Disposable Food Packaging and Serving Materials—Trends and Biodegradability
Abstract
:1. Introduction
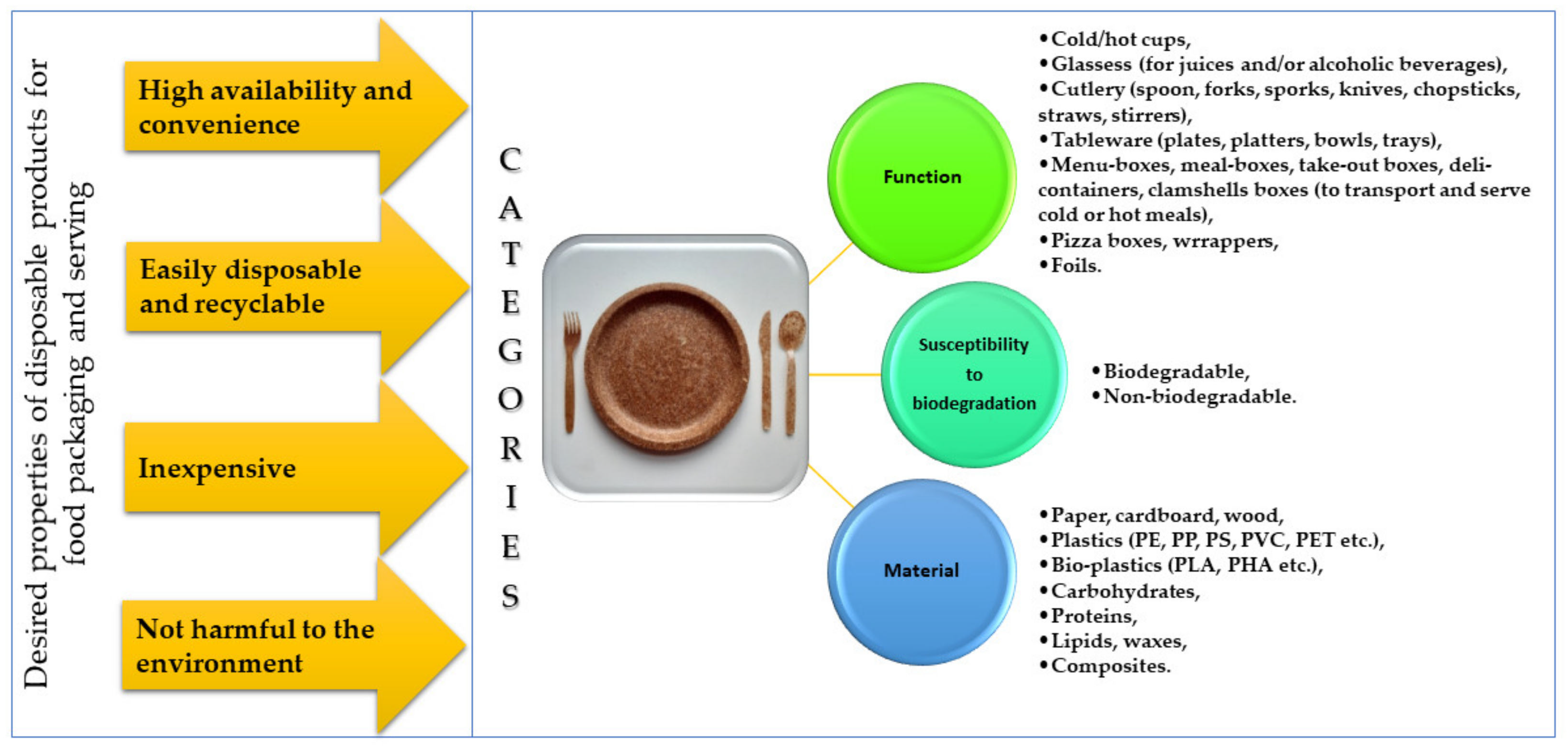
2. Disposable Tableware and Cutlery—Categories and Classification
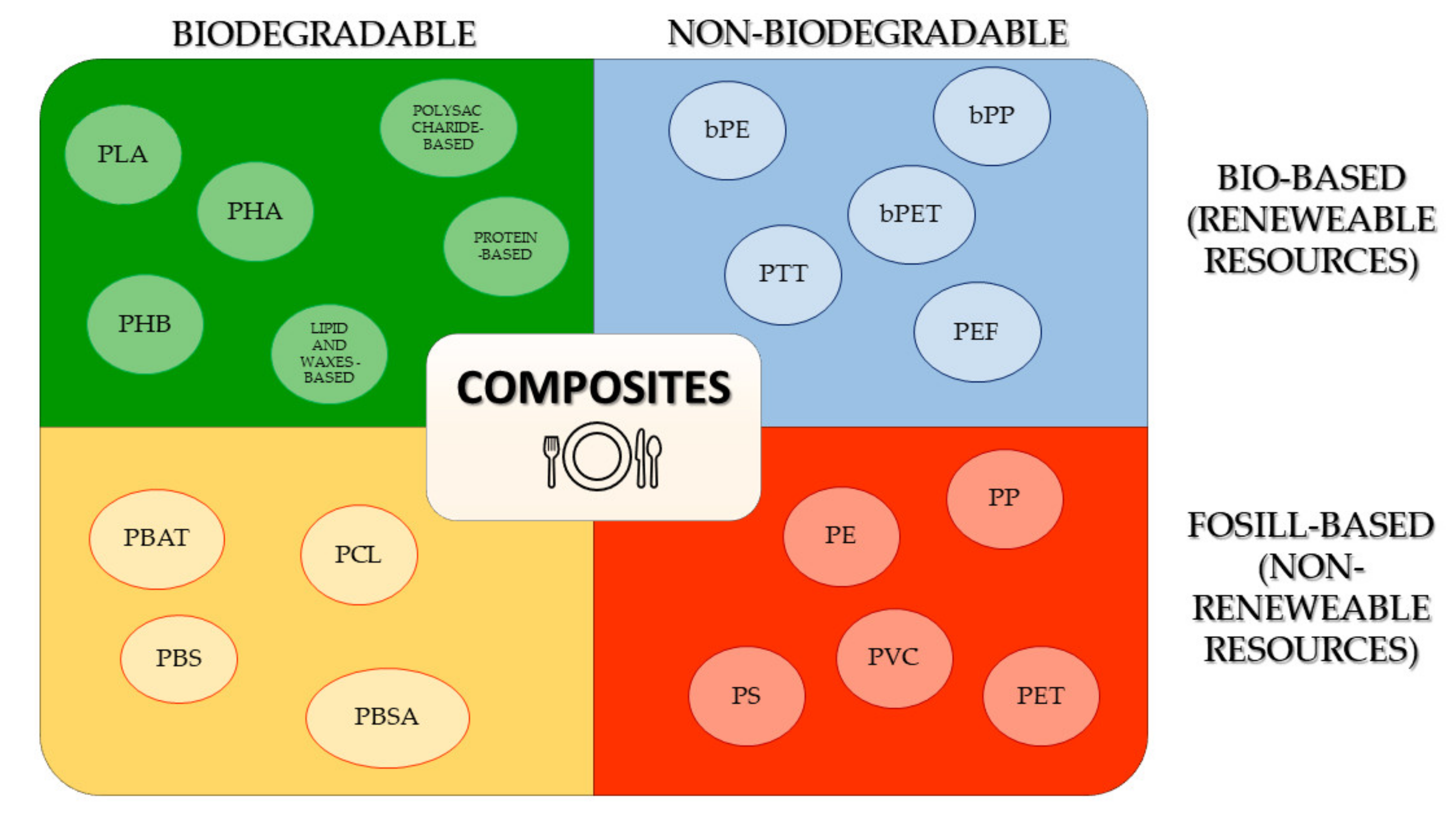
3. Conventional Materials Used in the Production of Dishes and Cutlery
3.1. Classical Polymers
3.1.1. Polypropylene
3.1.2. Polyethylene
3.1.3. Polyvinyl Chloride
3.1.4. Polyethylene Terephthalate
3.1.5. Polystyrene
3.2. Bio-Based, Biodegradable Polymers
3.2.1. Natural Polymers Produced by Living Organisms
Polysaccharides
Proteins
3.2.2. Synthetically Produced Polymers
Polylactide (PLA)
Polyethylene Furanoate (PEF)
Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA)
4. Dishes Made of Wastes from the Agro-Food Industry
5. Edible Tableware and Cutlery—Strength and Microbiological Safety
- physical and mechanical parameters of these products such as the strength on flexural and resistant to leakage or changeable temperature,
- biological and chemical safety of ingredients included in their composition,
- the way they are produced, packaged, and transported to the customer.
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Parliament. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment (Text with EEA Relevance); PE/11/2019/REV/1; European Parliament: Brussels, Belgium, 2019. [Google Scholar]
- Statista. Annual Production of Plastics Worldwide from 1950 to 2020. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 10 April 2021).
- Weston, J.N.J.; Carrillo-Barragan, P.; Linley, T.D.; Reid, W.D.K.; Jamieson, A.J. New species of Eurythenes from hadal depths of the mariana trench, pacific ocean (Crustacea: Amphipoda). Zootaxa 2020, 4748, 163–181. [Google Scholar] [CrossRef] [Green Version]
- Galloway, T.M. Micro- and nano-plastics and human health. In Marine Anthropogenic; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 343–366. ISBN 978-3-319-16509-7. [Google Scholar]
- Eurostat. How Much are Households Spending on Eating-Out? Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/EDN-20200101-2 (accessed on 13 January 2021).
- ResearchAndMarkets.com. Food Delivery Sector Sees a Huge Rise in Orders as a Result of Covid-19 Quarantine. Available online: https://www.prnewswire.com/news-releases/food-delivery-sector-sees-a-huge-rise-in-orders-as-a-result-of-covid-19-quarantine---researchandmarketscom-301034104.html (accessed on 13 January 2021).
- Beat Plastic Pollution. Our Planet is Drowning in Plastic Pollution—It’s Time for Change! Available online: https://www.unep.org/interactive/beat-plastic-pollution/ (accessed on 13 January 2021).
- United Nations Environment Programme. Single-Use Plastic Take-Away Food Packaging and Its Alternatives—Recommendations from Life Cycle Assessments. 2020. Available online: https://www.lifecycleinitiative.org/wp-content/uploads/2020/12/SUPP-Take-Away-food-containers-15.12.20.pdf (accessed on 4 August 2021).
- Mendes, A.C.; Pedersen, G.A. Perspectives on sustainable food packaging:– is bio-based plastics a solution? Trends Food Sci. Technol. 2021, 112, 839–846. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market/ (accessed on 4 May 2021).
- United Nations Environment Programme. Single-Use Plastic Tableware and Its Alternatives—Recommendations from Life Cycle Assessments. 2021. Available online: https://www.lifecycleinitiative.org/wp-content/uploads/2021/03/UNEP-D001-Tableware-Report_Lowres.pdf (accessed on 4 August 2021).
- Ritchie, H.; Roser, M. Plastic Pollution. Available online: https://ourworldindata.org/plastic-pollution#citation (accessed on 4 April 2021).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statista. Distribution of Plastics Converter Demand in the European Union (EU-28) from 2016 to 2019, by Polymer Type. Available online: https://www.statista.com/statistics/869574/polymer-plastics-converter-demand-european-union/ (accessed on 4 April 2021).
- PlasticsEurope. Plastics—The Facts 2019. An Analysis of European Plastics Production, Demand and Waste. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 4 April 2021).
- Statista. Production Volume of Polypropylene Resin Worldwide in 2018 and 2026. Available online: www.statista.com/statistics/1103529/global-polypropylene-production/ (accessed on 14 March 2021).
- Pielichowski, J.; Puszyński, A. Technologia Tworzyw Sztucznych; WNT: Warszawa, Polska, 1980; ISBN 83-204-1710-4. [Google Scholar]
- Tice, P. Polypropylene as a packaging material for foods and beverages. In ILSI Europe Report on Packaging Materials; Yates, K., Ed.; ILSI Press: Brussel, Belgium, 2002; pp. 1–25. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent updates on the barrier properties of ethylene vinyl alcohol copolymer (EVOH): A Review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef] [Green Version]
- Decker, W.; Roy, D.; Voght, C.; Roy, C.; Dabbert, P. Metallized Polymer Films as Replacement for Aluminum Foil in Packaging Applications. In Proceedings of the 47th Annual Technical Conference Proceedings, Society of Vacuum Coaters, Dallas, TX, USA, 24–29 April 2004; pp. 594–599, ISSN 0737-5921. [Google Scholar]
- Marsh, K.; Bugusu, B. Food Packaging - Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Maddah, H. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC.; European Parliament: Brussels, Belgium, 2004. [Google Scholar]
- Daggar, J. Recycling Symbols Explained. Available online: https://www.gwp.co.uk/guides/recycling-symbols-on-packaging/ (accessed on 14 March 2021).
- Statista. Polyethylene and Polypropylene Production Capacity Worldwide from 2017 to 2023. Available online: https://www.statista.com/statistics/1118115/global-polyethylene-polypropylene-capacity/ (accessed on 14 March 2021).
- Chitalia, A. Why Polyolefins Are the Polymers to Watch. Available online: https://www.woodmac.com/news/opinion/why-polyolefins-are-the-polymers-to-watch/ (accessed on 14 March 2021).
- Goldstain Market Intelligence. Global Polyethylene Industry Analysis by Product Type (HDPE, LDPE, LLDPE) by End-User Industry, by Technologies & by Region with COVID-19 Impact. Market Outlook 2017–2030. Available online: https://www.goldsteinresearch.com/report/global-polyethylene-market (accessed on 14 March 2021).
- Agboola, O.; Sadiku, R.; Mokrani, T.; Amer, I.; Imoru, O. Polyolefins and the environment. In Polyolefin Fibres; Woodhead Publishing: London, UK, 2017; pp. 89–133. ISBN 9780081011324. [Google Scholar]
- Tice, P. Polyethylene for food packaging applications. In Packaging Materials; Yates, K., Ed.; ILSI Press: Brussels, Belgium, 2003; ISBN 1-57881-155-4. [Google Scholar]
- Ojeda, T.; Freitas, A.; Birck, K.; Dalmolin, E.; Jacques, R.; Bento, F.; Camargo, F. Degradability of linear polyolefins under natural weathering. Polym. Degrad. Stab. 2011, 96, 703–707. [Google Scholar] [CrossRef] [Green Version]
- Manjula, B.; Reddy, A.B.; Sadiku, E.R.; Sivanjineyulu, V.; Molelekwa, G.F.; Jayaramudu, J.; Raj Kumar, K. Use of polyolefins in hygienic applications. In Polyolefin Fibres; Woodhead Publishing: London, UK, 2017; pp. 539–560. ISBN 9780081011324. [Google Scholar]
- Mieth, A.; Hoekstra, E.; Simoneau, C. Guidance for the Identification of Polymers in Multilayer Films Used in Food Contact Materials; Publications Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-57561-7. [Google Scholar]
- Wołosiak-Hnat, A.; Zych, K.; Mężyńska, M.; Kifonidis, A.; Dajworski, M.; Lisiecki, S.; Bartkowiak, A. LDPE/PET laminated films modified with FeO(OH) × H2O, Fe2O3, and ascorbic acid to develop oxygen scavenging system for food packaging. Packag. Technol. Sci. 2019, 32, 457–469. [Google Scholar] [CrossRef]
- Kang, G.B.; Kim, M.H.; Son, Y.; Park, O.O. Extrusion coating performances of iPP/LDPE blends. J. Appl. Polym. Sci. 2009, 111, 3121–3127. [Google Scholar] [CrossRef]
- Olafsson, G.; Jägerstad, M.; Öste, R.; Wesslén, B. Delamination of polyethylene and aluminum foil layers of laminated packaging material by acetic acid. J. Food Sci. 1993, 58, 215–219. [Google Scholar] [CrossRef]
- Brody, A.L. Commercial uses of active food packaging and modified atmosphere packaging systems. In Innovations in Food Packaging; Han, J., Han, J., Eds.; Elsevier: London, UK, 2005; pp. 457–474. ISBN 9780080455174. [Google Scholar]
- The Ellen MacArthur Foundation. The New Plastics Economy Rethinking the Future of Plastics. Available online: https://ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 15 March 2021).
- Statista. Global Consumption of Polyvinyl Chloride Projection 2019–2021. Available online: https://www.statista.com/statistics/1170567/global-polyvinyl-chloride-consumption/ (accessed on 15 March 2021).
- Yusheng Machinery. Polyvinyl Chloride (PVC) Properties, Production, Price, Market, and Uses. Available online: https://www.bellingmachinery.com/news/polyvinyl-chloride-pvc-properties-production-price-market-and-uses.html (accessed on 15 March 2021).
- Aster, N. Polyvinyl Chloride (PVC) Market: Key Trends and Latest News. Market Research Reports-Analytics & News. Available online: https://marketpublishers.com/lists/23819/news.html (accessed on 15 March 2021).
- Mordor Intelligence. Polyvinyl Chloride (PVC) Market—Growth, Trends, COVID-19 Impact, and Forecast (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/polyvinyl-chloride-pvc-market (accessed on 15 March 2021).
- Leadbitter, J. Polyvinyl chloride (PVC) for food packaging applications. In Packaging Materials; Yates, K., Ed.; ILSI Europe Report Series: Brussels, Belgium, 2003; pp. 1–21. ISBN 1-57881-161-9. [Google Scholar]
- The European Council of Vinyl Manufacturers PVC in Packaging. Available online: https://pvc.org/pvc-applications/pvc-in-packaging/ (accessed on 15 May 2021).
- Rubio, M. Recycling of PVC—Prospects and Challenges. Available online: https://www.ecomena.org/recycling-pvc/ (accessed on 15 May 2021).
- AcmePalstics.com. Your Guide to Plastic Recycling Symbols. Available online: https://www.acmeplastics.com/content/your-guide-to-plastic-recycling-symbols/ (accessed on 15 May 2021).
- Statista. Demand for Polyethylene Terephthalate Worldwide from 2010 to 2020, with a Forecast for 2021 to 2030. Available online: https://www.statista.com/statistics/1128658/polyethylene-terephthalate-demand-worldwide/ (accessed on 15 May 2021).
- Matthews, V. Polyethylene terephthalate (PET) for food packaging. In Packaging Materials; Yates, K., Ed.; ILSI Press: Brussels, Belgium, 2000; pp. 1–15. ISBN 1-57881-092-2. [Google Scholar]
- Tsochatzis, E.D.; Alberto Lopes, J.; Kappenstein, O.; Tietz, T.; Hoekstra, E.J. Quantification of PET cyclic and linear oligomers in teabags by a validated LC-MS method – In silico toxicity assessment and consumer’s exposure. Food Chem. 2020, 317, 126427. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, R.; Hajee, M.; Horrocks, A.R.; Kandola, B.K. Effect of compatibilizers on lignin/bio-polyamide blend carbon precursor filament properties and their potential for thermostabilisation and carbonisation. Polym. Test. 2021, 95, 107133. [Google Scholar] [CrossRef]
- Um, H.-J.; Hwang, Y.-T.; Choi, K.-H.; Kim, H.-S. Effect of crystallinity on the mechanical behavior of carbon fiber reinforced polyethylene-terephthalate (CF/PET) composites considering temperature conditions. Compos. Sci. Technol. 2021, 207, 108745. [Google Scholar] [CrossRef]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Dowling, D.P.; Monahan, F.J. PET trays coated with citrus extract exhibit antioxidant activity with cooked turkey meat. LWT 2012, 47, 471–477. [Google Scholar] [CrossRef]
- Hering, W.; Koecher, R.; Kretzschmar, B.S.M.; Gruenler, B.; Spange, S. Multi-layer hybrid coatings with high gas barrier properties and optical quality. Thin Solid Films 2020, 710, 138261. [Google Scholar] [CrossRef]
- Block, C.; Brands, B.; Gude, T. Report on Packaging Materials: 1. Polystyrene (PS) for food packaging applications. In Packaging Materials; Yates, K., Ed.; ILSI: Brussels, Belgium, 2017; pp. 1–36. ISBN 1-57881-092-2. [Google Scholar]
- Statista. Production Capacity of Polystyrene Worldwide from 2018 to 2024. Available online: https://www.statista.com/statistics/1065889/global-polystyrene-production-capacity/ (accessed on 15 May 2021).
- Mikloskova, H.; Witte, F.; Joeres, E.; Terjung, N. Storage stability of plain stirred whole milk yoghurt (3.7% fat) packed in polylactic acid and polystyrene. Int. Dairy J. 2021, 120, 105088. [Google Scholar] [CrossRef]
- Anonymous. Properties, Performance and Design Fundamentals of Expanded Polystyrene Packaging; EPS Industry Alliance: Crofton, MD, USA, 2016. [Google Scholar]
- Polymer Properties Database. Polymerization of Styrene. Available online: http://polymerdatabase.com/polymer%20chemistry/Polystyrene.html (accessed on 15 May 2021).
- Pilevar, Z.; Bahrami, A.; Beikzadeh, S.; Hosseini, H.; Jafari, S.M. Migration of styrene monomer from polystyrene packaging materials into foods: Characterization and safety evaluation. Trends Food Sci. Technol. 2019, 91, 248–261. [Google Scholar] [CrossRef]
- Liew, C.V.; Chan, L.W.; Ching, A.L.; Heng, P.W.S. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int. J. Pharm. 2006, 309, 25–37. [Google Scholar] [CrossRef]
- Nechita, P.; Roman, M. Review on polysaccharides used in coatings for food packaging papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Ferreira, A.; Alves, V.; Coelhoso, I. Polysaccharide-based membranes in food packaging applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Marchiore, N.G.; Manso, I.J.; Kaufmann, K.C.; Lemes, G.F.; de Oliveira Pizolli, A.P.; Droval, A.A.; Bracht, L.; Gonçalves, O.H.; Leimann, F.V. Migration evaluation of silver nanoparticles from antimicrobial edible coating to sausages. LWT Food Sci. Technol. 2017, 76, 203–208. [Google Scholar] [CrossRef]
- Viña, S.Z.; Mugridge, A.; García, M.A.; Ferreyra, R.M.; Martino, M.N.; Chaves, A.R.; Zaritzky, N.E. Effects of polyvinylchloride films and edible starch coatings on quality aspects of refrigerated Brussels sprouts. Food Chem. 2007, 103, 701–709. [Google Scholar] [CrossRef]
- Kakar, M.U.; Kakar, I.U.; Mehboob, M.Z.; Zada, S.; Soomro, H.; Umair, M.; Iqbal, I.; Umer, M.; Shaheen, S.; Syed, S.F.; et al. A review on polysaccharides from Artemisia sphaerocephala Krasch seeds, their extraction, modification, structure, and applications. Carbohydr. Polym. 2021, 252, 117113. [Google Scholar] [CrossRef]
- Roszowska-Jarosz, M.; Masiewicz, J.; Kostrzewa, M.; Kucharczyk, W.; Żurowski, W.; Kucińska-Lipka, J.; Przybyłek, P. Mechanical properties of bio-composites based on epoxy resin and nanocellulose fibres. Materials 2021, 14, 3576. [Google Scholar] [CrossRef]
- Yang, B.; Dai, Z.; Ding, S.-Y.; Wyman, C.E. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2011, 2, 421–449. [Google Scholar] [CrossRef] [Green Version]
- Ivanković, A.; Zeljko, K.; Talic, S.; Bevanda, A.; Lasic, M. Biodegradable packaging in the food industry. J. Food Saf. Food Qual. 2017, 68, 26–28. [Google Scholar] [CrossRef]
- Heidarian, P.; Behzad, T.; Sadeghi, M. Investigation of cross-linked PVA/starch biocomposites reinforced by cellulose nanofibrils isolated from aspen wood sawdust. Cellulose 2017, 24, 3323–3339. [Google Scholar] [CrossRef]
- He, Y.; Fei, X.; Li, H. Carboxymethyl cellulose-based nanocomposites reinforced with montmorillonite and ε-poly-L-lysine for antimicrobial active food packaging. J. Appl. Polym. Sci. 2020, 137, 48782. [Google Scholar] [CrossRef]
- He, B.; Wang, W.; Song, Y.; Ou, Y.; Zhu, J. Structural and physical properties of carboxymethyl cellulose/gelatin films functionalized with antioxidant of bamboo leaves. Int. J. Biol. Macromol. 2020, 164, 1649–1656. [Google Scholar] [CrossRef]
- Ribeiro, D.C.M.; Rebelo, R.C.; De Bon, F.; Coelho, J.F.J.; Serra, A.C. Process development for flexible films of industrial cellulose pulp using superbase ionic liquids. Polymers 2021, 13, 1767. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Wojnowska-Baryła, I.; Kulikowska, D.; Bernat, K. Effect of bio-based products on waste management. Sustainability 2020, 12, 2088. [Google Scholar] [CrossRef] [Green Version]
- Atta, O.M.; Manan, S.; Ahmed, A.A.Q.; Awad, M.F.; Ul-Islam, M.; Subhan, F.; Ullah, M.W.; Yang, G. Development and characterization of yeast-incorporated antimicrobial cellulose biofilms for edible food packaging application. Polymers 2021, 13, 2310. [Google Scholar] [CrossRef]
- Liu, C.; Luan, P.; Li, Q.; Cheng, Z.; Sun, X.; Cao, D.; Zhu, H. Biodegradable, hygienic, and compostable tableware from hybrid sugarcane and bamboo fibers as plastic alternative. Matter 2020, 3, 2066–2079. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Khaneghah, A.M. Role of green polymers in food packaging. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 305–319. ISBN 9780128131961. [Google Scholar]
- Lin, D.; Liu, Z.; Shen, R.; Chen, S.; Yang, X. Bacterial cellulose in food industry: Current research and future prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Revin, V.V.; Dolganov, A.V.; Liyaskina, E.V.; Nazarova, N.B.; Balandina, A.V.; Devyataeva, A.A.; Revin, V.D. Characterizing bacterial cellulose produced by Komagataeibacter sucrofermentans H-110 on molasses medium and obtaining a biocomposite based on it for the adsorption of fluoride. Polymers 2021, 13, 1422. [Google Scholar] [CrossRef]
- La China, S.; De Vero, L.; Anguluri, K.; Brugnoli, M.; Mamlouk, D.; Gullo, M. Kombucha tea as a reservoir of cellulose producing bacteria: Assessing diversity among Komagataeibacter isolates. Appl. Sci. 2021, 11, 1595. [Google Scholar] [CrossRef]
- Gullo, M.; La China, S.; Petroni, G.; Di Gregorio, S.; Giudici, P. Exploring K2G30 genome: A high bacterial cellulose producing strain in glucose and mannitol based media. Front. Microbiol. 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; La China, S.; Falcone, P.M.; Giudici, P. Biotechnological production of cellulose by acetic acid bacteria: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6885–6898. [Google Scholar] [CrossRef]
- La China, S.; Zanichelli, G.; De Vero, L.; Gullo, M. Oxidative fermentations and exopolysaccharides production by acetic acid bacteria: A mini review. Biotechnol. Lett. 2018, 40, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Skiba, E.A.; Shavyrkina, N.A.; Budaeva, V.V.; Sitnikova, A.E.; Korchagina, A.A.; Bychin, N.V.; Gladysheva, E.K.; Pavlov, I.N.; Zharikov, A.N.; Lubyansky, V.G.; et al. Biosynthesis of bacterial cellulose by extended cultivation with multiple removal of BC pellicles. Polymers 2021, 13, 2118. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial nanocellulose—A biobased polymer for active and intelligent food packaging applications: Recent advances and developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef]
- Kingkaew, J.; Kirdponpattara, S.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Effect of molecular weight of chitosan on antimicrobial properties and tissue compatibility of chitosan-impregnated bacterial cellulose films. Biotechnol. Bioprocess Eng. 2014, 19, 534–544. [Google Scholar] [CrossRef]
- Chang, S.-T.; Chen, L.-C.; Lin, S.-B.; Chen, H.-H. Nano-biomaterials application: Morphology and physical properties of bacterial cellulose/gelatin composites via crosslinking. Food Hydrocoll. 2012, 27, 137–144. [Google Scholar] [CrossRef]
- Gu, J.; Catchmark, J.M. Impact of hemicelluloses and pectin on sphere-like bacterial cellulose assembly. Carbohydr. Polym. 2012, 88, 547–557. [Google Scholar] [CrossRef]
- Dayal, M.S.; Catchmark, J.M. Mechanical and structural property analysis of bacterial cellulose composites. Carbohydr. Polym. 2016, 144, 447–453. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Sa Moreira de Souza Filho, M.; de Freitas Rosa, M. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.A.; dos Santos, G.R.; Soeiro, V.S.; dos Santos, J.R.; de Araujo Rebelo, M.; Chaud, M.V.; Gerenutti, M.; Grotto, D.; Pandit, R.; Rai, M.; et al. Bacterial nanocellulose membranes combined with nisin: A strategy to prevent microbial growth. Cellulose 2018, 25, 6681–6689. [Google Scholar] [CrossRef]
- Razavi, M.S.; Golmohammadi, A.; Nematollahzadeh, A.; Fiori, F.; Rovera, C.; Farris, S. Preparation of cinnamon essential oil emulsion by bacterial cellulose nanocrystals and fish gelatin. Food Hydrocoll. 2020, 109, 106111. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Arserim-Uçar, D.K.; Korel, F.; Liu, L.; Yam, K.L. Characterization of bacterial cellulose nanocrystals: Effect of acid treatments and neutralization. Food Chem. 2021, 336, 127597. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; González, G. Effects of exposure to pulsed light on surface and structural properties of edible films made from cassava and taro starch. Food Bioprocess Technol. 2016, 9, 1812–1824. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Sadeghizadeh-Yazdi, J.; Habibi, M.; Kamali, A.A.; Banaei, M. Application of edible and biodegradable starch-based films in food packaging: A systematic review and meta-analysis. Curr. Res. Nutr. Food Sci. J. 2019, 7, 624–637. [Google Scholar] [CrossRef]
- Phan, T.D.; Debeaufort, F.; Luu, D.; Voilley, A. Functional properties of edible agar-based and starch-based films for food quality preservation. J. Agric. Food Chem. 2005, 53, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J. Surface and nutraceutical properties of edible films made from starchy sources with and without added blackberry pulp. Carbohydr. Polym. 2017, 165, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Huntrakul, K.; Yoksan, R.; Sane, A.; Harnkarnsujarit, N. Effects of pea protein on properties of cassava starch edible films produced by blown-film extrusion for oil packaging. Food Packag. Shelf Life 2020, 24, 100480. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of yerba mate extract on the performance of starch films obtained by extrusion and compression molding as active and smart packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Estevez-Areco, S.; Goyanes, S. Potato starch-based biocomposites with enhanced thermal, mechanical and barrier properties comprising water-resistant electrospun poly(vinyl alcohol) fibers and yerba mate extract. Carbohydr. Polym. 2019, 215, 377–387. [Google Scholar] [CrossRef]
- Righetti, M.; Cinelli, P.; Mallegni, N.; Massa, C.; Aliotta, L.; Lazzeri, A. Thermal, mechanical, viscoelastic and morphological properties of poly(lactic acid) based biocomposites with potato pulp powder treated with waxes. Materials 2019, 12, 990. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Ching, Y.C.; Chuah, C.H.; Liou, N.-S. Preparation and characterization of starch/empty fruit bunch-based bioplastic composites reinforced with epoxidized oils. Polymers 2020, 13, 94. [Google Scholar] [CrossRef]
- Osman, A.F.; Siah, L.; Alrashdi, A.A.; Ul-Hamid, A.; Ibrahim, I. Improving the tensile and tear properties of thermoplastic starch/dolomite biocomposite film through sonication process. Polymers 2021, 13, 274. [Google Scholar] [CrossRef]
- Ren, J.; Dang, K.; Pollet, E.; Avérous, L. Preparation and characterization of thermoplastic potato starch/halloysite nano-biocomposites: Effect of plasticizer nature and nanoclay content. Polymers 2018, 10, 808. [Google Scholar] [CrossRef] [Green Version]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev. Int. 2020, 1–31. [Google Scholar] [CrossRef]
- Di Donato, P.; Poli, A.; Taurisano, V.; Nicolaus, B. Polysaccharides: Applications in biology and biotechnology/polysaccharides from bioagro-waste new biomolecules-life. In Polysaccharides; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–29. ISBN 978-3-319-03751-6. [Google Scholar]
- Tamaki, Y.; Konishi, T.; Fukuta, M.; Tako, M. Isolation and structural characterisation of pectin from endocarp of Citrus depressa. Food Chem. 2008, 107, 352–361. [Google Scholar] [CrossRef]
- Willats, W.G.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Application and evaluation of a pectin-based edible coating process for quality change kinetics and shelf-life extension of lime fruit (Citrus aurantifolium). Coatings 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Manrich, A.; Moreira, F.K.V.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H.C. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshi, R.; Kim, S.-M.; Rhim, J.-W. Pectin/pullulan blend films for food packaging: Effect of blending ratio. Food Chem. 2021, 347, 129022. [Google Scholar] [CrossRef]
- Ramos-García, M.; Bosquez-Molina, E.; Hernández-Romano, J.; Zavala-Padilla, G.; Terrés-Rojas, E.; Alia-Tejacal, I.; Barrera-Necha, L.; Hernández-López, M.; Bautista-Baños, S. Use of chitosan-based edible coatings in combination with other natural compounds, to control Rhizopus stolonifer and Escherichia coli DH5α in fresh tomatoes. Crop Prot. 2012, 38, 1–6. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Gumienna, M.; Górna, B. Antimicrobial food packaging with biodegradable polymers and bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef] [PubMed]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and antimicrobial activity of chitosan and its derivatives: A concise review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef]
- de la Paz Salgado-Cruz, M.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Martínez, M.C.; Yáñez-Fernández, J. Chitosan as a coating for biocontrol in postharvest products: A bibliometric review. Membranes 2021, 11, 421. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries—A review. J. Food Sci. Technol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, S.; Mobashir, M. LC–MS and docking profiling reveals potential difference between the pure and crude fucoidan metabolites. Int. J. Biol. Macromol. 2020, 143, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Rafiquzzaman, S.M.; Ahmed, R.; Lee, J.M.; Noh, G.; Jo, G.; Kong, I.-S. Improved methods for isolation of carrageenan from Hypnea musciformis and its antioxidant activity. J. Appl. Phycol. 2016, 28, 1265–1274. [Google Scholar] [CrossRef]
- Bhowmick, B.; Sarkar, G.; Rana, D.; Roy, I.; Saha, N.R.; Ghosh, S.; Bhowmik, M.; Chattopadhyay, D. Effect of carrageenan and potassium chloride on an in situ gelling ophthalmic drug delivery system based on methylcellulose. RSC Adv. 2015, 5, 60386–60391. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Saxena, A. Bacterial carrageenases: An overview of production and biotechnological applications. 3 Biotech 2016, 6, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Corino, C.; Di Giancamillo, A.; Modina, S.C.; Rossi, R. Prebiotic effects of seaweed polysaccharides in pigs. Animals 2021, 11, 1573. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J. Biomed. Biotechnol. 2010, 2010, 525291. [Google Scholar] [CrossRef] [Green Version]
- Cindana Mo’o, F.R.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a polysaccharide from macroalga Ulva sp.: A review of chemistry, biological activities and potential for food and biomedical applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Amin, H.H. Biosynthesized silver nanoparticles using Ulva lactuca as a safe synthetic pesticide (in vitro). Open Agric. 2020, 5, 291–299. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Sankaranarayanan, S.; Gajaria, T.K.; Li, G.; Kujawski, W.; Kujawa, J.; Navia, R. A Short review on the valorization of green seaweeds and ulvan: Feedstock for chemicals and biomaterials. Biomolecules 2020, 10, 991. [Google Scholar] [CrossRef]
- Morelli, A.; Massironi, A.; Puppi, D.; Creti, D.; Domingo Martinez, E.; Bonistalli, C.; Fabroni, C.; Morgenni, F.; Chiellini, F. Development of ulvan-based emulsions containing flavour and fragrances for food and cosmetic applications. Flavour Fragr. J. 2019, 34, 411–425. [Google Scholar] [CrossRef]
- Shalaby, S.; Amin, H.H. Potential using of ulvan polysaccharide from Ulva lactuca as a prebiotic in synbiotic yogurt production. J. Probiotics Heal. 2019, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gruskiene, R.; Kavleiskaja, T.; Staneviciene, R.; Kikionis, S.; Ioannou, E.; Serviene, E.; Roussis, V.; Sereikaite, J. Nisin-loaded ulvan particles: Preparation and characterization. Foods 2021, 10, 1007. [Google Scholar] [CrossRef]
- Guidara, M.; Yaich, H.; Richel, A.; Blecker, C.; Boufi, S.; Attia, H.; Garna, H. Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel ulvan films. Int. J. Biol. Macromol. 2019, 135, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Ramu Ganesan, A.; Shanmugam, M.; Bhat, R. Producing novel edible films from semi refined carrageenan (SRC) and ulvan polysaccharides for potential food applications. Int. J. Biol. Macromol. 2018, 112, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Gomez, C.G.; Pérez Lambrecht, M.V.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction–purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Andriamanantoanina, H.; Rinaudo, M. Characterization of the alginates from five madagascan brown algae. Carbohydr. Polym. 2010, 82, 555–560. [Google Scholar] [CrossRef]
- Łabowska, B.; Michalak, M.; Detyna, I. Methods of extraction, physicochemical properties of alginates and their applications in biomedical field—A review. Open Chem. 2019, 17, 738–762. [Google Scholar] [CrossRef] [Green Version]
- Peteiro, C. Alginate production from marine macroalgae, with emphasis on kelp farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 27–66. ISBN 978-981-10-6910-9. [Google Scholar]
- Konno, A.; Azechi, Y.; Kimura, H. Properties of curdlan gel. Agric. Biol. Chem. 1979, 43, 101–104. [Google Scholar] [CrossRef]
- West, T.P. Production of the polysaccharide curdlan by Agrobacterium species on processing coproducts and plant lignocellulosic hydrolysates. Fermentation 2020, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lu, M.; Fang, Y.; Wu, L.; Xu, Y.; Wang, S. Production of curdlan grown on cassava starch waste hydrolysates. J. Polym. Environ. 2018, 26, 33–38. [Google Scholar] [CrossRef]
- Lee, I.-Y.; Seo, W.T.; Kim, G.J.; Kim, M.K.; Park, C.S.; Park, Y.H. Production of curdlan using sucrose or sugar cane molasses by two-step fed-batch cultivation of Agrobacterium species. J. Ind. Microbiol. Biotechnol. 1997, 18, 255–259. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Castillo, E.; Felix, M.; Bengoechea, C.; Guerrero, A. Proteins from agri-food industrial biowastes or co-products and their applications as green materials. Foods 2021, 10, 981. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Curti, P.; Meniqueti, A.; Martins, A.; Rubira, A.; Muniz, E. Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [Green Version]
- Ben Shalom, T.; Belsey, S.; Chasnitsky, M.; Shoseyov, O. Cellulose nanocrystals and corn zein oxygen and water vapor barrier biocomposite films. Nanomaterials 2021, 11, 247. [Google Scholar] [CrossRef]
- Park, H.J.; Chinnan, M.S.; Shewfelt, R.L. Edible coating effects on storage life and quality of tomatoes. J. Food Sci. 1994, 59, 568–570. [Google Scholar] [CrossRef]
- Park, H.; Rhim, J.W.; Lee, H.Y. Edible coating effects on respiration rate and storage life of “Fuji” apples and “Shingo” pears. Foods Biotechnol. 1996, 5, 59–63. [Google Scholar]
- Vimala Bharathi, S.K.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Zein-based anti-browning cling wraps for fresh-cut apple slices. Int. J. Food Sci. Technol. 2020, 55, 1238–1245. [Google Scholar] [CrossRef]
- Ünalan, İ.U.; Arcan, I.; Korel, F.; Yemenicioğlu, A. Application of active zein-based films with controlled release properties to control Listeria monocytogenes growth and lipid oxidation in fresh Kashar cheese. Innov. Food Sci. Emerg. Technol. 2013, 20, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Budi Santosa, F.X.; Padua, G.W. Tensile properties and water absorption of zein sheets plasticized with oleic and linoleic acids. J. Agric. Food Chem. 1999, 47, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Oromiehie, A.R.; Musavi, M.; Falcone, P.M.; D-Jomeh, Z.E.; Rad, E.R. Study of mechanical properties, oxygen permeability and AFM topography of zein films plasticized by polyols. Packag. Technol. Sci. 2007, 20, 155–163. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Preparation and characterization of corn starch bio-active edible packaging films based on zein incorporated with orange-peel oil. Antioxidants 2019, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Arcan, I.; Yemenicioğlu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2011, 44, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, K.L.; Han, I.Y.; Dawson, P.L. Antimicrobial effects of corn zein films impregnated with nisin, lauric acid, and EDTA. J. Food Prot. 2001, 64, 885–889. [Google Scholar] [CrossRef]
- Milani, J.M.; Tirgarian, B. An overview of edible protein-based packaging: Main sources, advantages, drawbacks, recent progressions and food applications. J. Packag. Technol. Res. 2020, 4, 103–115. [Google Scholar] [CrossRef]
- Redl, A.; Morel, M.H.; Bonicel, J.; Vergnes, B.; Guilbert, S. Extrusion of wheat gluten plasticized with glycerol: Influence of process conditions on flow behavior, rheological properties, and molecular size distribution. Cereal Chem. J. 1999, 76, 361–370. [Google Scholar] [CrossRef]
- Mioduszewski, Ł.; Cieplak, M. Viscoelastic properties of wheat gluten in a molecular dynamics study. PLoS Comput. Biol. 2021, 17, e1008840. [Google Scholar] [CrossRef]
- Apichartsrangkoon, A.; Ledward, D. Dynamic viscoelastic behaviour of high pressure treated gluten–soy mixtures. Food Chem. 2002, 77, 317–323. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Domenek, S.; Feuilloley, P.; Gratraud, J.; Morel, M.-H.; Guilbert, S. Biodegradability of wheat gluten based bioplastics. Chemosphere 2004, 54, 551–559. [Google Scholar] [CrossRef]
- Alonso-González, M.; Ramos, M.; Bengoechea, C.; Romero, A.; Guerrero, A. Evaluation of composition on processability and water absorption of wheat gluten-based bioplastics. J. Polym. Environ. 2021, 29, 1434–1443. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239, 117994. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Mendieta, J.R.; Ortega-Toro, R. In-depth study from gluten/PCL-based food packaging films obtained under reactive extrusion conditions using chrome octanoate as a potential food grade catalyst. Food Hydrocoll. 2021, 111, 106255. [Google Scholar] [CrossRef]
- Rafieian, F.; Shahedi, M.; Keramat, J.; Simonsen, J. Mechanical, thermal and barrier properties of nano-biocomposite based on gluten and carboxylated cellulose nanocrystals. Ind. Crops Prod. 2014, 53, 282–288. [Google Scholar] [CrossRef]
- Chevillard, A.; Angellier-Coussy, H.; Cuq, B.; Guillard, V.; César, G.; Gontard, N.; Gastaldi, E. How the biodegradability of wheat gluten-based agromaterial can be modulated by adding nanoclays. Polym. Degrad. Stab. 2011, 96, 2088–2097. [Google Scholar] [CrossRef]
- Tanada-Palmu, P.S.; Grosso, C.R.F. Effect of edible wheat gluten-based films and coatings on refrigerated strawberry (Fragaria ananassa) quality. Postharvest Biol. Technol. 2005, 36, 199–208. [Google Scholar] [CrossRef]
- Zhang, X.; Do, M.D.; Kurniawan, L.; Qiao, G.G. Wheat gluten-based renewable and biodegradable polymer materials with enhanced hydrophobicity by using epoxidized soybean oil as a modifier. Carbohydr. Res. 2010, 345, 2174–2182. [Google Scholar] [CrossRef]
- Guillaume, C.; Pinte, J.; Gontard, N.; Gastaldi, E. Wheat gluten-coated papers for bio-based food packaging: Structure, surface and transfer properties. Food Res. Int. 2010, 43, 1395–1401. [Google Scholar] [CrossRef]
- Chavoshizadeh, S.; Pirsa, S.; Mohtarami, F. Sesame oil oxidation control by active and smart packaging system using wheat gluten/chlorophyll film to increase shelf life and detecting expiration date. Eur. J. Lipid Sci. Technol. 2020, 122, 1900385. [Google Scholar] [CrossRef]
- Ansorena, M.R.; Zubeldía, F.; Marcovich, N.E. Active wheat gluten films obtained by thermoplastic processing. LWT Food Sci. Technol. 2016, 69, 47–54. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2015, 6, 1. [Google Scholar] [CrossRef]
- Chao, Z.; Yue, M.; Xiaoyan, Z.; Dan, M. Development of soybean protein-isolate edible films incorporated with beeswax, Span 20, and Glycerol. J. Food Sci. 2010, 75, C493–C497. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Hwang, K.T.; Weller, C.L.; Hanna, M.A. Preparation and characterization of soy protein isolate films modified with sorghum wax. J. Am. Oil Chem. Soc. 2002, 79, 615–619. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, G. Biodegradable protein-based films from plant resources: A review. Environ. Prog. Sustain. Energy 2010, 29, 203–220. [Google Scholar] [CrossRef]
- Wan, V.C.-H.; Kim, M.S.; Lee, S.-Y. Water vapor permeability and mechanical properties of soy protein isolate edible films composed of different plasticizer combinations. J. Food Sci. 2006, 70, e387–e391. [Google Scholar] [CrossRef]
- Kim, K.M.; Marx, D.B.; Weller, C.L.; Hanna, M.A. Influence of sorghum wax, glycerin, and sorbitol on physical properties of soy protein isolate films. J. Am. Oil Chem. Soc. 2003, 80, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Ghorpade, V.M.; Li, H.; Gennadios, A.; Hanna, M.A. Chemically modified soy protein films. Trans. ASAE 1995, 38, 1805–1808. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Chiou, B.-S.; Avena-Bustillos, R.J.; Bechtel, P.J.; Jafri, H.; Narayan, R.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Cold water fish gelatin films: Effects of cross-linking on thermal, mechanical, barrier, and biodegradation properties. Eur. Polym. J. 2008, 44, 3748–3753. [Google Scholar] [CrossRef]
- Wang, L.Z.; Liu, L.; Holmes, J.; Kerry, J.F.; Kerry, J.P. Assessment of film-forming potential and properties of protein and polysaccharide-based biopolymer films. Int. J. Food Sci. Technol. 2007, 42, 1128–1138. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Liu, A.; Wang, W. Improved thermal-stability and mechanical properties of type I collagen by crosslinking with casein, keratin and soy protein isolate using transglutaminase. Int. J. Biol. Macromol. 2017, 98, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassan, A.A.; Norziah, M.H. Starch–gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Shin, Y.J.; Jang, S.-A.; Song, K.B. Preparation and mechanical properties of rice bran protein composite films containing gelatin or red algae. Food Sci. Biotechnol. 2011, 20, 703–707. [Google Scholar] [CrossRef]
- Xia, C.; Wang, W.; Wang, L.; Liu, H.; Xiao, J. Multilayer zein/gelatin films with tunable water barrier property and prolonged antioxidant activity. Food Packag. Shelf Life 2019, 19, 76–85. [Google Scholar] [CrossRef]
- Rangaraj, M.V.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Biscarat, J.; Charmette, C.; Sanchez, J.; Pochat-Bohatier, C. Development of a new family of food packaging bioplastics from cross-linked gelatin based films. Can. J. Chem. Eng. 2015, 93, 176–182. [Google Scholar] [CrossRef]
- Gómez-Estaca, I.; López de Lacey, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microb. 2010, 27, 889–896. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-Anan, M. An approach for the enhancement of the mechanical properties and film coating efficiency of shellac by the formation of composite films based on shellac and gelatin. J. Food Eng. 2012, 108, 94–102. [Google Scholar] [CrossRef]
- Pranoto, Y.; Lee, C.M.; Park, H.J. Characterizations of fish gelatin films added with gellan and κ-carrageenan. LWT Food Sci. Technol. 2007, 40, 766–774. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Grosso, C.R.F. Gelatin-based films containing hydrophobic plasticizers and saponin from Yucca schidigera as the surfactant. Food Res. Int. 2010, 43, 1710–1718. [Google Scholar] [CrossRef]
- Shankar, S.; Jaiswal, L.; Rhim, J.-W. Gelatin-based nanocomposite films. In Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 339–348. ISBN 9780128007235. [Google Scholar]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/gelatin/silver nanoparticles composites films for biodegradable food packaging applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Kazimierczak, W. Gum arabic/gelatin and water-soluble soy polysaccharides/gelatin blend films as carriers of astaxanthin—A comparative study of the kinetics of release and antioxidant properties. Polymers 2021, 13, 1062. [Google Scholar] [CrossRef]
- Sáenz-Santos, C.M.; Opemipo Oyedara, O.; García-Tejeda, Y.V.; Romero-Bastida, C.A.; García-Oropesa, E.M.; Villalobo, E.; Rodríguez-Pérez, M.A. Active biopolymeric films inoculated with Bdellovibrio bacteriovorus, a predatory bacterium. Coatings 2021, 11, 605. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of active fish gelatin films with anthocyanins by compression molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.L. Physicochemical and microstructural characterization of whey protein films formed with oxidized ferulic/tannic acids. Foods 2021, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Minj, S.; Anand, S. Whey proteins and its derivatives: Bioactivity, functionality, and current applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Socaciu, M.-I.; Fogarasi, M.; Simon, E.L.; Semeniuc, C.A.; Socaci, S.A.; Podar, A.S.; Vodnar, D.C. Effects of whey protein isolate-based film incorporated with tarragon essential oil on the quality and shelf-life of refrigerated brook trout. Foods 2021, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Mchugh, T.H.; Aujard, J.-F.; Krochta, J.M. Plasticized whey protein edible films: Water vapor permeability properties. J. Food Sci. 1994, 59, 416–419. [Google Scholar] [CrossRef]
- Jooyandeh, H. Whey protein films and coatings: A review. Pak. J. Nutr. 2011, 10, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Bahram, S.; Rezaei, M.; Soltani, M.; Kamali, A.; Ojagh, S.M.; Abdollahi, M. Whey protein concentrate edible film activated with cinnamon essential oil. J. Food Process. Preserv. 2014, 38, 1251–1258. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Gago, M.B.; Krochta, J.M. Lipid particle size effect on water vapor permeability and mechanical properties of whey protein/beeswax emulsion films. J. Agric. Food Chem. 2001, 49, 996–1002. [Google Scholar] [CrossRef]
- Fernández, L.; de Apodaca, E.D.; Cebrián, M.; Villarán, M.C.; Maté, J.I. Effect of the unsaturation degree and concentration of fatty acids on the properties of WPI-based edible films. Eur. Food Res. Technol. 2007, 224, 415–420. [Google Scholar] [CrossRef]
- Lara, B.R.B.; Dias, M.V.; Guimarães Junior, M.; de Andrade, P.S.; de Souza Nascimento, B.; Ferreira, L.F.; Yoshida, M.I. Water sorption thermodynamic behavior of whey protein isolate/polyvinyl alcohol blends for food packaging. Food Hydrocoll. 2020, 103, 105710. [Google Scholar] [CrossRef]
- Lara, B.R.B.; de Andrade, P.S.; Guimarães Junior, M.; Dias, M.V.; Alcântara, L.A.P. Novel whey protein isolate/polyvinyl biocomposite for packaging: Improvement of mechanical and water barrier properties by incorporation of nano-silica. J. Polym. Environ. 2021, 29, 2397–2408. [Google Scholar] [CrossRef]
- Vanden Braber, N.L.; Di Giorgio, L.; Aminahuel, C.A.; Díaz Vergara, L.I.; Martín Costa, A.O.; Montenegro, M.A.; Mauri, A.N. Antifungal whey protein films activated with low quantities of water soluble chitosan. Food Hydrocoll. 2021, 110, 106156. [Google Scholar] [CrossRef]
- Potrč, S.; Fras Zemljič, L.; Sterniša, M.; Smole Možina, S.; Plohl, O. Development of biodegradable whey-based laminate functionalised by chitosan–natural extract formulations. Int. J. Mol. Sci. 2020, 21, 3668. [Google Scholar] [CrossRef] [PubMed]
- Ozer, B.B.P.; Uz, M.; Oymaci, P.; Altinkaya, S.A. Development of a novel strategy for controlled release of lysozyme from whey protein isolate based active food packaging films. Food Hydrocoll. 2016, 61, 877–886. [Google Scholar] [CrossRef] [Green Version]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel active food packaging films based on whey protein incorporated with seaweed extract: Development, characterization, and application in fresh poultry meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Aguirre-Joya, J.A.; Rojas, R.; Vicente, A.; Aguilar-González, M.A.; Rodríguez-Herrera, R.; Alvarez-Perez, O.B.; Torres-León, C.; Aguilar, C.N. Candelilla wax edible coating with flourensia cernua bioactives to prolong the quality of tomato fruits. Foods 2020, 9, 1303. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, M.M.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Lizardi-Mendoza, J.; Miranda, R.; Ayala-Zavala, J.F. Individual and combined coatings of chitosan and carnauba wax with oregano essential oil to avoid water loss and microbial decay of fresh cucumber. Coatings 2020, 10, 614. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Paidari, S.; Zamindar, N.; Tahergorabi, R.; Kargar, M.; Ezzati, S.; Shirani, N.; Musavi, S.H. Edible coating and films as promising packaging: A mini review. J. Food Meas. Charact. 2021. [Google Scholar] [CrossRef]
- Hasan, M.; Kumar, V.A.; Maheshwari, C.; Mangraj, S. Biodegradable and edible film: A counter to plastic pollution. Int. J. Chem. Stud. 2020, 8, 2242–2245. [Google Scholar] [CrossRef] [Green Version]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Gutiérrez-Cortez, E. Effect of nano-edible coating based on beeswax solid lipid nanoparticles on strawberry’s preservation. Coatings 2020, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Steinbüchel, A.; Hein, S. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. In Biopolyesters. Advances in Biochemical Engineering/Biotechnology; Babel, W., Steinbüchel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 81–123. ISBN 978-3-540-40021-9. [Google Scholar]
- Wynn, J.; Anderson, A. Microbial polysaccharides and single cell oils. In Basic Biotechnology; Ratledge, C., Kristiansen, B., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 381–403. [Google Scholar]
- Jung, Y.-M.; Lee, Y.-H. Utilization of oxidative pressure for enhanced production of poly-β-hydroxybutyrate and poly(3-hydroxybutyrate-3-hydroxyvalerate) in Ralstonia eutropha. J. Biosci. Bioeng. 2000, 90, 266–270. [Google Scholar] [CrossRef]
- Priyadarshi, S.; Shukla, A.; Borse, B.B. Polyhydroxyalkanoates: Role of Ralstonia eutropha. Int. J. Biomed. Adv. Res. 2014, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Masood, F. Polyhydroxyalkanoates in the food packaging industry. In Nanotechnology Applications in Food; Grumezescu, A., Oprea, A., Eds.; Elsevier: London, UK, 2017; pp. 153–177. ISBN 9780128119433. [Google Scholar]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofuels Bioprod. Biorefining 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef]
- Eş, I.; Mousavi Khaneghah, A.; Barba, F.J.; Saraiva, J.A.; Sant’Ana, A.S.; Hashemi, S.M.B. Recent advancements in lactic acid production—A review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef]
- Komesu, A.; de Oliveira, J.A.R.; da Silva Martins, L.H.; Wolf Maciel, M.R.; Maciel Filho, R. Lactic acid production to purification: A review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Grand View Research. Polyethylene Furanoate Market Size, Share & Trends Analysis Report by Application (Bottles, Fibers, Films), by Region (North America, Europe, APAC, Central & South America, MEA), and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/polyethylene-furanoate-pef-market (accessed on 17 July 2021).
- Höhnemann, T.; Steinmann, M.; Schindler, S.; Hoss, M.; König, S.; Ota, A.; Dauner, M.; Buchmeiser, M.R. Poly(ethylene ruranoate) along its life-cycle from a polycondensation approach to high-performance yarn and its recyclate. Materials 2021, 14, 1044. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-based packaging: Materials, modifications, industrial applications and sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Aeschelmann, F.; Carus, M. Biobased building blocks and polymers in the world: Capacities, production, and applications–status quo and trends towards 2020. Ind. Biotechnol. 2015, 11, 154–159. [Google Scholar] [CrossRef]
- Transparency Market Research In-Depth Analysis, Accurate Results. Polybutylene Succinate (PBS) Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2018–2026. Available online: https://www.transparencymarketresearch.com/polybutylene-succinate-market.html (accessed on 14 July 2021).
- Industry Arc. Polybutylene Succinate Market Forecast (2021–2026). Available online: https://www.industryarc.com/Report/16097/polybutylene-succinate-market.html (accessed on 17 July 2021).
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and supramolecular changes in polybutylene succinate (PBS) and polybutylene succinate adipate (PBSA) Copolymer during degradation in various environmental conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A review on properties and application of bio-based poly(butylene succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-B.; Li, Y.-D.; Zhu, Q.-Y.; Yang, K.-K.; Wang, X.-L.; Wang, Y.-Z. A novel biodegradable multiblock poly(ester urethane) containing poly(l-lactic acid) and poly(butylene succinate) blocks. Polymer 2009, 50, 1178–1186. [Google Scholar] [CrossRef]
- Nam, T.H.; Ogihara, S.; Tung, N.H.; Kobayashi, S. Effect of alkali treatment on interfacial and mechanical properties of coir fiber reinforced poly(butylene succinate) biodegradable composites. Compos. Part B Eng. 2011, 42, 1648–1656. [Google Scholar] [CrossRef]
- Frollini, E.; Bartolucci, N.; Sisti, L.; Celli, A. Poly(butylene succinate) reinforced with different lignocellulosic fibers. Ind. Crops Prod. 2013, 45, 160–169. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H.-T.; Jhang, J.-J. Palm fibre-reinforced hybrid composites of poly(butylene succinate): Characterisation and assessment of mechanical and thermal properties. Polym. Bull. 2013, 70, 3443–3462. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal degradation mechanism of poly(ethylene succinate) and poly(butylene succinate): Comparative study. Thermochim. Acta 2005, 435, 142–150. [Google Scholar] [CrossRef]
- Song, L.; Qiu, Z. Crystallization behavior and thermal property of biodegradable poly(butylene succinate)/functional multi-walled carbon nanotubes nanocomposite. Polym. Degrad. Stab. 2009, 94, 632–637. [Google Scholar] [CrossRef]
- Jamaluddin, N.; Razaina, M.T.; Ishak, Z.M. Mechanical and morphology behaviours of polybutylene (succinate)/thermoplastic polyurethane blend. Procedia Chem. 2016, 19, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Kim, H.-J.; Lee, J.-W.; Choi, I.-G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Cao, A.; Wang, J. In vitro evaluation of biodegradable poly(butylene succinate) as a novel biomaterial. Macromol. Biosci. 2005, 5, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventós, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralla, T.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Oat bran extract (Avena sativa L.) from food by-product streams as new natural emulsifier. Food Hydrocoll. 2018, 81, 253–262. [Google Scholar] [CrossRef]
- Gustafsson, J.; Landberg, M.; Bátori, V.; Åkesson, D.; Taherzadeh, M.J.; Zamani, A. Development of bio-based films and 3D objects from apple pomace. Polymers 2019, 11, 289. [Google Scholar] [CrossRef] [Green Version]
- Buxoo, S.; Jeetah, P. Feasibility of producing biodegradable disposable paper cup from pineapple peels, orange peels and Mauritian hemp leaves with beeswax coating. SN Appl. Sci. 2020, 2, 1359. [Google Scholar] [CrossRef]
- Gautam, A.M.; Caetano, N. Study, design and analysis of sustainable alternatives to plastic takeaway cutlery and crockery. Energy Procedia 2017, 136, 507–512. [Google Scholar] [CrossRef]
- Olt, J.; Maksarov, V.V.; Soots, K.; Leemet, T. Technology for the production of environment friendly tableware. Environ. Clim. Technol. 2020, 24, 57–66. [Google Scholar] [CrossRef]
- Wysocki, J. Material for making biodegradable mouldings from bran and method thereof. Patent No. WO/2001/039612, 7 July 2001. [Google Scholar]
- Chindyasov, V. Biodegradable disposable tableware and methods for making same. Patent No. US 8524130, 3 September 2013. [Google Scholar]
- Kong, Z. Edible and biodegradable utensils. Patent No. WO/2018/157119, 30 August 2018. [Google Scholar]
- Bhagat, M.; Zafari, R. Edible eating device and method of making. Patent No. US 2019/0380519 A1f281, 19 December 2019. [Google Scholar]
- BIOTREM. Producer Webpage. Available online: http://biotrem.pl/pl/ (accessed on 17 July 2021).
- Borgobello, B. Reinvented with Eco-Friendly and Edible Packaging In-Flight Meal Tray Reinvented with Eco-Friendly and Edible Packaging. Available online: https://newatlas.com/environment/priestmangoode-eco-friendly-in-flight-tray-concept/ (accessed on 17 July 2021).
- PriestmanGoode. Producer Webpage. Available online: https://www.priestmangoode.com/project/get-onboard/ (accessed on 17 July 2021).
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and functionalized films/coatings—Performances and perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Research and Markets. Edible Cutlery Market by Product, Raw Material and Application: Global Opportunity Analysis and Industry Forecast, 2019–2026. Available online: https://www.researchandmarkets.com/reports/5019925/edible-cutlery-market-by-product-raw-material (accessed on 17 July 2021).
- Walther, B.A.; Kunz, A.; Hu, C.-S. Type and quantity of coastal debris pollution in Taiwan: A 12-year nationwide assessment using citizen science data. Mar. Pollut. Bull. 2018, 135, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Munin, S. Edible Cutlery: The Future of Eco-Friendly Utensils. Available online: https://www.kickstarter.com/projects/1240116767/edible-cutlery-the-future-of-eco-friendly-utensils (accessed on 17 July 2021).
- Rayah, L. Cutlery You Can Eat: One Company’s Approach to the Plastic Pollution Problem. Available online: https://www.cbc.ca/news/science/bakeys-edible-cutlery-1.4763171 (accessed on 17 July 2021).
- Steffen, L. Edible Plates and Chopsticks Made from Potato Starch in Japan. Available online: https://www.intelligentliving.co/edible-plates-and-chopsticks (accessed on 17 July 2021).
- Menon, A. Chew on Some Dinner Plates. Available online: https://www.thehindu.com/life-and-style/food/get-tableware-you-can-eat-to-save-the-planet-from-plastic-pollution-say-edible-tableware-manufacturers/article34669333.ece (accessed on 17 July 2021).
- Garfield, L. These Real-Life Willy Wonka Edible Spoons Could Help Solve Our Growing Plastic Problem. Available online: https://www.businessinsider.com/bakeys-makes-edible-cutlery-and-launches-kickstarter-2016-3?IR=T (accessed on 17 July 2021).
- Edibles by Jack. Producer Webpage. Available online: https://ediblesbyjack.com/ (accessed on 17 July 2021).
- Edible Pro. Producer Webpage. Available online: https://ediblepro.com/ (accessed on 17 July 2021).
- Dordevic, D.; Necasova, L.; Antonic, B.; Jancikova, S.; Tremlová, B. Plastic cutlery alternative: Case study with biodegradable spoons. Foods 2021, 10, 1612. [Google Scholar] [CrossRef]
- Evoware. Seaweed-Based Packaging. Available online: https://www.webpackaging.com/en/portals/evoware/ (accessed on 17 July 2021).
- Loliware. Producer Webpage. Available online: https://www.loliware.com/the-straw (accessed on 17 July 2021).
- Notpla. Producer Webpage. Available online: https://www.notpla.com/ (accessed on 17 July 2021).
- Patel, P. Edible packaging. ACS Cent. Sci. 2019, 5, 1907–1910. [Google Scholar] [CrossRef] [Green Version]
- Sloan, W. Candy Cutlery Cuts Plastic Waste. Available online: https://www.waste360.com/waste-reduction/candy-cutlery-cuts-plastic-waste (accessed on 17 July 2021).
- Du, W.-X.; Olsen, C.W.; Avena-Bustillos, R.J.; Friedman, M.; McHugh, T.H. Physical and antibacterial properties of edible films formulated with apple skin polyphenols. J. Food Sci. 2011, 76, M149–M155. [Google Scholar] [CrossRef]
- Puscaselu, R.; Gutt, G.; Amariei, S. Rethinking the future of food packaging: Biobased edible films for powdered food and drinks. Molecules 2019, 24, 3136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Zhang, J.; Cheng, F. Cross-linked starch-based edible coating reinforced by starch nanocrystals and its preservation effect on graded Huangguan pears. Food Chem. 2020, 311, 125891. [Google Scholar] [CrossRef] [PubMed]
- Regalado, C.; Perez-Perez, C.; Lara-Cortes, E.; Garcia-Almendarez, B. Whey protein based edible food packaging films and coatings. In Advanced Agriculture Food Biotechnology; Guevara-Gonzalez, R., Torres-Pacheco, I., Eds.; Research Signpost: Kerala, India, 2006; pp. 237–262. ISBN 81-7736-269-0. [Google Scholar]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dybka-Stępień, K.; Antolak, H.; Kmiotek, M.; Piechota, D.; Koziróg, A. Disposable Food Packaging and Serving Materials—Trends and Biodegradability. Polymers 2021, 13, 3606. https://doi.org/10.3390/polym13203606
Dybka-Stępień K, Antolak H, Kmiotek M, Piechota D, Koziróg A. Disposable Food Packaging and Serving Materials—Trends and Biodegradability. Polymers. 2021; 13(20):3606. https://doi.org/10.3390/polym13203606
Chicago/Turabian StyleDybka-Stępień, Katarzyna, Hubert Antolak, Magdalena Kmiotek, Dominik Piechota, and Anna Koziróg. 2021. "Disposable Food Packaging and Serving Materials—Trends and Biodegradability" Polymers 13, no. 20: 3606. https://doi.org/10.3390/polym13203606
APA StyleDybka-Stępień, K., Antolak, H., Kmiotek, M., Piechota, D., & Koziróg, A. (2021). Disposable Food Packaging and Serving Materials—Trends and Biodegradability. Polymers, 13(20), 3606. https://doi.org/10.3390/polym13203606









