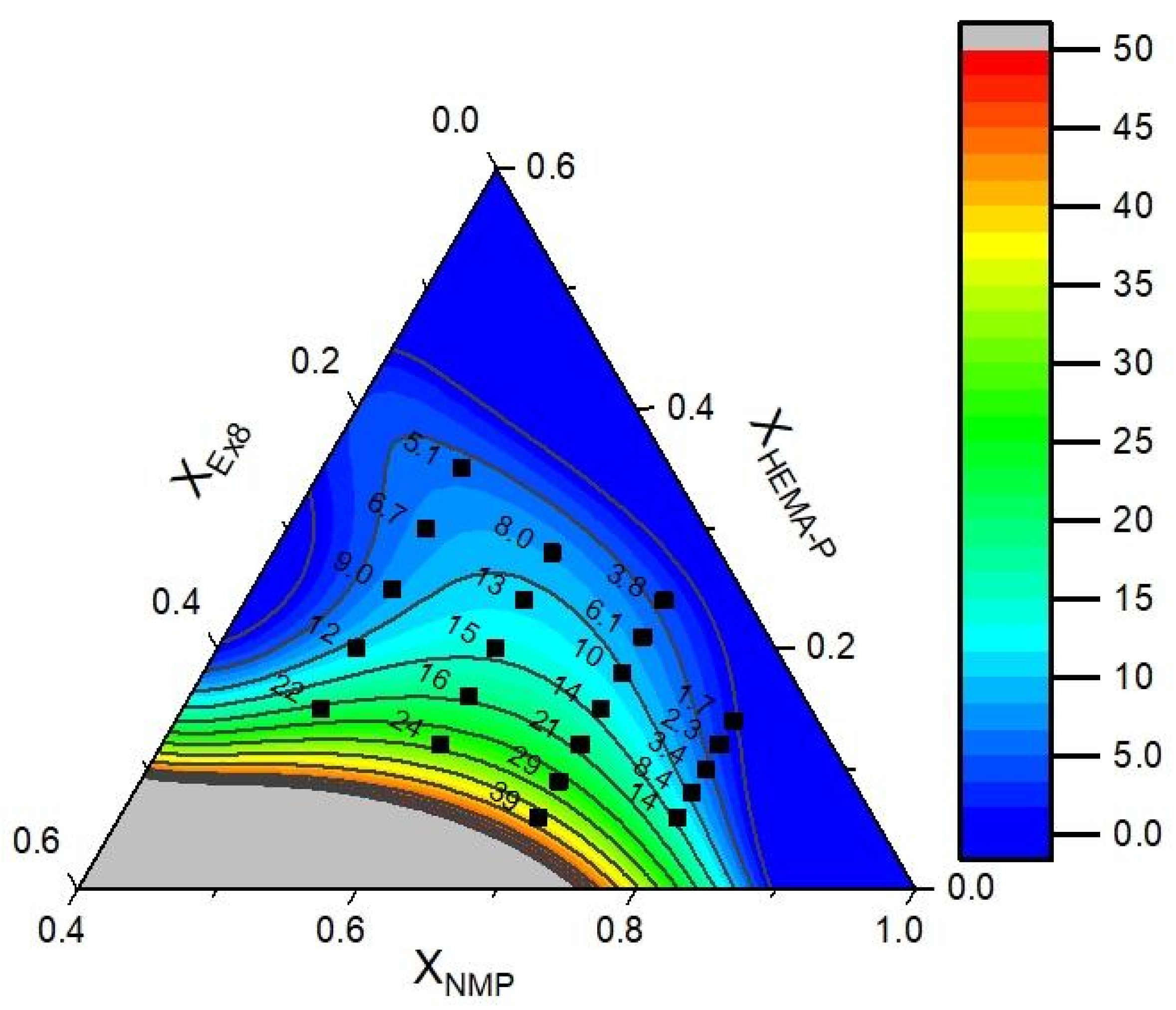
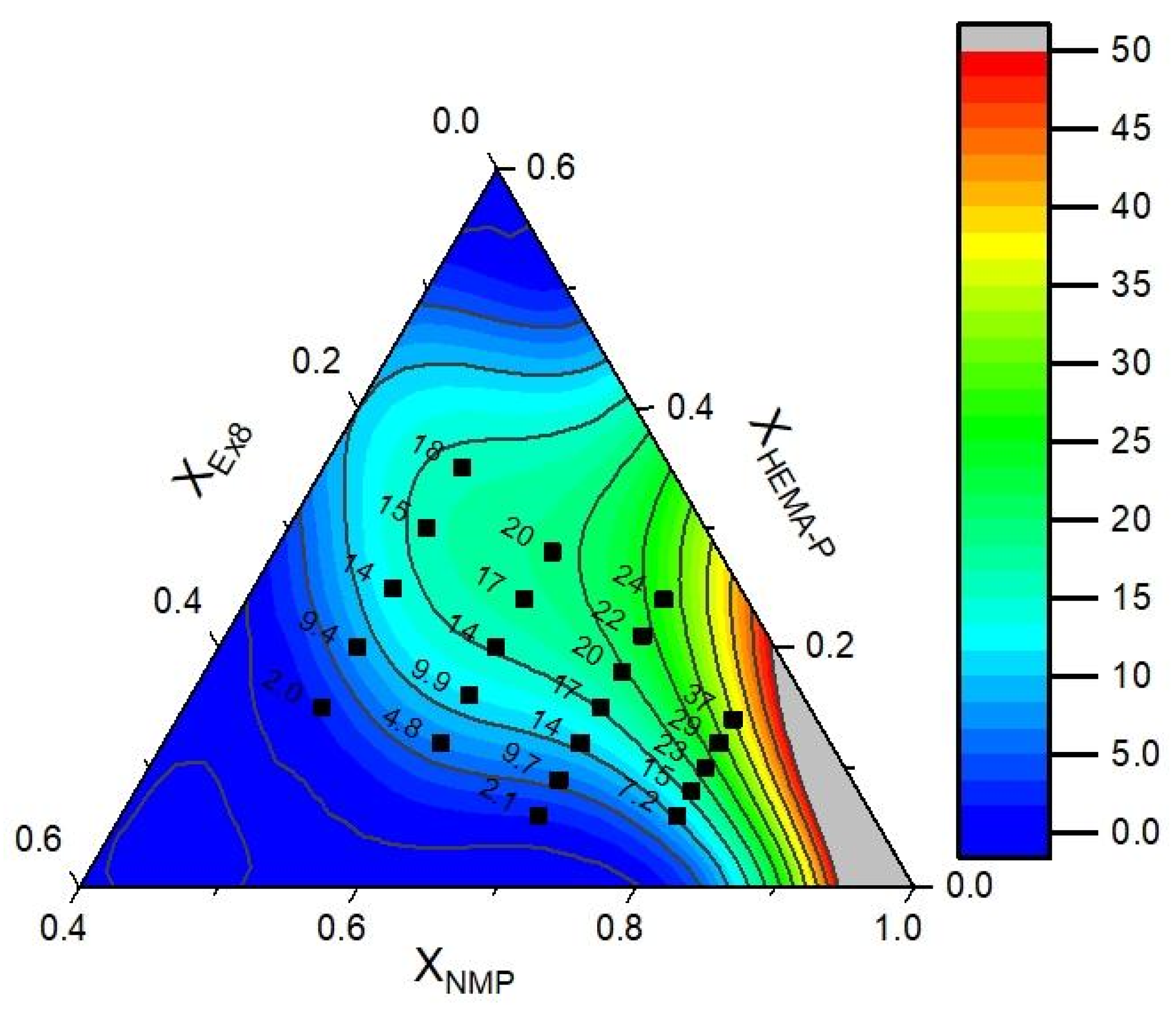
2.2.8. Optimization of Hydrogel Polymer Electrolyte Composition
b23·XHEMA-P·XEx8 + d12·XNMP·XHEMA-P·(XNMP − XHEMA-P) + d13·XNMP·XEx8·(XNMP − XEx8) +
d23·XHEMA-P·XEx8·(XHEMA-P − XEx8) + b123·XNMP·XHEMA-P·XEx8
2.2.9. Electrochemical Measurements of Capacitors
Preparation of Electrodes
Electrochemical Investigations
3. Results
3.1. Characterization of Reagents
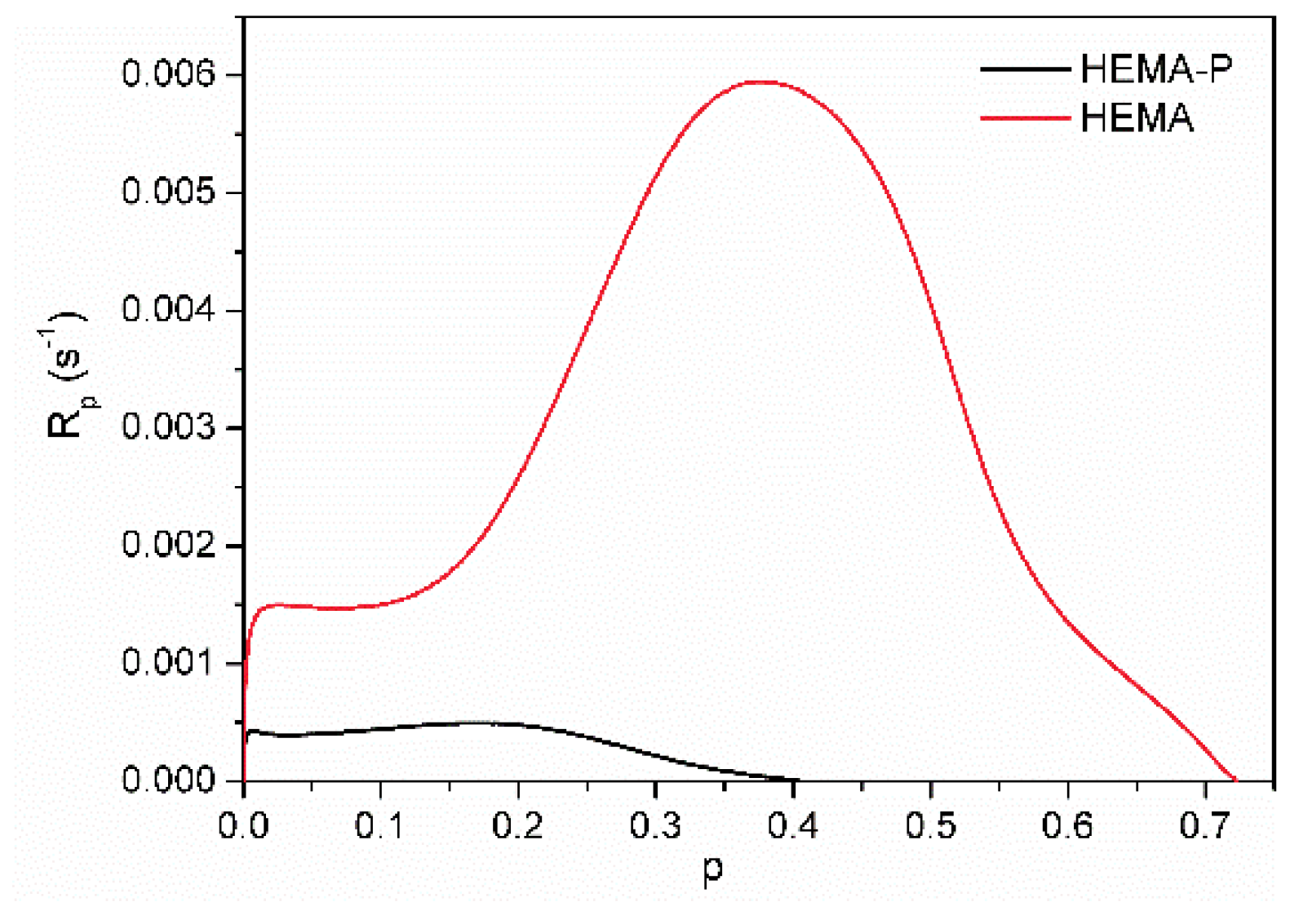
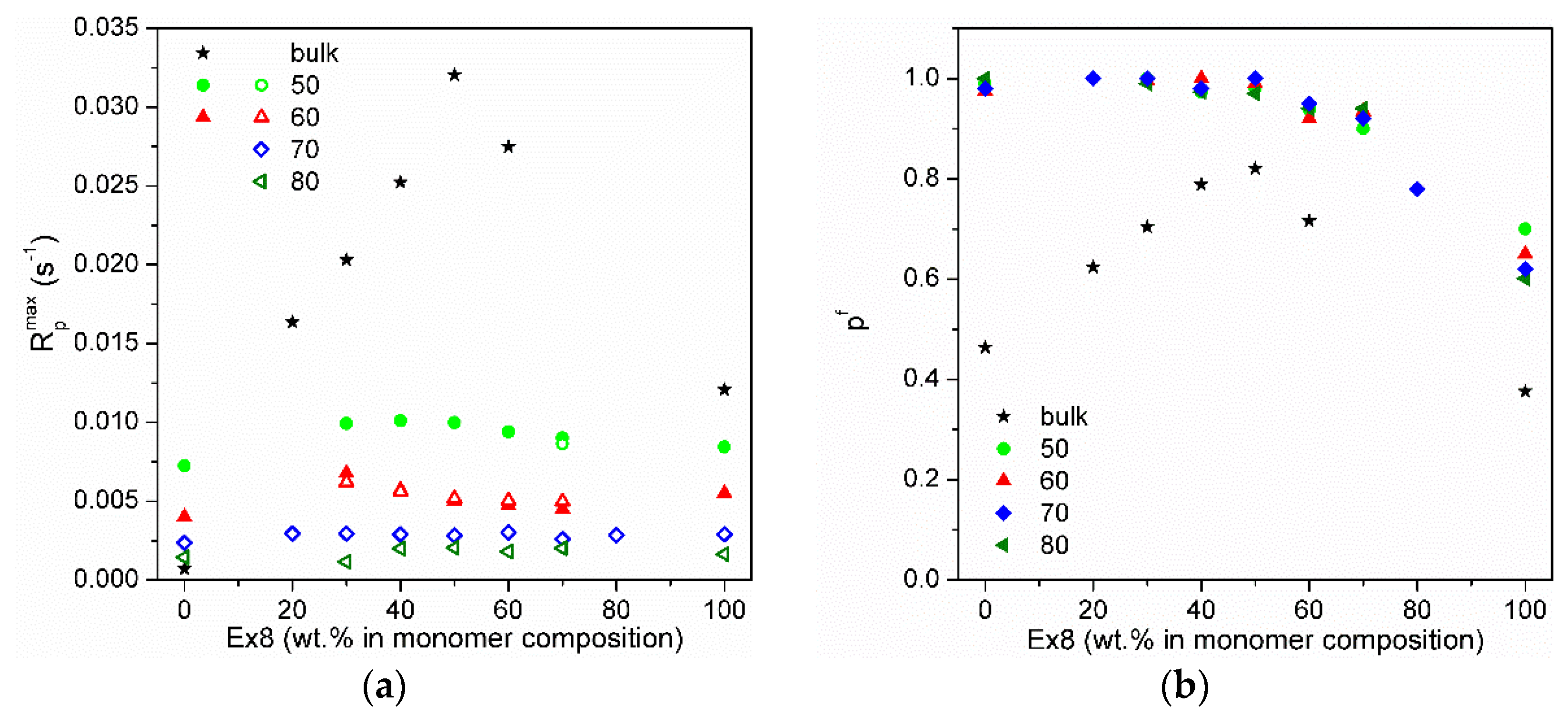
3.2. Photopolymerization Kinetic
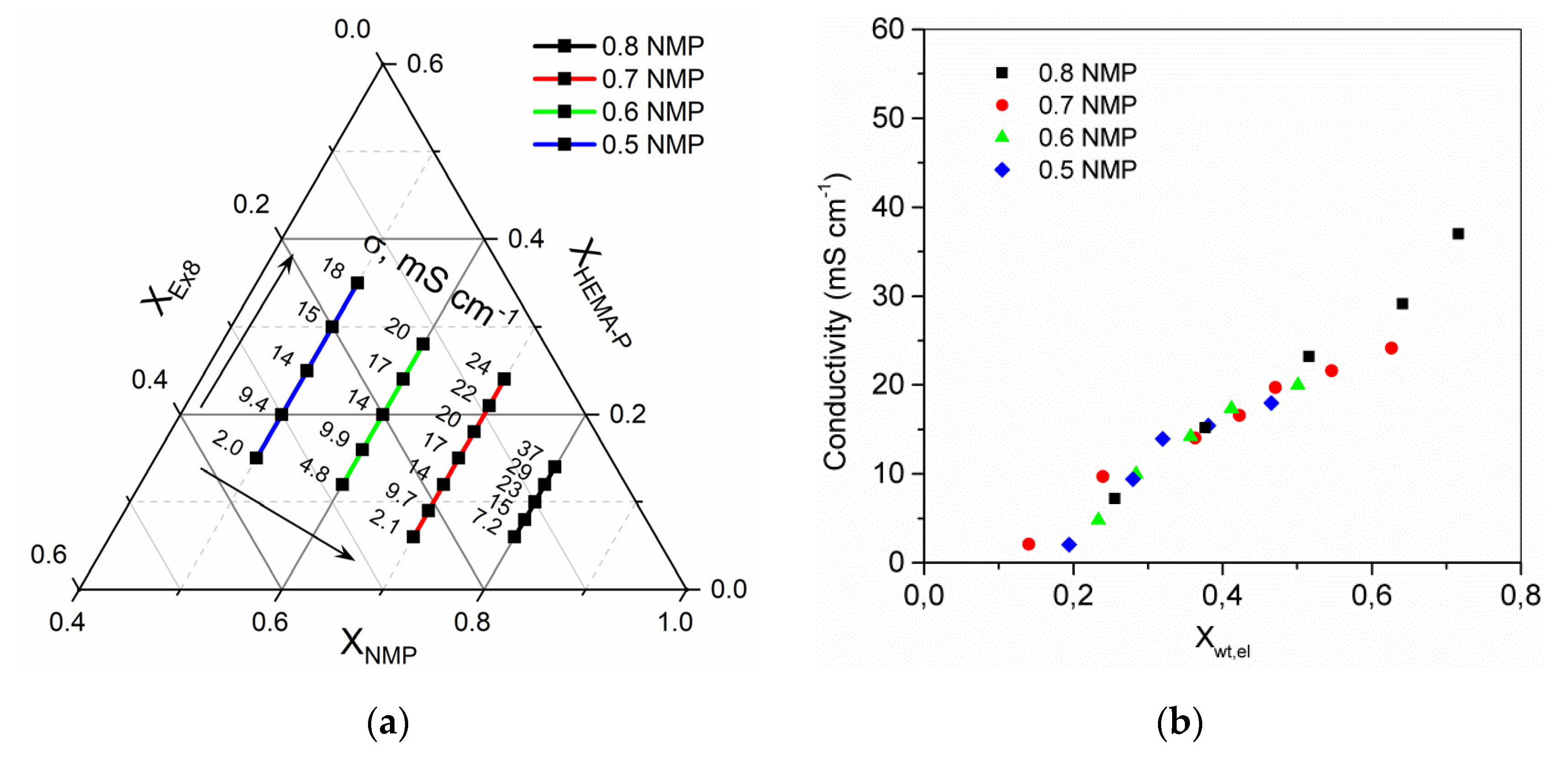
3.3. Electrolyte Sorption and Conductivity
3.4. Mechanical Properties
3.5. Optimization of the Photocurable Mixture Composition
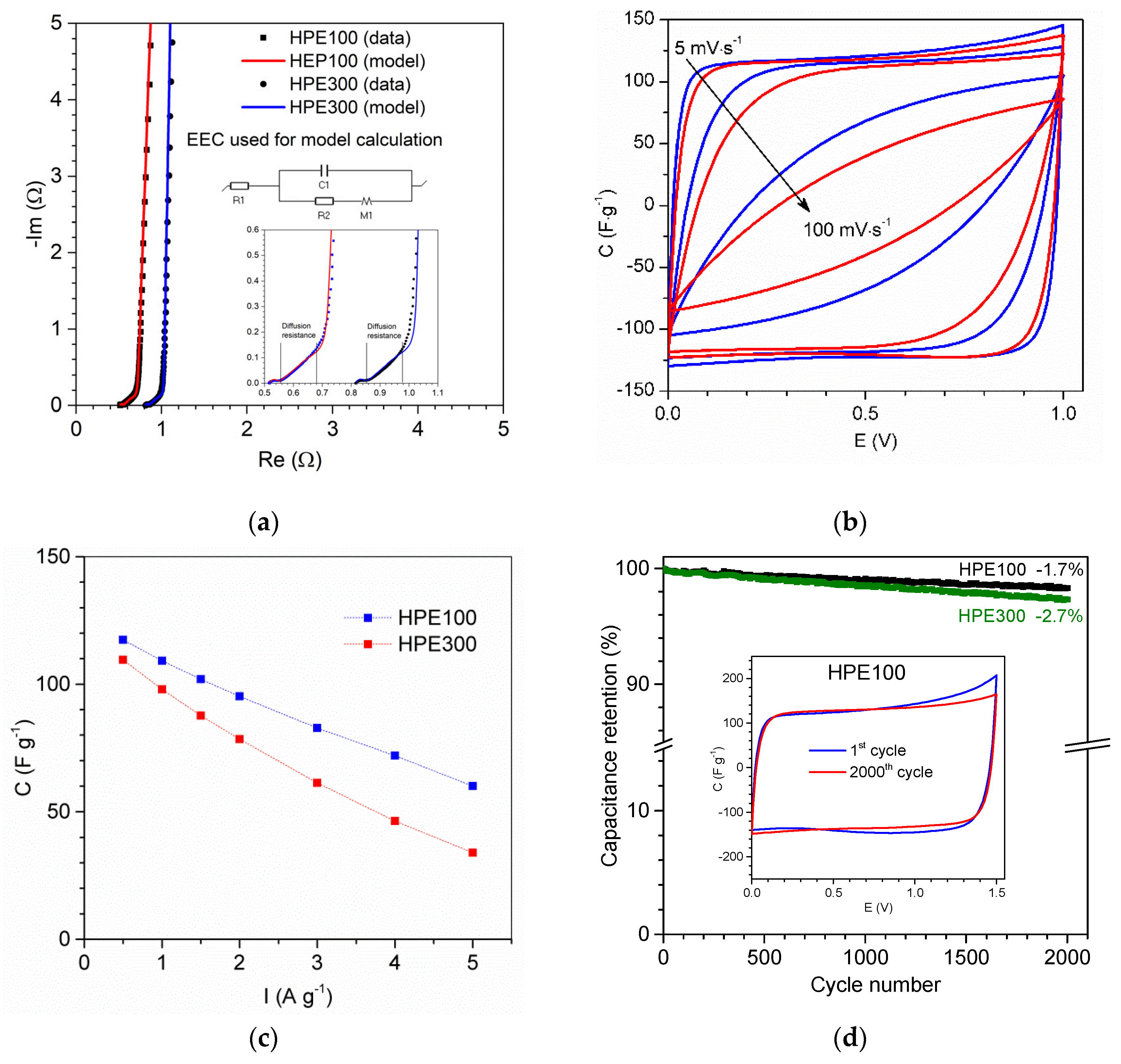
3.6. Electrochemical Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Jiang, C.; Sun, N.; Tan, D.; Li, Q.; Bi, S.; Song, J. Recent progress in multifunctional hydrogel-based supercapacitors. J. Sci. Adv. Mater. Devices 2021, 6, 338–350. [Google Scholar] [CrossRef]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical supercapacitors for energy storage and conversion. In Handbook of Clean Energy Systems; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–25. [Google Scholar] [CrossRef]
- Lin, Z.; Taberna, P.-L.; Simon, P. Electrochemical double layer capacitors: What is next beyond the corner? Curr. Opin. Electrochem. 2017, 6, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.; Choudhury, N.A.; Shukla, A.K. Hydrogel membrane electrolyte for electrochemical capacitors. J. Chem. Sci. 2009, 121, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, N.A.; Sampath, S.; Shukla, A.K. Hydrogel-polymer electrolytes for electrochemical capacitors: An overview. Energy Environ. Sci. 2008, 2, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhang, X.; Li, C.; Zhang, H.; Sun, X.; Xu, N.; Ma, Y. Flexible solid-state supercapacitors based on a conducting polymer hydrogel with enhanced electrochemical performance. J. Mater. Chem. A 2014, 2, 19726–19732. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, X.; Dong, B.; Sun, N.; Zheng, L. Poly(ionic liquid) hydrogels exhibiting superior mechanical and electrochemical properties as flexible electrolytes. J. Mater. Chem. A 2015, 4, 1112–1118. [Google Scholar] [CrossRef]
- Wada, H.; Nohara, S.; Furukawa, N.; Inoue, H.; Sugoh, N.; Iwasaki, H.; Morita, M.; Iwakura, C. Electrochemical characteristics of electric double layer capacitor using sulfonated polypropylene separator impregnated with polymer hydrogel electrolyte. Electrochim. Acta 2004, 49, 4871–4875. [Google Scholar] [CrossRef]
- Fei, H.; Yang, C.; Bao, H.; Wang, G. Flexible all-solid-state supercapacitors based on graphene/carbon black nanoparticle film electrodes and cross-linked poly(vinyl alcohol)—H2SO4 porous gel electrolytes. J. Power Sources 2014, 266, 488–495. [Google Scholar] [CrossRef]
- Wada, H.; Yoshikawa, K.; Nohara, S.; Furukawa, N.; Inoue, H.; Sugoh, N.; Iwasaki, H.; Iwakura, C. Electrochemical characteristics of new electric double layer capacitor with acidic polymer hydrogel electrolyte. J. Power Sources 2006, 159, 1464–1467. [Google Scholar] [CrossRef]
- Batisse, N.; Raymundo-Piñero, E. A self-standing hydrogel neutral electrolyte for high voltage and safe flexible supercapacitors. J. Power Sources 2017, 348, 168–174. [Google Scholar] [CrossRef]
- Stepniak, I.; Ciszewski, A. Electric double layer capacitors with polymer hydrogel electrolyte based on poly(acrylamide) and modified electrode and separator materials. Electrochim. Acta 2009, 54, 7396–7400. [Google Scholar] [CrossRef]
- Gomez, I.; Alesanco, Y.; Blázquez, J.A.; Viñuales, A.; Colmenares, L.C. Room-Temperature Self-Standing Cellulose-Based Hydrogel Electrolytes for Electrochemical Devices. Polymers 2020, 12, 2686. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.A.; Tan, B.; Freeman, A.W.; Gagnon, J.C.; Hoffman, C.; Logan, M.W.; Maranchi, J.P.; Gerasopoulos, K. UV-cured gel polymer electrolytes with improved stability for advanced aqueous Li-ion batteries. Chem. Commun. 2019, 55, 13085–13088. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Rana, H.; Lee, J.Y.; Park, H.S. Renewable flexible supercapacitors based on all-lignin-based hydrogel electrolytes and nanofiber electrodes. J. Mater. Chem. A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Liu, J.; Zhang, J.; Ma, X.; Huang, Y. An intrinsically compressible and stretchable all-in-one configured supercapacitor. Chem. Commun. 2018, 54, 6200–6203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, Q. An Omni-Healable Supercapacitor Integrated in Dynamically Cross-Linked Polymer Networks. Adv. Funct. Mater. 2017, 27, 1700690. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Pan, Q. An all-in-one self-healable capacitor with superior performance. J. Mater. Chem. A 2018, 6, 2500–2506. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Lewandowska, A.; Gajewski, P.; Szcześniak, K.; Sadej, M.; Patelski, P.; Marcinkowska, A. Modification of Thiol-Ene Ionogels with Octakis(methacryloxypropyl) Silsesquioxane. Polymers 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Esstech, Inc. Item #X-891-0000, Exothane 8 On Esstech, Inc. Available online: https://catalog.esstechinc.com/item/all-categories/esstech-specialty-chemicals-exothane/x-891-0000-2 (accessed on 11 September 2021).


| Formulation | Ex8 | HEMA-P | NMP |
|---|---|---|---|
| wt.% | |||
| 30Ex:70HP_50NMP | 15 | 35 | 50 |
| 40Ex:60HP_50NMP | 20 | 30 | 50 |
| 50Ex:50HP_50NMP | 25 | 25 | 50 |
| 60Ex:40HP_50NMP | 30 | 20 | 50 |
| 70Ex:30HP_50NMP | 35 | 15 | 50 |
| 30Ex:70HP_60NMP | 12 | 28 | 60 |
| 40Ex:60HP_60NMP | 16 | 24 | 60 |
| 50Ex:50HP_60NMP | 20 | 20 | 60 |
| 60Ex:40HP_60NMP | 24 | 16 | 60 |
| 70Ex:30HP_60NMP | 28 | 12 | 60 |
| 20Ex:80Ex_70NMP | 6 | 24 | 70 |
| 30Ex:70HP_70NMP | 9 | 21 | 70 |
| 40Ex:60HP_70NMP | 12 | 18 | 70 |
| 50Ex:50HP_70NMP | 15 | 15 | 70 |
| 60Ex:40HP_70NPM | 18 | 12 | 70 |
| 70Ex:30HP_70NMP | 21 | 9 | 70 |
| 80Ex:20HP_70NMP | 24 | 6 | 70 |
| 30Ex:70HP_80NMP | 6 | 14 | 80 |
| 40Ex:60HP_80NMP | 8 | 12 | 80 |
| 50Ex:50HP_80NMP | 10 | 10 | 80 |
| 60Ex:40HP_80NMP | 12 | 8 | 80 |
| 70Ex:30HP_80NMP | 14 | 6 | 80 |
| Reagent | Viscosity, mPas |
|---|---|
| Ex8 | 151,000 |
| HEMA-P | 578 |
| HEMA | 5.74 |
| NMP | 1.81 |
| Parameter | Mathematical Model of Puncture Resistance RMS = 4.0, R2 = 0.95 | Mathematical Model of Conductivity RMS = 3.8, R2 = 0.95 | ||
|---|---|---|---|---|
| Value | p-Value 1 | Value | p-Value 1 | |
| b1 | −41.8 | <0.001 | 92.8 | <0.001 |
| b2 | −346 | 0.022 | −93.2 | 0.001 |
| b3 | 230 | <0.001 | −1282 | 0.008 |
| b12 | 600 | <0.001 | - | - |
| b13 | 146 | 0.002 | 2228 | 0.034 |
| b23 | - | - | 1480 | 0.022 |
| b123 | −1128 | <0.001 | - | - |
| d12 | - | - | −41.5 | 0.025 |
| d13 | - | - | −1874 | 0.001 |
| d23 | 2584 | 0.033 | - | - |
| Optimal Mixture Composition | Conductivity and Puncture Resistance | |
|---|---|---|
| Mathematical Model | Experimental Value | |
| XNMP = 0.66 XHEMA-P = 0.17 XEx8 = 0.17 | σ = 15.5 ± 1.3 mS·cm−1 | σ = 15.7 ± 1.1 mS·cm−1 |
| F = 14.9 ± 0.9 N | F = 16.5 ± 1.4 N | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewski, P.; Lewandowska, A.; Szcześniak, K.; Przesławski, G.; Marcinkowska, A. Optimization of the Properties of Photocured Hydrogels for Use in Electrochemical Capacitors. Polymers 2021, 13, 3495. https://doi.org/10.3390/polym13203495
Gajewski P, Lewandowska A, Szcześniak K, Przesławski G, Marcinkowska A. Optimization of the Properties of Photocured Hydrogels for Use in Electrochemical Capacitors. Polymers. 2021; 13(20):3495. https://doi.org/10.3390/polym13203495
Chicago/Turabian StyleGajewski, Piotr, Aneta Lewandowska, Katarzyna Szcześniak, Grzegorz Przesławski, and Agnieszka Marcinkowska. 2021. "Optimization of the Properties of Photocured Hydrogels for Use in Electrochemical Capacitors" Polymers 13, no. 20: 3495. https://doi.org/10.3390/polym13203495
APA StyleGajewski, P., Lewandowska, A., Szcześniak, K., Przesławski, G., & Marcinkowska, A. (2021). Optimization of the Properties of Photocured Hydrogels for Use in Electrochemical Capacitors. Polymers, 13(20), 3495. https://doi.org/10.3390/polym13203495