Review on the Accelerated and Low-Temperature Polymerization of Benzoxazine Resins: Addition Polymerizable Sustainable Polymers
Abstract
:1. Introduction
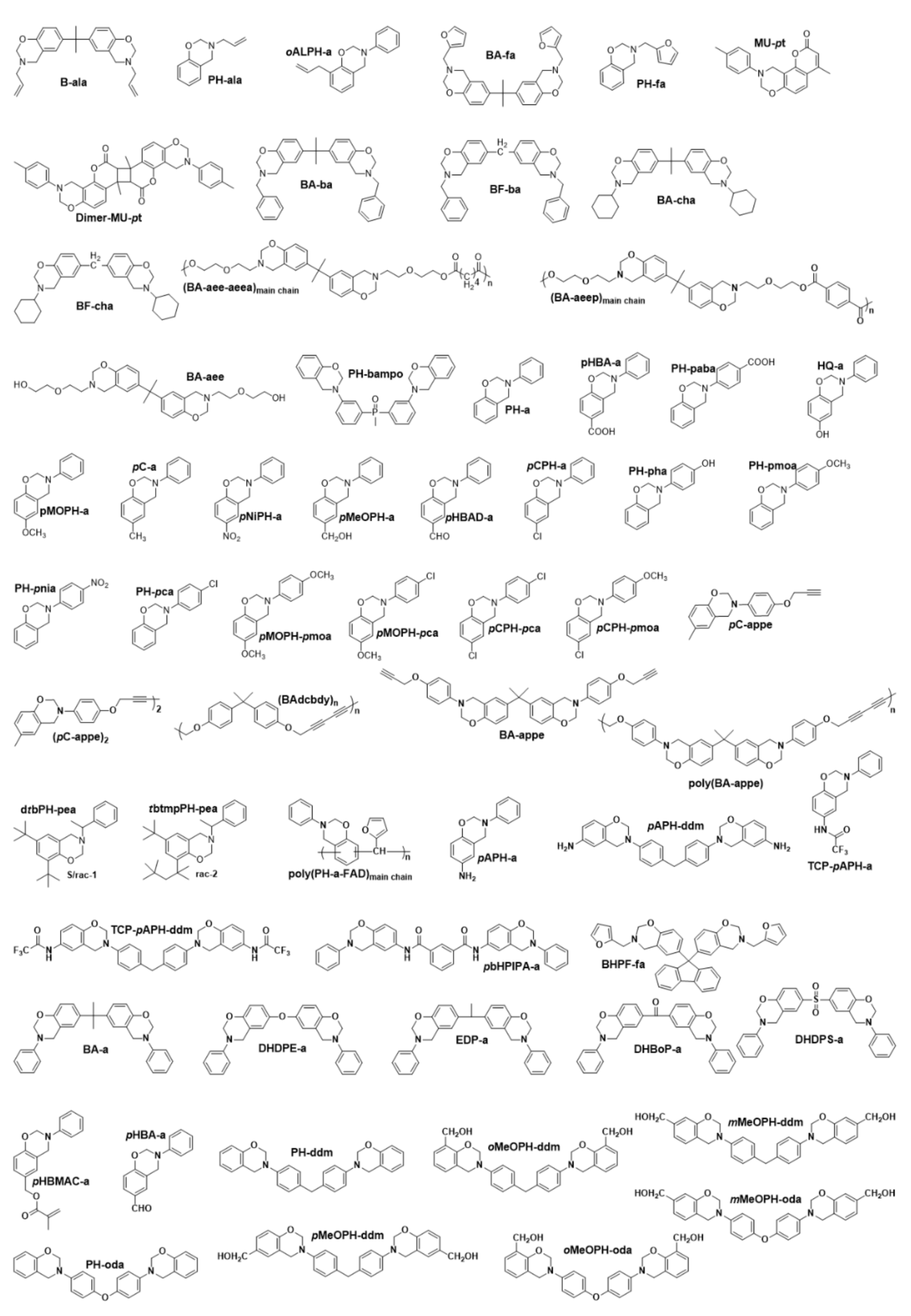
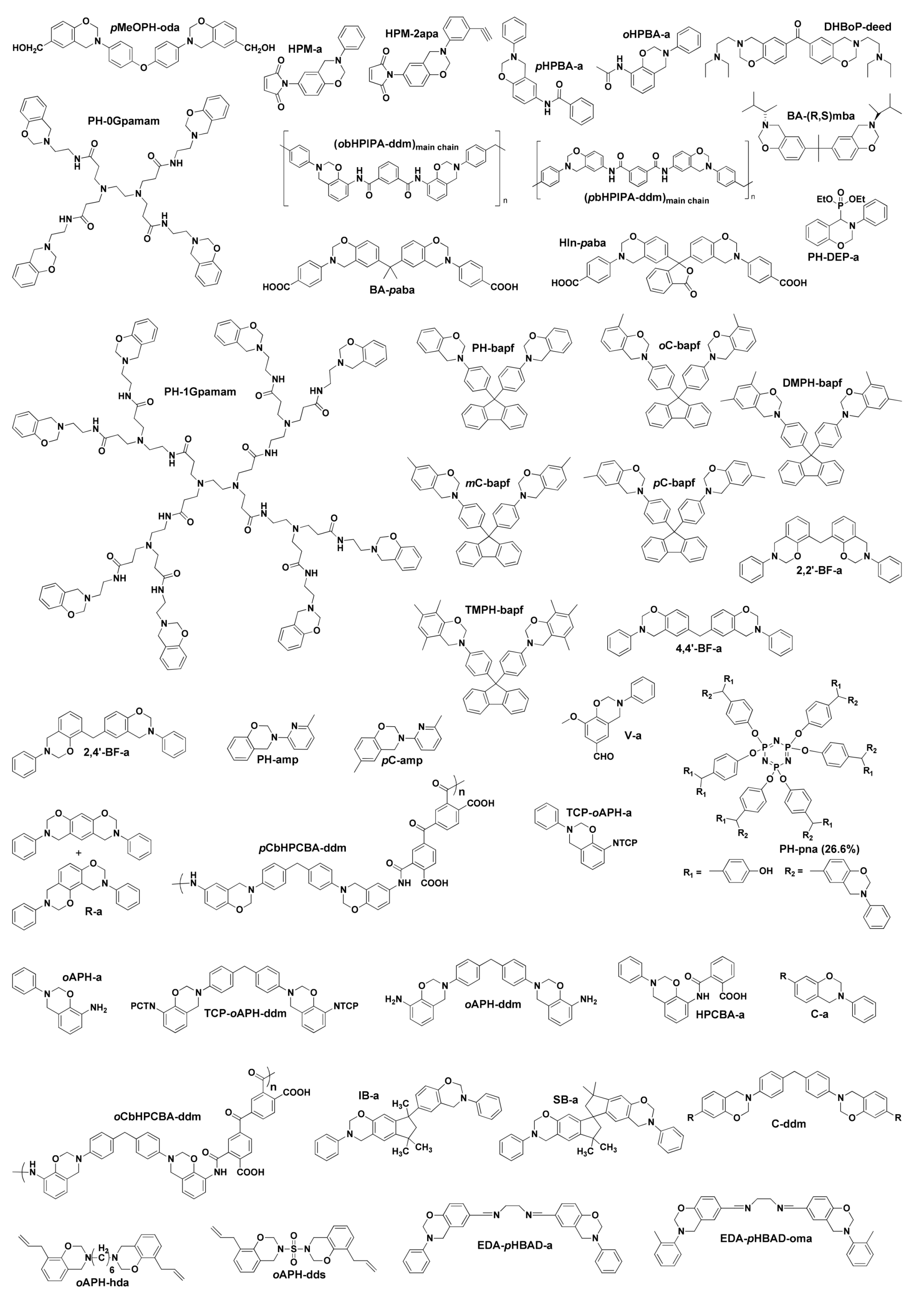
2. Classification of Benzoxazine Monomers
2.1. First Generation Benzoxazines
2.1.1. General Benzoxazine Synthesis and Structure
2.1.2. Mono-Oxazine Functional Monomers
2.1.3. Multi-Oxazine Functional Monomers
2.2. Second Generation Benzoxazines
2.2.1. Multiple Polymerization Mechanisms
2.2.2. Smart Benzoxazine Monomers
2.3. Third Generation Benzoxazines
2.4. Fourth Generation
2.4.1. Smart Benzoxazines
2.4.2. Oxazine Ring Substituted Benzoxazine
2.4.3. Fused Ring Benzoxazines
3. Acceleration of the Rate of Polymerization via Intermolecular Interaction
3.1. Use of Cationic Initiators
3.1.1. Ordinary Acids
3.1.2. Thiols and Elemental Sulfur
3.1.3. Brønsted Acids
3.1.4. Others
3.2. Use of Catalysts
3.2.1. Lewis Acids
3.2.2. Amines
3.2.3. Latent Catalysts
Heterogeneous Latent Catalysts
Homogeneous Latent Catalysts
3.2.4. Nanomaterials
3.2.5. Others
3.3. Others
3.3.1. Intermolecular Influence on Oxazine Ring-Opening Equilibrium (OH Groups)
3.3.2. Participation of the Non-Oxazine Functional Group on the Polybenzoxazine Formation
3.3.3. Influence by Molecular Alignment or Packing
4. Acceleration of the Rate of Polymerization via Intramolecular Interaction
4.1. Modification of Monomer Structures by Electron-Donating or -Withdrawing Groups
4.2. Design of Monomer Structure to Influence the Intermolecular Packing (Rigid Groups)
4.3. Influence of Oxazine Ring-Opening by Intramolecular Interactions: Neighboring Group Effect and 5- or 6-Membered Ring H-Bonding
4.3.1. Background of Intramolecular H-bonding in Benzoxazines
4.3.2. Smart Benzoxazines
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 0G | Zero generation |
| 1G | First generation |
| 35x | 3,5-Xylidine |
| 22PP | 2,2′-Biphenol |
| α-ZrP | α-Zirconium phosphate nanoplatelets |
| a | Aniline |
| A | Arbutin |
| aa | Amic acid |
| aap | Aminoacetophenone |
| aba | Aminobenzoic acid |
| AC | Acacia catechu |
| acac | Acetylacetonate |
| aee | Aminoethoxyethanol |
| aeea | bis(2-(2-aminoethoxy)ethyl) adipate |
| aeep | bis(2-(2-aminoethoxy)ethyl) terephthalate |
| AEPA | α,ω-aminoligo(ethylene terephthalamide) |
| ala | Allylamine |
| ALPH | Allylphenol |
| AMIM+PF6 | 1-allyl-3-methylimidazolium hexafluorophosphate |
| Amp | 2-Amino-6-methylpyridine |
| ap | 2-Aminopyridine |
| apa | 3-Aminophenylacetylene |
| apd | Aminopyrrolidine |
| APH | Aminophenol |
| appe | Aminophenyl propargyl ether |
| appn | 3-Aminophenoxyl-o-phthalonitrile |
| aptes | Aminopropyltriethoxysilane |
| ArIFB | Diaryliodonium tetrafluoroborate |
| ATPEG | Amine terminated poly(ethylene glycol) |
| ba | Benzylamine |
| BA | Bisphenol A (for the sake of convenience bisphenol A is written as BA instead of BPA) |
| BADCY | Bisphenol A dicyanate ester |
| BAEPA | bis-(aminoethyl) terephthalamide |
| bampo | bis(m-aminophenyl)methylphosphine oxide |
| BA-NH2 | 4-(4-(2-(4-(4-aminophenoxy)phenyl)propan-2-yl) phenoxy)benzenamine |
| BAPBACP | 3,3′-(((Propane-2,2-diylbis(4,1-phenylene))bis(oxy))bis(4,1-phenylene))bis(3,4-dihydro-2H-benzo[e][1,3]oxazine-6-carbonitrile) |
| Bapf | 9,9-Bis-(4-aminophenyl)-fluorene |
| BAPh | Bisphenol A phthalonitrile |
| BEM | BA-a/DGEBA (2 wt%)/Imidazole (1.5 wt%) |
| BEP | Benzoxazine/Epoxy/Phenolic (6/4/1 by weight) |
| BF | Bisphenol F |
| BHPe | 1,5-Bis(4-hydroxyphenyl)penta-1,4-dien-3-one |
| BHPr | 1,3-Bis(4-hydroxyphenyl)propanone |
| BHPF | 9,9-Bis(4-hydroxyphenyl)fluorene |
| BHPICA | N,2-bis(2-hydroxyphenyl)-1,3-dioxoisoindoline-5-carboxamide |
| bHPIITO | 2,7-Bis(2-hydroxyphenyl)isoindolo[5,6-f]isoindole-1,3,6,8(2H,7H)-tetraone |
| bHPIPA | N1,N3-bis(4-hydroxyphenyl)isophthalamide |
| BHPPA | N1,N3-bis(2-hydroxyphenyl)isophthalamide |
| BHPPIO | 2,6-Bis(2-hydroxyphenyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone |
| BMI | 4,4′-Bismaleimidodiphenylmethane |
| BO | Butoxyphenol |
| BoP | Benzophenone |
| BTDA | 3,3′,4,4′-Benzophenonetetracarboxylic dianhydride |
| Bz | Benzoxazine |
| BZ-CN | Bisphenol A-based benzoxazine-functionalized phthalonitrile |
| C | Cardanol |
| ca | Chloroaniline |
| cab | Cholesteryl 4-aminobenzoate |
| CbHPCBA | 4,4’-Carbonylbis(2-((4-hydroxyphenyl)carbamoyl)benzoicacid) |
| CH | Cyclohexyl |
| cha | Cyclohexylamine |
| Char yield | Yc |
| CHOTs | Cyclohexyl p-toluenesulfonate |
| CF | Carbon fibre |
| cna | Cyanatoaniline |
| CNSL | Cashew nut shell liquid |
| CNT | Carbon nanotubes |
| COLBERT | Catalytic opening of the lateral benzoxazine rings by thiols |
| cPBO | Crosslinked polybenzoxazole |
| CPH | Chlorophenol |
| cPI | Crosslinked polyimide |
| cpl-dmapa | Caprolactum modified dmapa |
| CSR | Core shell rubber |
| dab | 1,4-Diaminobutane |
| dadd | 1,12-Diaminododecane |
| dcbdy | 1,4-Dichlorobuta-1,3-diyne |
| ddbep | Bis-(4-(4-aminophenoxy)phenyl)ether |
| ddbem | Bis-(3-(4-aminophenoxy)phenyl)ether |
| dde | 4,4′-Diaminodiphenylether |
| ddm | 4,4′-Diaminodiphenylmethane |
| dds | 4,4′-Diaminodiphenylsulfone |
| deed | N, N′-diethylethylenediamine |
| DEP | Diethylphosphite |
| DGEBA | Diglycidylether of bisphenol A |
| DHBoP | 4,4′-Dihydroxybenzophenone |
| DHDPE | 4,4′-Dihydroxydiphenylether |
| DHDPS | 4,4′-Dihydroxydiphenylsulfone |
| DHNP | 2,7-Dihydroxy naphthalene |
| dma | Dimethylaniline |
| dmapa | N, N′-dimethylaminopropylamine |
| DMPH | Dimethylphenol |
| DPA | 4,4′-Bis(4-hydroxyphenyl)pentanoic acid |
| DSC | Differential scanning calorimetry |
| dtbPH | 2,4-Di-tert-butylphenol |
| ea | Ethanolamine |
| ECC | 3,4-Epoxycyclohexylmethyl-3,4-epoxycyclohexanecarboxylate |
| eda | Ethylenediamine |
| EDP | 4,4’-(Ethane-1,1-diyl)diphenol |
| EMI | 2-Ethyl-4-methylimidazole |
| fa | Furfurylamine |
| FAD | Furfuraldehyde |
| FB | Tetrafluoroborate |
| (Fe)0 | Iron oxide nanoparticle |
| (Fe)TA | Terephthalic acid coated iron oxide nanoparticle |
| (Fe)ATA | Aminoterephthalic acid coated iron oxide nanoparticle |
| G | Guaiacol |
| ha | Hydroxyaniline |
| hap | Hydroxyacetophenone |
| HBA | Hydroxybenzoic acid |
| HBMAC | Hydroxybenzyl methacrylate |
| HBP | Hydroxyl-ended hyperbranched polyesters |
| HBAD | Hydroxybenzaldehyde |
| HBN | Hydroxybenzonitrile |
| hda | 1,6-Hexanediamine |
| HIn | Phenolphthalein |
| HCHAL | Dihydroxychalcone |
| HPAMPH | 4-Hydroxy(phenylaminomethyl)phenol |
| HPBA | Hydroxyphenylbenzamide |
| HPCBA | 2-((2-Hydroxyphenyl)carbamoyl)benzoic acid |
| HPIO | Hydroxyphenylisoindoline-1,3-dione |
| HPM | Hydroxyphenylmaleimide |
| HPNI | o-Hydroxyphenylnadimide |
| HQ | Hydroquinone |
| HRC | Heat release capacity |
| IB | Indane bisphenol |
| LC | Liquid crystal |
| LSS | Lap shear strength |
| M | Magnolol |
| ma | Methylamine |
| MAF | 5-Furfuryl-2,2-dimethyl-[1,3]dioxane-4,6-dione |
| mba | 3-Methylbutan-2-amine |
| MBM | Phenol- N,N’-dimethyl-1,3-propanediamine Bz |
| mda | Methylenedianiline |
| MeOPH | Hydroxymethylphenol |
| MeOTs | Methyl-p-toluene sulfonate |
| mepu | 4,4′-Methylenebis(3-ethylamine-1-phenylurea) |
| MMA | Methyl methacrylate |
| moa | Methoxyaniline |
| MOF5 | [Zn4O(BDC)3], BDC=1,4-Benzenedicarboxylate |
| MOPH | Methoxyphenol |
| MR | 2-Methylresorcinol |
| MSA | Methanesulfonic acid |
| MU | 4-Methylumbelliferone |
| MWCNT | Multiwalled carbon nanotube |
| [(nbd)RhCl]2 | [(Norbornadiene)rhodium(I) chloride]2 |
| NH2-H(PAM)2PH | 4-Amino-2-(((4-hydroxy-3-((phenylamino)methyl)phenyl)amino)-methyl)phenol |
| NH2-PAMPH | 4-Amino-2-((phenylamino)methyl)phenol |
| nia | Nitroaniline |
| NiPH | Nitrophenol |
| N, N-DBA | N, N′-dibenzylaniline |
| NP | Naphthol |
| Oda | 4, 4′-Oxydianiline |
| oMBA | o-Methyl bisphenol A |
| oTFHPA | 2,2,2-Trifluoro-N-(2-hydroxyphenyl)acetamide |
| pamam | Polyamindoamine dendrimer |
| PBA | Phenylboronic acid |
| PBO | Polybenzoxazole |
| PBOM | 2,6-Diphenylbenzo[1,2-d:5,4-d’]bis(oxazole) |
| PBz | Polybenzoxazine |
| pC | p-Cresol |
| pC-a-pC | Ring-opened pC-a Bz with p-Cresol |
| (pC-appe)2 | Dimer of pC-appe |
| PCL | poly(ε-Caprolactone) |
| pda | Phenylenedianiline |
| pea | Phenylethanamine |
| PF | Hexafluorophosphate |
| PH | Phenol |
| Ph2I+PF6- | Diphenyliodonium hexafluorophosphate |
| Ph3S+AsF6- | Triphenylsulfonium hexafluorophosphate |
| PG | Pyrogallol |
| PGU | Phloroglucinol |
| PGU-NH2 | 1,3,5-Tris(4-aminophenoxy)benzene |
| PIPF | Diphenyliodonium hexafluorophosphate |
| PIFA | Diphenyliodonium hexafluoroarsenate |
| pJeff-V-a | p-Jeffamine funcionalized V-a |
| PN | Phosphazene |
| pna | Phosphonitrilic amine |
| POSS | Polyhedral oligomeric silsesquioxane |
| Pra | n-Propylamine |
| PRB | poly(Resorcinol phenylboronate) |
| Pyoa | 4-(Prop-2-yn-1-yloxy)aniline |
| PU | Polyurethane |
| PVPH | Poly(4-vinylphenol) |
| R | Resorcinol |
| RES | Resveratrol |
| ROP | Ring-opening polymerization |
| SB | Spirobiindane bisphenol |
| St | Stearate |
| SP(St-DVB) | Sulfonated poly (styrene-divinylbenzene) microspheres |
| t | Toluidine |
| T | 1,3,5-Tri(p-hydroxyphenyl)benzene |
| TA | p-Toluic acid |
| T5% | 5% Weight reduction temperature |
| T10% | 10% Weight reduction temperature |
| Tg | Glass transition temperature |
| To | Onset temperature of polymerization |
| Tp | Exothermic peak temperature of polymerization |
| TBMI | N,N′-(2,2,4-trimethylhexan-1,6-diyl)bismaleimide |
| tbtmpPH | 4-(tert-Butyl)-2-(2,4,4-trimethylpentan-2-yl)phenol |
| TCP | Tetrachlorophthalic anhydride |
| TCP-pAPH-a | TCP protected pAPH-a |
| TDA | 3,3′-Thiodipropionic acid |
| TDPH | 4,4′-Thiodiphenol |
| TEOS | Tetraethoxysilane |
| tepa | Tetraethylenepentamine |
| tapm | Tetra-p-aminophenylmethane |
| TFAPH | Trifluoroacetamidophenol |
| TIPO | Titanium isopropoxide |
| tma | Trimethylaniline |
| TMPH | Trimethylphenol |
| tpa | Triphenylamine |
| TPHB | Triphenylbenzene |
| trisapm | p-Rosaniline amine |
| TsOH | p-Toluenesulfonic acid |
| U | Umbelliferone |
| V | Vanillin |
| va | Vinylaniline |
| XY | Xylenol |
Appendix A
| Year | Monomer/Aid | Category | Curing Parameters | Ref. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| To | Tp | Δ H(J/g) | T5% | T10% | |||||||||||
| 1995 | BA-a a | Monomer | 188 | 226 | 313 | - | - | [266] | |||||||
| BA-a/adipic acid, sebacic acid, 2,2′-dihydroxybiphenyl (6 mol%) | Brønsted acid | 150–154 | 188–203 | 352–322 | - | - | |||||||||
| 1999 | 2, 4-XY-cha/BF3.OEt2 | Lewis acid | Curing time studied | [257] | |||||||||||
| 1999 | pC-ma/CF3COOH (10 mol%) Sebacic acid (9 mol%) p-cresol (10 mol%) | Brønsted acid and phenol | Catalysis studied with curing time | [256] | |||||||||||
| 1999 | BA-a | Monomer | - | 231 | - | - | |||||||||
| BA-a/MeOTs (5 wt%) | Brønsted acid | ~80 | 144, 179, 200 | - | - | [258] | |||||||||
| 1999 | BA-a/PCl5 (20:1 mole ratio, 2wt%) | Lewis acid | 122, 189, 264 | - | - | - | [255,416] | ||||||||
| PCl3, POCl3, TiCl4, AlCl3, SbCl5/oxetane, MeOTs, MeOTf, CF3COOH, BF3.OEt2, (C6H5)3CSbCl6, MeI, BuLi, (C6H5CO)2O2 | Lewis acids, akylating agent, covalent initiator, anionic initiator, free radical initiator | DSC data not available | |||||||||||||
| 2001 | BA-a | Monomer | - | - | ~320 | - | - | [80] | |||||||
| Ba-A/PCL (11–100 wt%) | Blend | - | - | ~270–88 | - | - | |||||||||
| 2002 | BA-a | Monomer | >360 min | [417] | |||||||||||
| BA-a/Oxalic acid (0.07 wt%) | Acid | 283 min. | Gelation time at 140 °C | ||||||||||||
| N,N-dimethylamine (1 wt%) | Amine | 104 min. | |||||||||||||
| Epoxy resin (10 wt%) N,N′- dimethylbenzylamine (1 wt%) | Blends | 193 min. | |||||||||||||
| 2003 | PH-a/Ph2I+PF6− (0.1–2.4 molL/Ph2I+PF6−1) | Onium salts | 17–72% % conversion at 300 nm | [299] | |||||||||||
| Ph3S+AsF6/Ph2I+PF6− (2.4 molL/Ph2I+PF6−1) | Onium salts | 63% | |||||||||||||
| 2003 | BA-a/Organically modified clay | Nanocomposite | 144 b | 217 | 298 | - | - | [302] | |||||||
| 2004 | BA-a/TIPO (10 wt%)? | Nanocomposite | 146 | 220 | 51 cal/g | 350 | 425 | [304] | |||||||
| 2005 | BA-a | Monomer | 220 b | 239 | - | - | - | [305] | |||||||
| BA-a/CF (59 wt%) | Nanocomposite | 176 | 214 | - | - | - | |||||||||
| 2006 | BA-a | Monomer | ~220 b | ~255 | - | 315 | - | [191] | |||||||
| MBM/BA-a/clay nanocomposite (10 wt%) a | Nanocomposite | ~185 | 225 | - | 331 | - | |||||||||
| 2005 | BA-a | Monomer | ~190 b | ~240 | - | - | - | [52] | |||||||
| BA-a/CuCl, CuCl2 | Transition metalsLewis acid | ~160 | ~223 | - | - | - | |||||||||
| 2006 | BA-a | Monomer | ~230 b | ~257 | 309 | - | - | [312] | |||||||
| BA-a/MWCNT (1 wt%) | Nanocomposite | 240 | 248 | 286 | - | - | |||||||||
| 2006 | BA-a | Monomer | 2 h at 200 °C, heating | [301] | |||||||||||
| BA-a/SiC (4 wt%) | Microwave radiation at 270 W | <20 min. | |||||||||||||
| 2008 | BA-a/Epoxy | Blends | ~210 b | ~260 | - | 309 | [335] | ||||||||
| BA-a (50 mol%)/Epoxy (50 mol%)/salt of diethanolamine and TsOH (10 wt%) | Latent curing agent | ~130 b | 210 | - | 302 | ||||||||||
| 2008 | PH-pyoa | Monomer | 225 | 240 | 772 | 348 | 386 | [418] | |||||||
| PH-pyoa/([(nbd)RhCl]2) (10 µmol) | Transition metal | 188 | 221 | 130 | 330 | 371 | |||||||||
| 2008 | BEP | Monomer | - | 241 | - | 360 | - | [345] | |||||||
| BEP/CNT:CSR (1:0,0:11, 1:11 wt%) | Nanocomposite | - | 233–236 | - | 360 | - | |||||||||
| 2010 | BA-a | Monomer | - | 263 | - | - | - | [406] | |||||||
| BA-a/EMI (5 mol%) | Amine | - | 235 | - | - | - | |||||||||
| BA-a/TsOH. H2O (5 mol%) | Brønsted acid | - | 212 | - | - | - | |||||||||
| BA-a/Mn(acac)2, Fe(acac)3 (5 mol%) | Lewis acid | - | 203–209 | - | - | - | |||||||||
| 2010 | BA-a | Monomer | 187 | 219 | 271 | - | - | [86] | |||||||
| C-a | 273 | 263 | 71 | - | - | ||||||||||
| C-a/BA-a (38 wt%) | Blending | 233 | 250 | 114 | - | - | |||||||||
| 2011 | BA-a | Monomer | 221 | 249 | - | - | - | [419] | |||||||
| BA-a/TEOS (39 wt%) | Blending | 169 | 223 | - | - | - | |||||||||
| BA-a/TEOS (39 wt%)/PH-aptes (20 mol%) | 191 | 242 | - | - | - | ||||||||||
| 2011 | BA-a | Monomer | ~115 b | 191 | - | 283 | 293 | [404] | |||||||
| BA-a/resorcinol (10 mol%) | Urethane derivatives | ~120 | 177 | - | 279 | 290 | |||||||||
| BA-a/resorcinol:phenyl isocyanate (10:10 mol%) | ~125 | ~150 | - | 275 | 288 | ||||||||||
| 2011 | pC-a/TsOH, MeOTs, CHOTs | Alkyl sulfonates | 32–95% conversion at 100 °C in 1 day | [290] | |||||||||||
| 2011 | BA-a | Monomer | ~180 b | 237 | 277 | - | - | [274] | |||||||
| BA-a/CNSL (different ratios) | Phenol | ~130–170 | 197–216 | 194–246 | - | - | |||||||||
| 2011 | BA-a/BADCY, epoxy | Blend | ~200–215 b | ~240–260 | - | 310–330 | - | [175] | |||||||
| 2012 | BA-a | Monomer | ~200 b | 242 | 313 | - | - | [420] | |||||||
| BA-a (100)/TDPH (10 wt%) | Blend | ~130 | 218 | 361 | 218 | - | |||||||||
| BA-a/DGEBA (different ratios) | ~225 | 258–275 | 276–326 | - | - | ||||||||||
| BA-a/DGEBA/TDPH (different ratios) | ~160 | 228–239 | 342–348 | - | - | ||||||||||
| 2012 | BA-a | Monomer | 178 b | 227 | 232 | - | - | [421] | |||||||
| BA-a/Lignin (50 wt%) | Phenol | 112 | 211 | 64 | - | - | |||||||||
| 2012 | PH-pra/2-methylresorcinol (2:1 mol ratio) | % conversion at RT in methanol at different time interval | [422] | ||||||||||||
| 2012 | PH-a | Monomer | 247 | 254 | 298 | - | - | [423] | |||||||
| BA-a | 249 | 261 | 277 | - | - | ||||||||||
| PH-a, BA-a/Lignin (30 wt%) | Polyphenolic materials | 175–176 | 203–212 | 202–303 | - | - | |||||||||
| 2012 | BA-a | Monomer | 240 b | 261 | - | - | - | [424] | |||||||
| BA-a/FeCl3 (1 wt%) | Lewis Acid | 135 | ~205 | - | - | - | |||||||||
| 2012 | BA-ala | Monomer | 150 | 230, 278 | - | 334 | 363 | [283] | |||||||
| (BA-ala)/1,2- ethanedithiol | Blend/Thiol-ene reaction | 140, 225 | 175, 260 | - | - | - | |||||||||
| 2013 | BA-mda | Monomer | ~175 b | ~250 | 232 | 362 | 384 | [347] | |||||||
| BA-mda/GO (1–5 wt%) | Nanocomposite | ~180–~190 | ~250 | 224–155 | 365–366 | 389 | |||||||||
| 2013 | PH-a | Monomer | 0% | % conversion at 60 °C for 2 days | [314] | ||||||||||
| PH-a, PH-ma, PH-ba, pC-a, BA-a/BF3.OEt2 (40:1 mol ratio) | Blend | 0–98% | |||||||||||||
| 2013 | Bz (Epsilon 99100 RTM) | Monomer | 214 | - | 329 | - | - | [425] | |||||||
| Bz (Epsilon 99100RTM)/CNT (0.1–1 wt%) | Nanocomposites | 200 | - | 316–327 | - | - | |||||||||
| 2013 | G-fa | Monomer | 219 a | 240 | 62 | 332 | 401 | [334] | |||||||
| G-fa/MSA, TsOH, MeOTs (different ratios) | Acids | 125–191 | 173–203 | 103–115 | 313–335 | 407–450 | |||||||||
| 2012 | BEM-80/140/180 | Epoxy/amine | ~185–~200 | 227–244 | 169–204 | 192–199 | 243–275 | [326] | |||||||
| 2013 | BA-a | Monomer | 203 | 240 | 299 | 240 | 305 | [275] | |||||||
| BF-a | 170 | 232 | 311 | 301 | 353 | ||||||||||
| BA-a, BF-a/TDPH (12 mol%) | Blend with acid | 132–172 | 218–222 | 300–331 | 214–252 | 308–312 | |||||||||
| BA-a, BF-a/TDA (15 mol%) | 130–145 | 196–198 | 294–388 | 200–265 | 246–338 | ||||||||||
| TDPH-a | 173 | 221 | 350 | 313 | 334 | ||||||||||
| TDPH-a/TDPH (12 mol%) | 144 | 209 | 316 | 381 | 311 | ||||||||||
| TDPH-a/TDA (15 mol%) | 146 | 191 | 315 | 280 | 316 | ||||||||||
| 2013 | pC-a | Monomer | 262 | 269 | 77 kJ/mol | - | - | [426] | |||||||
| pC-a/(MX, M = Na, Li, NH4+, Zn+2, Cu+2, Al+3, Fe+3 Ag+, Co+2; X = I, ClO4, SCN, Br, OPh, SPh, Cl, OAc, OTf, DMAP, EMI, 2-/3-/4-hydroxypyridine, TsOH.2H2O | Catalysts | 168–262 | 197–269 | 67–88 kJ/mol | - | - | |||||||||
| 2013 | C-a | Monomer | 242 | 263 | 71 | 358 | 391 | [11] | |||||||
| pHBA-a | 158 | 165 | 177 | 290 | 330 | ||||||||||
| BA-a | 187 | 217 | 271 | 313 | 342 | ||||||||||
| C-a, BA-a: pHBA-a (1:0.1) | Binary blends | 175–513 | 220–255 | 54–262 | 307–343 | 374–382 | |||||||||
| C-a:BA-a (1:3–3:1) | 211–235 | 250–281 | 74–223 | 287–345 | 353–389 | ||||||||||
| C-a:BA-a: pHBA-a (1–3:1–6:0.05–0.3) | Ternary blends | 161–201 | 209–235 | 200–129 | 258–346 | 339–421 | |||||||||
| 2013 | PH-a/BF3.OEt2 (40/1 wt%) | Cationic initiator | Polymer yield 98% at 60 °C in two days | [314] | |||||||||||
| 2013 | BA-a a | Monomer | ~200 b | ~240 | - | - | - | [346] | |||||||
| BA-a/MWCNT (1:1 wt%) | Nanocomposites | 160 | 230 | - | - | - | |||||||||
| BA-a/MWCNT (1:1 wt%) thermal curing | No exotherm in DSC | - | - | ||||||||||||
| BA-a/MWCNT (1:1 wt%) microwave curing | 417 | - | |||||||||||||
| 2013 | BA-a/TBMI (1/1 mol) | Blend | ~190 b | 247 | - | - | - | [427] | |||||||
| BA-a/TBMI/Imidazole (1/1 mol/3 wt%) | Ternary blend | 100 | 180, 220 | - | - | - | |||||||||
| 2013 | BA-a | Monomer | 241 | 261 | 149 KJ/mol | - | - | [428] | |||||||
| BA-a/TBMI (1:1 mol ratio) | Blend | 194 | 247 | 121 | - | - | |||||||||
| 2013 | BMI/BA-a (2:1 wt ratio)/BADCY (30 wt%) | Ternary blend | 150 b | ~220 | - | 333 | - | [429] | |||||||
| 2013 | BA-a | Monomer | - | 261 | - | 297 | - | [346] | |||||||
| BA-a/GO (0.5–3wt%) | Nanocomposites | - | 236–250 | - | 307-ND | - | |||||||||
| BA-a/Graphite (0.5–3 wt%) | - | 264–266 | - | 300-ND | - | ||||||||||
| 2014 | pC-a | At 150 °C for 6 h, without promoter | 0% | [278] | |||||||||||
| pC-a/Thiophenol, p-nitrothiophenol (10 mol%) | Acids at 150 °C for 6 h | 97–99% | |||||||||||||
| 2014 | BA-a | Monomer | - | ~250 | - | - | - | [430] | |||||||
| BA-a/PBOM (5–20 wt%) | Composite | - | 210–220 | - | - | - | |||||||||
| 2014 | PH-a | Monomer | ~250 b | 265 | - | - | - | [431] | |||||||
| 2,4-XY-tma | - | - | - | - | - | ||||||||||
| BADCY | ~290 | 331 | - | - | - | ||||||||||
| PH-a, 2,4-XY-tma/BADCY (1:1 mol%) | Blend | ~175 b | 225–246 | - | - | - | |||||||||
| 2015 | BA-had a | Monomer | 178 | 255 | 193 | 162 | 271 | [432] | |||||||
| BA-hda/1,2-ethanethiol; 1,6-hexanedithiol (2.4 wt%) | Thiols | 165–169 | 205, 269 | 155–185 | 161–258 | 207–280 | |||||||||
| 2015 | PU/PH-ma (70:30 wt%) | Blends | 110 b | 191 | 52 | - | - | [367] | |||||||
| PU-Phenol/PH-ma (70:30 wt%) | 140 | 215 | 18 | - | - | ||||||||||
| 2015 | BA-a | Monomer | 213 | 234 | 309 | 293 | 331 | [323] | |||||||
| BA-a/aromatic and aliphatic diamine | Blend | 57–206 | 80–254 | 4–70 | 251–318 | 266–351 | |||||||||
| 2015? | BA | Monomer | 228 | 248 | 325 | 360 | 393 | [319] | |||||||
| BA/Phenylboronic acid (5–20 wt%) | Acid | 165–209 | 211–232 | 230–287 | 284–330 | 356–412 | |||||||||
| 2015 | PH-apa | Lewis acid | ~175 b | 247 | 879 | - | - | [316] | |||||||
| PH-apa/Ni(acac) (0.01mol)/triphenyl phosphine (0.01mol) | ~140 b | 184, 228 | 878 | - | - | ||||||||||
| 2015 | BAPBACP | Monomer | 240, 341 | 246, 62 | - | - | [317] | ||||||||
| BAPBACP/Fe(acac)3 (3.5wt%) | Lewis acids | 218, 351 | 72, 46 | - | - | ||||||||||
| 2016 | BA-ala | Monomer | 203 | 266 | −316 | 342 | 362 | [285] | |||||||
| BA-ala/S8 (90 wt%) | Chemical linking | 161 | 182 | −11 | 219 | 283 | |||||||||
| 2016 | pC-pt | Monomer | ~242 b | 273 | 220 | - | - | [286] | |||||||
| pC-pt/S8 (0.5–5 wt%) | Blend | ~170 | ~227–~250 | - | - | ||||||||||
| HQ-a | Monomer | ~230 | ~260 | - | - | - | |||||||||
| HQ-a/S8 (0.5–5 wt%) | Blend | ~190–210 | 220–242 | - | - | - | |||||||||
| 2016 | C-a | Monomer | 240 | 263 | - | 345 | 393 | [310] | |||||||
| C-a/MOF5 (1–15 wt%) | Lewis acids | 178–228 | 227–251 | - | 416-ND | 429-ND | |||||||||
| 2016 | C-a/S8 (80 wt%) | Chemically linked sulfur | No exotherm and endotherm, cured during reaction | - | - | [21] | |||||||||
| 2016 | T-a | Monomer | 208 | 238 | - | 396 | 424 | [145] | |||||||
| T-a/Ph, MR, HQ, PG, pMOPH (1.5 eq.) | Blend | 88–165 | 115–237 | - | - | - | |||||||||
| 2016 | BA-a | Monomer | 212 | 245 | 322 | 289 | 323 | [294] | |||||||
| BA-a/BA (5–25%) | Blend | 122–180 | 192–229 | 333–349 | 265–297 | 293–325 | |||||||||
| 2016 | BA-a/amine, NaI, diacid, indole, imidazole, p-tert-butyl phenol (10 mol%) | Amines, acid, phenol | 216–254 | - | - | - | [330] | ||||||||
| 2016 | BF-a | Monomer | 202 | 228 | 254 | 195 | 235 | [340] | |||||||
| BF-a/m-xylylenediamine, trimethylhexamethylenediamine (0.011 mol) | Basic catalyst | 71–211 | 131–242 | 7–47 | 159–175 | 201–219 | |||||||||
| 2016 | BA Bz (Huntsman) | Monomer | 203 b | 243 | - | - | - | [300] | |||||||
| BA Bz/ArIFB (1–3 wt%) | Diaryliodinium salts | 166–169 | 213–225 | - | - | - | |||||||||
| BA Bz/PIPF (1–3 wt%) | 160–162 | 212–218 | - | - | - | ||||||||||
| BA Bz/PIFA (1–3 wt%) | 164–169 | 218–226 | - | - | - | ||||||||||
| 2017 | BA-a/PH-a/CY-179/High molecular weight phenoxy resin/XS-EP-7 (35:25:12.5:12.5:15 wt%) | Flame Retardant composition Blend | 160 b | ~220 | - | - | - | [433] | |||||||
| 2018 | PH-a, PH-dmapa, PH-cpl-dmapa | Brønsted acids | 160–202 | 195–238 | 36–262 | 310–331 | - | [273] | |||||||
| BA-a, BA-dmapa, BA-cpl-dmapa | 148–196 | 190–247 | 32–195 | 260–339 | - | ||||||||||
| BF-a, BF-dmapa, BF-cpl-dmapa | 122–152 | 196–225 | 37–189 | 193–391 | - | ||||||||||
| 4,4-BF-a, 4,4-BF-dmapa, 4,4-BF-cpl-dmapa | 150–156 | 186–213 | 44–147 | 262–331 | - | ||||||||||
| 2017 | PH-pt | Monomer | 190 | 224 | 124 | 275 | 305 | [336] | |||||||
| PH-pt/NH3OHCl, PhNH3Cl, PhNHNH3Cl, NH4Cl, EtNH3Cl (5 mol%) | Amine salts | 158–173 | 185–205 | 74–161 | 242–261 | 284–294 | |||||||||
| BA-a | Monomer | 211 | 237 | 125 | - | - | |||||||||
| BA-a/PhNH3OHCl, NH3Cl, PhNHNH3Cl, NH4Cl, EtNH3Cl (5 mol%) | Amine salts | 170–181 | 208–214 | 35–124 | - | - | |||||||||
| 2017 | C-a:BA-a (3:1) | Monomer blend | 218 | 241 | 40 | 338 | 389 | [13] | |||||||
| C-a:BA-a (3:1)/nanoalumina (1–5 wt%) | Nanoparticle | 195–212 | 239–244 | 46–95 | 389-ND | 412-ND | |||||||||
| C-a:BA-a (1:3) | 211 | 231 | 223 | 291 | 356 | ||||||||||
| C-a:BA-a (1:3)/nanoalumina (1–5 wt%) | 191–196 | 228–231 | 110–165 | 285-ND | 359-ND | ||||||||||
| 2017 | PH-ddm/2-phenyl-1,3,2-benzenediolborane | Boron based catalyst | 197 | 238 | 124 | 186 | [320] | ||||||||
| 2017 | Bz-CN | Blend | ~160 | 230, 265 | - | - | - | [172] | |||||||
| Bz-CN/SH-2100 (10–50 wt%) | Thiol | ~155–130 | ~210–183, ~257–248 | - | - | - | |||||||||
| 2017 | PH-fa/MAF (1:1 mol ratio) | Blend | - | - | - | - | - | [296] | |||||||
| 2017 | BA-a (Huntsman) | Monomer | 186 | 227 | 324 | 325 | 347 | [351] | |||||||
| BA-a/α-ZrP (1–3 wt%) | Nanocomposite | 117–158 | 209–214 | 348–351 | 332-ND | 355-ND | |||||||||
| 2017 | BA-a | Monomer | 173 | 235 | 295 | 295 | 332 | [434] | |||||||
| BA-a/m-phenylenediamine formaldehyde oligomer | Blend | 130 | 215 | 23 | 322 | 346 | |||||||||
| 2018 | BA-a | Monomer | 212 | 237 | 201 | 326 | 364 | [315] | |||||||
| BA-a/B(C6F5)3 (3–10 mol%) | Lewis acid Blend | 125–180 | 174–217 | 103–156 | 294–323 | 340–347 | |||||||||
| PH-ba | Monomer | 242 | 261 | 35 | 257 | 267 | |||||||||
| PH-ba/B(C6F5)3 (3–10 mol%) | Lewis acid Blend | 163–217 | 215–251 | 45–130 | 244–258 | 263–297 | |||||||||
| 2018 | BA/ECC (50 wt%) (BA-Huntsman) | Blend | ~150 b | ~255 | - | 310 | - | [352] | |||||||
| BA/ECC)/HBP-AMIM+PF6− (different wt%) | ~125–175 | ~230–255 | - | 332–345 | - | ||||||||||
| 2018 | BA-a | Monomer | 186 b | 235 | 391 | 303 | 335 | [435] | |||||||
| BA-a/AC (1–5 wt%) | Acid | 164–179 | 224–230 | 303–376 | 306–321 | 338–355 | |||||||||
| 2018 | C-trisapm | Monomer | 254 | 272 | 108 | 387 | 421 | [313] | |||||||
| C-trisapm/Iron nanoparticles | Nanoparticles blend | 172–206 | 218–246 | 71–89 | 306–340 | 365–386 | |||||||||
| 2018 | C-a | Monomer | 242 | 263 | 63 | 292 | 331 | [311] | |||||||
| C-a/Stearic acid; Stearate salts (1–10 wt%) | Lewis acid | 169–226 | 202–247 | 68–97 | 351-ND | 390-ND | |||||||||
| 2018 | BA-a (Polaris tech.) | Monomer | ~195 b | ~230 | - | - | - | [297] | |||||||
| BA-a/ SP(St-DVB) (5–15 wt%) | Nanocomposite | ~175–130 | ~220–205 | - | - | - | |||||||||
| 2019 | V-fa/CS (0–50:0–50 wt%) | Composite | 190-ND | 204-ND | 35.5-ND | - | 91-389 | [361] | |||||||
| 2019 | BA-tepa/Amino cellulose | Composite | 181–226 | 224–256 | - | - | - | [363] | |||||||
| 2019 | BA-a/4-nitrophthalonitrile | Composite | - | 219 | - | 346 | 372 | [57] | |||||||
| Year | Monomer/Polymer | To (°C) | Tp (°C) | ΔH (kJ/mol) | T5% (°C) | T10% (°C) | Ref. |
|---|---|---|---|---|---|---|---|
| 2003 | BA, PH-ala | 145 | 207–265 | 351, 531 | 343, 348 | 367, 374 | [53] |
| oALPH-a | 241 | 263 | 84 | 288 | 356 | ||
| 2005 | BA, PH-fa | ~233 | 241, 247 | 250, 265 | 336, 347 | 382, 391 | [130] |
| 2007 | MU-pt | 225 | 229 | 292 | 304 | 323 | [208] |
| Dimer-MU-pt | 187.5 | 203 | 205 | 293 | 324 | ||
| 2007 | BA, BF-ba | - | 213, 249 | 67, 81 | - | - | [140] |
| BA, BF-cha | - | 237, 245 | 72,93 | - | - | ||
| 2008 | BA-aee | 180 | 202 | 75 | - | - | [217] |
| (BA-aee-aeea)main chain | 215 | 250 | 105 | 248 | 278 | ||
| (BA-aeep)main chain | 210 | 243 | 95 | 288 | 328 | ||
| 2008 | PH-bampo | ~210 | ~235 | - | - | - | [81] |
| 2008 | PH, pHBA -a | 262 c, 180 c | 85 d | - | 338, 330 | [292] | |
| PH, pHBA -paba | 204 c, 208 c | - | - | 282, 265 | |||
| 2008 | pCPH, pMeOPH, pHBAD, pNiPH, pC, pMOPH, HQ, PH-a | - | 175 a–273 a | 77 d–108 d | - | 142–490 | [138] |
| PH-pha, pmoa, pnia, pca | - | 195 a–289 a | 73 d–107 d | - | 335–375 | ||
| pMOPH-pmoa, pca | - | 254 a, 274 a | 72 d, 73 d | - | 315, 302 | ||
| pCPH-pca, pmoa | - | 269 a, 257 a | 80 d, 76 d | - | 300, 321 | ||
| 2009 | pC-appe | 191 | 235 | - | 339 | 356 | [380] |
| (pC-appe)2 | 140 | 215 | 340d | 349 | 368 | ||
| 2009 | (BAdcbdy)n | - | 185, 260 | - | - | - | [413] |
| BA-appe | - | 241 | - | - | - | ||
| Poly(BA-appe) | 125 | 185 | - | 367 | - | ||
| 2009 | PH-eda | 185 | 185 | 444 | 291 | - | [137] |
| pC-dab | 193 | 193 | 316 | 251 | - | ||
| oC-eda | 234 | 234 | 317 | 258 | - | ||
| mC-eda | 197 | 197 | 321 | 273 | - | ||
| PH-hda | 225 | 292 | 280 | - | |||
| pC-hda | 244 | 253 | 283 | - | |||
| oC-hda | 250 | 250 | 277 | - | |||
| mC-hda | 238 | 261 | 272 | - | |||
| PH, pC, oC, mC- dadd | 241–262 | - | 215–229 | 281–290 | - | ||
| 2009 | dtbPH, tbtmpPH-pea | - | 263 b–294 b | 5.42–81.8 | - | - | [160] |
| 2010 | poly(PH-a-FAD)main chain | 198 | 241 | - | - | - | [232] |
| 2010 | pAPH-a, ddm | - | 96–243 | - | - | - | [322] |
| TCP-pAPH-a, ddm | 184, 236 | 210, 237 | - | - | - | ||
| pbHPIPA-a | 194 | 223 | - | 341 | 380 | ||
| 2010 | C-a:BA-a (0–3:0–3) | 187–242 | 219–263 | 71–271 | - | - | [86] |
| 2011 | BHPF-fa | 205 | 252 | - | 402 | 422 | [154] |
| 2011 | BA, BF, DHDPE, EDP, DHBoP, DHDPS -a | 206–261 | 215–267 | 157 d–144 d | - | - | [400] |
| 2011 | pHBMAC-a | 193 | 203 | 315 | - | - | [228] |
| 2011 | pHBA-a | 162 a,b | 196 | 64 d | - | - | [375] |
| 2012 | C-ddbep, ddbem, dds, ddm, a | 195–245 | 233–267 | 71–271 | 292–355 | 350–389 | [399] |
| 2012 | PH-0G, 1Gpamam | ~177 a,150 a | ~273, 271 | - | 265, 222 | 314, 304 | [143] |
| 2012 | PH, oMeOPH, mMeOPH, pMeOPH-ddm | 140–224 | 188–262 | 211–326 | 364–394 | 390–430 | [398] |
| P-oda | 134–227 | 162–253 | 262–305 | - | - | ||
| 2012 | HPM-a | 169c | 205, 245 | 172 | 344 | 377 | [389] |
| HPM-2apa | 196c | 209 | 568 | 412 | 432 | ||
| 2012 | PH, pHPBA, oHPBA-a | - | 187–263 | - | - | - | [193] |
| (obHPIPA, pbHPIPA-ddm)main chain | - | 212, 259 | - | - | - | ||
| 2012 | PH, oHBA, mHBA, pHBA-a | 151–237 | 196–255 | 201–336 | - | - | [403] |
| 2012 | DHBoP-deed | 142 | 174 | - | - | - | [237] |
| 2013 | BA, Hln-paba | 153 a, 166 a | 227, 238 | - | 339, 430 | 408, 506 | [293] |
| 2013 | BA-(R,S), (rac)mba | ~183 b, ~178 b | 223–238 | 114, 107 | 297, 298 | 329, 331 | [159] |
| 2013 | PH-DEP-a | ~ 190 | 210 | 83 d | - | - | [17] |
| 2013 | PH, oC, mC, pC, DMPH, TMPH-bapf | 253 c–279 c | 266–301 | 98–226 | 322–401 | 353–432 | [157] |
| 2014 | 4,4′, 2,4′, 2,2′-BF-a | 213–255 | 250–260 | 199–297 | 264–353 | 341–403 | [152] |
| 2014 | PH, pC-amp | ~200, 150 | 240, 208 | - | 306, 398 | 350, 475 | [376] |
| 2014 | V-a | ~200 c | 231 | 143 | - | - | [97] |
| pJeff-V-a | ~240 c | 256 | - | - | - | ||
| 2014 | PH-pna (26.6–75.2%) | 97–171 | 202–222 | - | 375–427 | 407–474 | [148] |
| 2014 | R-a | ~150 c, ~200 | 179, 229 | - | 267 | - | [402] |
| 2014 | pCbHPCBA-ddm | ~190 c | ~220 | - | 407 | 486 | [242] |
| 2014 | oAPH, TCP-oAPH-a | ~100 c, 194 c | 149–222 | - | - | - | [241] |
| oAPH, TCP-oAPH-ddm | ~110 c, ~225 | 151–248 | - | - | - | ||
| HPCBA-a | ~150 c | 210 | - | - | - | ||
| oCbHPCBA-ddm | ~190 c | 223 | 511 | 567 | |||
| 2015c | BA-a | 129 c | 215 | 213 | - | - | [401] |
| IB-a | 137 c | 214 | 143 | - | - | ||
| SB-a | 231 c | 259 | 134 | - | - | ||
| 2015 | C-a, -ddm/- trisapm/- tapm | 140–225 c | 190–265 | - | 355–391 | - | [87] |
| 2015 | oAPH-hda, dds | 185, 223 | 238, 281 | - | 242, 370 | - | [206] |
| 2015 | EDA-pHBAD-a/-oma, -ap | 205 c–236 | 229–252 | - | 128–395 | 287–420 | [388] |
| 2015 | BHPPA, BHPICA, BHPPIO-a | ~160 c–~240 c | 209–256 | 92–248 | 507–536 | 559–589 | [197] |
| 2015 | o-HPNI-a, ddm | ~210 c, ~210 c | 231, 245 | 155, 173 | ND, 463 | ND, 484 | [196] |
| 2016 | PH, MU, U-a | 215–255 | 220–261 | 288–320 | 343–361 | [210] | |
| 2016 | HPAMPH-a | 236 | 251 | 359 | 350 | 373 | [147] |
| HPAMPH-NH2-PAMPH | 224 | 242 | 311 | 353 | 377 | ||
| HPAMPH-NH2-H(PAM)2PH | 215 | 237 | 285 | 343 | 366 | ||
| 2016 | MU-a a | ~200 c, | 229 | 294 | - | - | [211] |
| MU-BA-NH2, MU-PGU-NH2 | ~220 c | 251, 241 | 90, 146 | - | - | ||
| 2016 | A-fa | 150 c | 207 | 220 | - | - | [110] |
| 2016 | PH, R, PGU-a | 172 a–234 a | ~214–237 | ~241–281 | [144] | ||
| 2016 | C-ATPEG-200–1500 | 152–172 | 220–242 | 41–94 | - | - | [238] |
| 2016 | C-BAEPA, AEPA | 114 c, 130 | 215, 238 | 107, 35 | 298, 380 | - | [116] |
| 2016 | 1-NP-pcna, apacn | ~160 c, ~130 c | 172–206 | 565, 603 | 332, 302 | 359, 323 | [182] |
| 2-NP-pcna, ocna | ~180 c, ~140 c | 174–215 | 537, 623 | 311, 309 | 330, 327 | ||
| 2016 | 2-NP-3-apd | 171 a | 183 | 167 | - | - | [333] |
| 2016 | pNiPH-pNia, mNia, paap | ~190 c, ~210 c | 249–286 | - | - | - | [386] |
| pHBA, phap-pNia | ~150 c, ~200 c | 344, 237 | - | - | - | ||
| 2017 | C-mepu | 103 | 202 | - | 322 | 369 | [225] |
| 2017 | BHPe-a | ~245 c | 265 | 23 | 408 | - | [213] |
| 2017 | BHPr-a | ~190 c | 220 | 257 | 431 | - | [212] |
| 2017 | oTFHPA-ddm | ~170 c | 207 | - | 471 | 512 | [341] |
| 2018 | pHBN-oda | 156 | 227 | 184 | [170] | ||
| 2018 | BAPh | - | - | - | [171] | ||
| BA, N, N-DBA, pC-a-pC/BAPh | 241–304 | 265–>350 | 31–103 | 413–479 | 451–521 | ||
| BA-appn | ~175 c | 220, 260 | - | 456 | 511 | ||
| 2018 | C-pha, -paba, -a | ~140 c–~200 c | 186–255 | - | - | 292–302 | [436] |
| 2018 | obHPIITO-a, -oda | ~150 c, ~250 c | 234, 314 | 123, 58 | - | 400, 460 | [244] |
| 2018 | pHBA-a | 201 | 216 | - | 395 | 436 | [353] |
| 2018 | HCHAL-a | ~120 c | 206 | - | 335 | 375 | [214] |
| 2018 | BAM-3–9 | 217 c–230 c | 225–243 | 234–266 | - | - | [437] |
| 2018 | PH-a | 202 | 238 | 262 | 331 | - | [273] |
| PH-dmapa, cpl-dmapa | 170, 160 | 205, 195 | 13, 36 | 323, 310 | - | ||
| BA-a, dmapa, cpl-dmapa | 148–196 | 190–247 | 32–195 | 260–339 | - | ||
| 4,4′-BF-a + 2,4′-BF-a | 152 | 225 | 189 | 391 | - | ||
| 4,4′-BF-dmapa + 2,4′-BF-dmapa | 135 | 219 | 169 | 249 | - | ||
| 4,4′-BF-cpl-dmapa + 2,4′-BF-cpl-dmapa | 122 | 196 | 37 | 193 | - | ||
| 4,4′-BF-a | 150 | 213 | 147 | 331 | |||
| 4,4′-BF-dmapa | 156 | 197 | 68 | 262 | - | ||
| 4,4′-BF-cpl-dmapa | 152 | 186 | 44 | 278 | - | ||
| 2018 | SBA-a | 170 c | 231 | 180 | ~60 | [192] | |
| 2018 | oHPNI-a | ~230 c | 245 | 173 | 371 | 431 | [245] |
| Poly(oHPNI-a)main chain | ~225 c | 241 | 120 | 319 | 366 | ||
| 2018 | PG-fa, a | 152, 166 | 177, 189 | - | - | - | [408] |
| 2019 | β-NP, G, C-ima | 238–246 | 253–266 | 81–183 | - | 318–358 | [89] |
| 2019 | M-fa | - | 229 | - | 440 | 463 | [105] |
| 2019 | RES-fa | 193 | 229 | 324 | 346 | 403 | [106] |
| 2019 | HPM-fa | - | 214 | - | 350 | 403 | [215] |
| Poly(HPM-fa)main chain | - | 217 | - | 336 | 370 |


References
- Holly, F.W.; Cope, A.C. Condensation products of aldehydes and ketones with o-aminobenzyl alcohol and o-hydroxybenzylamine. J. Am. Chem. Soc. 1944, 66, 1875–1879. [Google Scholar] [CrossRef]
- Burke, W. 3, 4-Dihydro-1, 3, 2H-Benzoxazines. Reaction of p-substituted phenols with N, N-dimethylolamines. J. Am. Chem. Soc. 1949, 71, 609–612. [Google Scholar] [CrossRef]
- Burke, W.; Weatherbee, C. 3, 4-Dihydro-1, 3, 2H-Benzoxazines. Reaction of Polyhydroxybenzenes with N-Methylolamines1. J. Am. Chem. Soc. 1950, 72, 4691–4694. [Google Scholar] [CrossRef]
- Schreiber, H. German Offen. p. 2,255,504, 1973. [Google Scholar]
- Schreiber, H. German Offen. p. 2,323,936, 1973. [Google Scholar]
- Higginbottom, H.P. Polymerizable Compositions Comprising Polyamines and Poly (Dihydrobenzoxazines). U.S. Patent 4,501,864, 26 February 1985. [Google Scholar]
- Ning, X.; Ishida, H. Phenolic materials via ring-opening polymerization: Synthesis and characterization of bisphenol-A based benzoxazines and their polymers. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1121–1129. [Google Scholar] [CrossRef]
- Ishida, H.; Froimowicz, P. Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ishida, H.; Low, H.Y. Synthesis of benzoxazine functional silane and adhesion properties of glass-fiber-reinforced polybenzoxazine composites. J. Appl. Polym. Sci. 1998, 69, 2559–2567. [Google Scholar] [CrossRef]
- Sarkar, S.; Adhikari, B. Lignin-modified phenolic resin: Synthesis optimization, adhesive strength, and thermal stability. J. Adhes. Sci. Technol. 2000, 14, 1179–1193. [Google Scholar] [CrossRef]
- Lochab, B.; Varma, I.K.; Bijwe, J. Blends of benzoxazine monomers. J. Therm. Anal. Calorim. 2013, 111, 1357–1364. [Google Scholar] [CrossRef]
- Li, H.; Gu, J.; Wang, D.; Qu, C.; Zhang, Y. Study on benzoxazine-based film adhesive and its adhesion properties with CFPR composites. J. Adhes. Sci. Technol. 2017, 31, 1796–1806. [Google Scholar] [CrossRef]
- Monisha, M.; Shukla, S.; Lochab, B. Nanoparticles as curing and adhesive aid for biobased and petrobased polybenzoxazines. Green Mater. 2017, 5, 94–102. [Google Scholar] [CrossRef]
- Xie, P.; Yao, Y.; Huang, Z.; Liu, Z.; Zhang, J.; Li, T.; Wang, G.; Shahbazian-Yassar, R.; Hu, L.; Wang, C. Highly efficient decomposition of ammonia using high-entropy alloy catalysts. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Liu, S.-Z.; Tian, D.-T.; Qiu, J.-J.; Liu, C.-M. Well-defined organic–inorganic hybrid benzoxazine monomers based on cyclotriphosphazene: Synthesis, properties of the monomers and polybenzoxazines. Polymer 2011, 52, 4235–4245. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Liu, S.-Z.; Guo, Y.-N.; Qiu, J.-J.; Liu, C.-M. Highly branched benzoxazine monomer based on cyclotriphosphazene: Synthesis and properties of the monomer and polybenzoxazines. Polymer 2011, 52, 1004–1012. [Google Scholar] [CrossRef]
- Lin, L.K.; Wu, C.S.; Su, W.C.; Liu, Y.L. Diethylphosphonate-containing benzoxazine compound as a thermally latent catalyst and a reactive property modifier for polybenzoxazine-based resins. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3523–3530. [Google Scholar] [CrossRef]
- Amarnath, N.; Appavoo, D.; Lochab, B. Eco-friendly halogen-free flame retardant cardanol polyphosphazene polybenzoxazine networks. ACS Sustain. Chem. Eng. 2017, 6, 389–402. [Google Scholar] [CrossRef]
- Shukla, S.; Ghosh, A.; Sen, U.K.; Roy, P.K.; Mitra, S.; Lochab, B. Cardanol benzoxazine-Sulfur Copolymers for Li-S batteries: Symbiosis of Sustainability and Performance. ChemistrySelect 2016, 1, 594–600. [Google Scholar] [CrossRef]
- Ghosh, A.; Shukla, S.; Khosla, G.S.; Lochab, B.; Mitra, S. Sustainable sulfur-rich copolymer/graphene composite as lithium-sulfur battery cathode with excellent electrochemical performance. Sci. Rep. 2016, 6, 25207. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Ghosh, A.; Roy, P.K.; Mitra, S.; Lochab, B. Cardanol benzoxazines–A sustainable linker for elemental sulphur based copolymers via inverse vulcanisation. Polymer 2016, 99, 349–357. [Google Scholar] [CrossRef]
- Je, S.H.; Hwang, T.H.; Talapaneni, S.N.; Buyukcakir, O.; Kim, H.J.; Yu, J.-S.; Woo, S.-G.; Jang, M.C.; Son, B.K.; Coskun, A. Rational sulfur cathode design for lithium–sulfur batteries: Sulfur-embedded benzoxazine polymers. ACS Energy Lett. 2016, 1, 566–572. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Li, Z.; Sheng, W.; Zhang, Y.; Gu, J.; Zheng, X.; Cao, F. Improved electrochemical performance of LiFePO4@ N-doped carbon nanocomposites using polybenzoxazine as nitrogen and carbon sources. ACS Appl. Mater. Interfaces 2016, 8, 26908–26915. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Shukla, S.; Monisha, M.; Kumar, A.; Lochab, B.; Mitra, S. Sulfur copolymer: A new cathode structure for room-temperature sodium–sulfur batteries. ACS Energy Lett. 2017, 2, 2478–2485. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, X.; Xin, Z.; Liu, J.; Zhang, Y. Hydrophobic benzoxazine-cured epoxy coatings for corrosion protection. Prog. Org. Coat. 2013, 76, 1178–1183. [Google Scholar] [CrossRef]
- Yildirim, C.; Erciyes, A.T.; Yagci, Y. Thermally curable benzoxazine-modified vegetable oil as a coating material. J. Coat. Technol. Res. 2013, 10, 559–569. [Google Scholar] [CrossRef]
- Caldona, E.B.; De Leon, A.C.C.; Thomas, P.G.; Naylor III, D.F.; Pajarito, B.B.; Advincula, R.C. Superhydrophobic rubber-modified polybenzoxazine/SiO2 nanocomposite coating with anticorrosion, anti-ice, and superoleophilicity properties. Ind. Eng. Chem. Res. 2017, 56, 1485–1497. [Google Scholar] [CrossRef]
- Renaud, A.; Bonnaud, L.; Dumas, L.; Zhang, T.; Fasano, F.; Kulyk, O.; Pospisilova, E.; Nysten, B.; Delcorte, A.; Bonifazi, D. A benzoxazine/substituted borazine composite coating: A new resin for improving the corrosion resistance of the pristine benzoxazine coating applied on aluminum. Eur. Polym. J. 2018, 109, 460–472. [Google Scholar] [CrossRef] [Green Version]
- Iguchi, D.; Ohashi, S.; Abarro, G.J.; Yin, X.; Winroth, S.; Scott, C.; Gleydura, M.; Jin, L.; Kanagasegar, N.; Lo, C. Development of Hydrogen-Rich Benzoxazine Resins with Low Polymerization Temperature for Space Radiation Shielding. ACS Omega 2018, 3, 11569–11581. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.-P.; Li, W.-C.; Qian, D.; Wang, G.-H.; Zhang, W.-P.; Zhang, T.; Wang, A.-Q.; Schüth, F.; Bongard, H.-J.; Lu, A.-H. Structurally designed synthesis of mechanically stable poly (benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. J. Am. Chem. Soc. 2011, 133, 11378–11388. [Google Scholar] [CrossRef] [PubMed]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon aerogels with excellent CO2 adsorption capacity synthesized from clay-reinforced biobased chitosan-polybenzoxazine nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Mohamed, M.G.; Kuo, S.-W. Directly synthesized nitrogen-doped microporous carbons from polybenzoxazine resins for carbon dioxide capture. Polym. Chem. 2017, 8, 5481–5489. [Google Scholar] [CrossRef]
- Manmuanpom, N.; Thubsuang, U.; Dubas, S.T.; Wongkasemjit, S.; Chaisuwan, T. Enhanced CO2 capturing over ultra-microporous carbon with nitrogen-active species prepared using one-step carbonization of polybenzoxazine for a sustainable environment. J. Environ. Manag. 2018, 223, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Konnola, R.; Anirudhan, T.S. Efficient carbon dioxide capture by nitrogen and sulfur dual-doped mesoporous carbon spheres from polybenzoxazines synthesized by a simple strategy. J. Environ. Chem. Eng. 2020, 8, 103614. [Google Scholar] [CrossRef]
- Chaisuwan, T.; Komalwanich, T.; Luangsukrerk, S.; Wongkasemjit, S. Removal of heavy metals from model wastewater by using polybenzoxazine aerogel. Desalination 2010, 256, 108–114. [Google Scholar] [CrossRef]
- Taskin, O.S.; Kiskan, B.; Aksu, A.; Balkis, N.; Weber, J.; Yagci, Y. Polybenzoxazine: A powerful tool for removal of mercury salts from water. Chem. A Eur. J. 2014, 20, 10953–10958. [Google Scholar] [CrossRef]
- Iguchi, D.; Salum, M.L.; Froimowicz, P. Application of Benzoxazine-Based Dimers, Oligomers, and Polymers as Chelating Agents. Macromol. Chem. Phys. 2019, 220, 1800366. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Chen, H.; Li, H. Benzoxazine monomers containing triphenylimidazole: Polymerization of monomers and properties of polybenzoxazines. Eur. Polym. J. 2019, 121, 109347. [Google Scholar] [CrossRef]
- Weigand, J.J.; Miller, C.I.; Janisse, A.P.; McNair, O.D.; Kim, K.; Wiggins, J.S. 3D printing of dual-cure benzoxazine networks. Polymer 2020, 189, 122193. [Google Scholar] [CrossRef]
- Rimdusit, S.; Ishida, H. Development of new class of electronic packaging materials based on ternary systems of benzoxazine, epoxy, and phenolic resins. Polymer 2000, 41, 7941–7949. [Google Scholar] [CrossRef]
- Chen, S.; Ren, D.; Li, B.; Li, K.; Chen, L.; Xu, M.; Liu, X. Benzoxazine Containing Fluorinated Aromatic Ether Nitrile Linkage: Preparation, Curing Kinetics and Dielectric Properties. Polymers 2019, 11, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, H.; Allen, D.J. Physical and mechanical characterization of near-zero shrinkage polybenzoxazines. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1019–1030. [Google Scholar] [CrossRef]
- Ran, Q.c.; Tian, Q.; Li, C.; Gu, Y. Investigation of processing, thermal, and mechanical properties of a new composite matrix-benzoxazine containing aldehyde group. Polym. Adv. Technol. 2010, 21, 170–176. [Google Scholar] [CrossRef]
- Hamerton, I.; Howlin, B.J.; Mitchell, A.L.; McNamara, L.T.; Takeda, S. Systematic examination of thermal, mechanical and dielectrical properties of aromatic polybenzoxazines. React. Funct. Polym. 2012, 72, 736–744. [Google Scholar] [CrossRef]
- Grishchuk, S.; Sorochynska, L.; Vorster, O.C.; Karger-Kocsis, J. Structure, thermal, and mechanical properties of DDM-hardened epoxy/benzoxazine hybrids: Effects of epoxy resin functionality and ETBN toughening. J. Appl. Polym. Sci. 2013, 127, 5082–5093. [Google Scholar] [CrossRef]
- Hassan, W.A.W.; Hamerton, I.; Howlin, B.J. Prediction of Selected Physical and Mechanical Properties of a Telechelic Polybenzoxazine by Molecular Simulation. PLoS ONE 2013, 8, e61179. [Google Scholar] [CrossRef] [Green Version]
- Rimdusit, S.; Punuch, W.; Jubsilp, C. Effects of Mono-And Dianhydrides on Thermal and Mechanical Properties Enhancement of Polybenzoxazine: A Property Comparison. Proc. Appl. Mech. Mater. 2014, 576, 63–67. [Google Scholar] [CrossRef]
- Xu, M.; Lei, Y.; Ren, D.; Chen, L.; Li, K.; Liu, X. Thermal stability of allyl-functional phthalonitriles-containing benzoxazine/bismaleimide copolymers and their improved mechanical properties. Polymers 2018, 10, 596. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.B.; Ishida, H. Development and characterization of high-performance polybenzoxazine composites. Polym. Compos. 1996, 17, 710–719. [Google Scholar] [CrossRef]
- Brunovska, Z.; Ishida, H. Thermal study on the copolymers of phthalonitrile and phenylnitrile-functional benzoxazines. J. Appl. Polym. Sci. 1999, 73, 2937–2949. [Google Scholar] [CrossRef]
- Kim, H.J.; Brunovska, Z.; Ishida, H. Dynamic mechanical analysis on highly thermally stable polybenzoxazines with an acetylene functional group. J. Appl. Polym. Sci. 1999, 73, 857–862. [Google Scholar] [CrossRef]
- Low, H.Y.; Ishida, H. Improved thermal stability of polybenzoxazines by transition metals. Polym. Degrad. Stab. 2006, 91, 805–815. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Synthesis and characterization of novel benzoxazine monomers containing allyl groups and their high performance thermosets. Macromolecules 2003, 36, 6010–6017. [Google Scholar] [CrossRef]
- Lorjai, P.; Wongkasemjit, S.; Chaisuwan, T.; Jamieson, A.M. Significant enhancement of thermal stability in the non-oxidative thermal degradation of bisphenol-A/aniline based polybenzoxazine aerogel. Polym. Degrad. Stab. 2011, 96, 708–718. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Du, W.; Huang, F.; Du, L. Thermal stability of the copolymers of silicon-containing arylacetylene resin and acetylene-functional benzoxazine. Polym. Degrad. Stab. 2011, 96, 2276–2283. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, J.; Li, S.; Shang, Z.; Wang, J. Remarkable improvement of thermal stability of main-chain benzoxazine oligomer by incorporating o-norbornene as terminal functionality. J. Appl. Polym. Sci. 2017, 134, 45408. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, R.; Yu, X.; Zhang, K. Modification of Solventless-Synthesized Benzoxazine Resin by Phthalonitrile Group: An Effective Approach for Enhancing Thermal Stability of Polybenzoxazines. Macromol. Chem. Phys. 2019, 220, 1800291. [Google Scholar] [CrossRef]
- Espinosa, M.; Galia, M.; Cadiz, V. Novel phosphorilated flame retardant thermosets: Epoxy–benzoxazine–novolac systems. Polymer 2004, 45, 6103–6109. [Google Scholar] [CrossRef]
- Lin, C.H.; Cai, S.X.; Leu, T.S.; Hwang, T.Y.; Lee, H.H. Synthesis and properties of flame-retardant benzoxazines by three approaches. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3454–3468. [Google Scholar] [CrossRef]
- Sponton, M.; Lligadas, G.; Ronda, J.; Galia, M.; Cadiz, V. Development of a DOPO-containing benzoxazine and its high-performance flame retardant copolybenzoxazines. Polym. Degrad. Stab. 2009, 94, 1693–1699. [Google Scholar] [CrossRef]
- Kim, H.D.; Ishida, H. Study on the chemical stability of benzoxazine-based phenolic resins in carboxylic acids. J. Appl. Polym. Sci. 2001, 79, 1207–1219. [Google Scholar] [CrossRef]
- Ramdani, N.; Derradji, M.; Wang, J.; Mokhnache, E.-O.; Liu, W.-B. Improvements of Thermal, Mechanical, and Water-Resistance Properties of Polybenzoxazine/Boron Carbide Nanocomposites. JOM 2016, 68, 2533–2542. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, F.; Bai, W.; Chen, J.; Xu, Y.; Lin, J. Preparation of porous urushiol-based polybenzoxazine films with chemical resistance by breath figures method. Polym. Bull. 2019, 76, 6459–6466. [Google Scholar] [CrossRef]
- Ishida, H.; Low, H.Y. A study on the volumetric expansion of benzoxazine-based phenolic resin. Macromolecules 1997, 30, 1099–1106. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y. Study on the volumetric change during ring-opening polymerization of benzoxazines. Acta Polym. Sin. 2000, 5, 612–619. [Google Scholar]
- Velez-Herrera, P.; Doyama, K.; Abe, H.; Ishida, H. Synthesis and characterization of highly fluorinated polymer with the benzoxazine moiety in the main chain. Macromolecules 2008, 41, 9704–9714. [Google Scholar] [CrossRef]
- Liu, H.-C.; Su, W.-C.; Liu, Y.-L. Self-assembled benzoxazine-bridged polysilsesquioxanes exhibiting ultralow-dielectric constants and yellow-light photoluminescent emission. J. Mater. Chem. 2011, 21, 7182–7187. [Google Scholar] [CrossRef]
- Vengatesan, M.; Devaraju, S.; Dinakaran, K.; Alagar, M. SBA-15 filled polybenzoxazine nanocomposites for low-k dielectric applications. J. Mater. Chem. 2012, 22, 7559–7566. [Google Scholar] [CrossRef]
- Alagar, M. Dielectric and thermal behaviors of POSS reinforced polyurethane based polybenzoxazine nanocomposites. RSC Adv. 2015, 5, 33008–33015. [Google Scholar]
- Wang, C.F.; Su, Y.C.; Kuo, S.W.; Huang, C.F.; Sheen, Y.C.; Chang, F.C. Low-surface-free-energy materials based on polybenzoxazines. Angew. Chem. Int. Ed. 2006, 45, 2248–2251. [Google Scholar] [CrossRef]
- Qu, L.; Xin, Z. Preparation and surface properties of novel low surface free energy fluorinated silane-functional polybenzoxazine films. Langmuir 2011, 27, 8365–8370. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Shen, T.Y.; Shih, Y.S.; Lin, H.T.; Wang, C.F. Flexible polybenzoxazine thermosets with high glass transition temperatures and low surface free energies. Polym. Chem. 2012, 3, 935–945. [Google Scholar] [CrossRef]
- Selvi, M.; Vengatesan, M.; Devaraju, S.; Kumar, M.; Alagar, M. In situ sol–gel synthesis of silica reinforced polybenzoxazine hybrid materials with low surface free energy. RSC Adv. 2014, 4, 8446–8452. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Recycling and self-healing of polybenzoxazines with dynamic sulfide linkages. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Feng, S.; Lu, Z. Reprocessable and degradable thermoset with high T g cross-linked via Si–O–Ph bonds. J. Mater. Chem. A 2019, 7, 17498–17504. [Google Scholar] [CrossRef]
- He, Y.; Gao, S.; Jubsilp, C.; Rimdusit, S.; Lu, Z. Reprocessable polybenzoxazine thermosets crosslinked by mussel-inspired catechol-Fe3+ coordination bonds. Polymer 2020, 122307. [Google Scholar] [CrossRef]
- Lyu, Y.; Ishida, H. Natural-sourced benzoxazine resins, homopolymers, blends and composites: A review of their synthesis, manufacturing and applications. Prog. Polym. Sci. 2019, 99, 101168. [Google Scholar] [CrossRef]
- Machado, I.S.; Hurdle, K.; Calado, V.; Ishida, H. Towards the Development of Green Flame Retardancy by Polybenzoxazines. Prog. Polym. Sci. 2021, in press. [Google Scholar]
- Ishida, H. Process for Preparation of Benzoxazine Compounds in Solventless Systems. U.S. Patent 5,543,516, 6 August 1996. [Google Scholar]
- Laobuthee, A.; Chirachanchai, S.; Ishida, H.; Tashiro, K. Asymmetric mono-oxazine: An inevitable product from Mannich reaction of benzoxazine dimers. J. Am. Chem. Soc. 2001, 123, 9947–9955. [Google Scholar] [CrossRef] [PubMed]
- Sponton, M.; Larrechi, M.; Ronda, J.; Galia, M.; Cádiz, V. Synthesis and study of the thermal crosslinking of bis (m-aminophenyl) methylphosphine oxide based benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7162–7172. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y. Effects of molecular structure parameters on ring-opening reaction of benzoxazines. Sci. China. Ser. B 2001, 44, 552–560. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Yang, P. Effect of methyl substituent on the curing of bisphenol-arylamine-based benzoxazines. Thermochim. Acta 2018, 662, 55–63. [Google Scholar]
- Hao, B.; Han, L.; Liu, Y.; Zhang, K. An apigenin-based bio-benzoxazine with three polymerizable functionalities: Sustainable synthesis, thermal latent polymerization, and excellent thermal properties of its thermosets. Polym. Chem. 2020, 11, 5800–5809. [Google Scholar] [CrossRef]
- Calò, E.; Maffezzoli, A.; Mele, G.; Martina, F.; Mazzetto, S.E.; Tarzia, A.; Stifani, C. Synthesis of a novel cardanol-based benzoxazine monomer and environmentally sustainable production of polymers and bio-composites. Green Chem. 2007, 9, 754–759. [Google Scholar]
- Lochab, B.; Varma, I.; Bijwe, J. Thermal behaviour of cardanol-based benzoxazines: Monomers and polymers. J. Therm. Anal. Calorim. 2010, 102, 769–774. [Google Scholar] [CrossRef]
- Shukla, S.; Mahata, A.; Pathak, B.; Lochab, B. Cardanol benzoxazines–interplay of oxazine functionality (mono to tetra) and properties. RSC Adv. 2015, 5, 78071–78080. [Google Scholar] [CrossRef]
- Puchot, L.; Verge, P.; Fouquet, T.; Vancaeyzeele, C.; Vidal, F.; Habibi, Y. Breaking the symmetry of dibenzoxazines: A paradigm to tailor the design of bio-based thermosets. Green Chem. 2016, 18, 3346–3353. [Google Scholar] [CrossRef]
- Amarnath, N.; Shukla, S.; Lochab, B. Isomannide derived chiral rigid fully bio-based polybenzoxazines. ACS Sustain. Chem. Eng. 2019, 7, 18700–18710. [Google Scholar] [CrossRef]
- Monisha, M.; Amarnath, N.; Mukherjee, S.; Lochab, B. Cardanol Benzoxazines: A Versatile Monomer with Advancing Applications. Macromol. Chem. Phys. 2019, 220, 1800470. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Liu, X.; Sudo, A.; Endo, T. Synthesis and copolymerization of fully bio-based benzoxazines from guaiacol, furfurylamine and stearylamine. Green Chem. 2012, 14, 2799–2806. [Google Scholar] [CrossRef]
- Phalak, G.A.; Patil, D.M.; Mhaske, S. Synthesis and characterization of thermally curable guaiacol based poly (benzoxazine-urethane) coating for corrosion protection on mild steel. Eur. Polym. J. 2017, 88, 93–108. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Sarojadevi, M. Synthesis and copolymerization of fully biobased benzoxazines from renewable resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Dumas, L.; Bonnaud, L.; Olivier, M.; Poorteman, M.; Dubois, P. Eugenol-based benzoxazine: From straight synthesis to taming of the network properties. J. Mater. Chem. A 2015, 3, 6012–6018. [Google Scholar] [CrossRef]
- Amarnath, N.; Shukla, S.; Lochab, B. Harvesting the benefits of inherent reactive functionalities in fully biosourced isomeric benzoxazines. ACS Sustain. Chem. Eng. 2018, 6, 15151–15161. [Google Scholar] [CrossRef]
- Sini, N.; Bijwe, J.; Varma, I.K. Renewable benzoxazine monomer from Vanillin: Synthesis, characterization, and studies on curing behavior. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 7–11. [Google Scholar] [CrossRef]
- Van, A.; Chiou, K.; Ishida, H. Use of renewable resource vanillin for the preparation of benzoxazine resin and reactive monomeric surfactant containing oxazine ring. Polymer 2014, 55, 1443–1451. [Google Scholar] [CrossRef]
- Sha, X.-L.; Yuan, L.; Liang, G.; Gu, A. Preparation of high performance bio-based benzoxazine resin through a green solvent-free strategy for shape memory application. Polymer 2020, 202, 122673. [Google Scholar] [CrossRef]
- Froimowicz, P.; Arza, C.R.; Han, L.; Ishida, H. Smart, Sustainable, and Ecofriendly Chemical Design of Fully Bio-Based Thermally Stable Thermosets Based on Benzoxazine Chemistry. ChemSusChem 2016, 9, 1921–1928. [Google Scholar] [CrossRef]
- Kotzebue, L.R.; de Oliveira, J.s.R.; da Silva, J.B.; Mazzetto, S.E.; Ishida, H.; Lomonaco, D. Development of fully biobased high-performance bis-benzoxazine under environmentally friendly conditions. ACS Sustain. Chem. Eng. 2018, 6, 5485–5494. [Google Scholar] [CrossRef]
- Comí, M.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable benzoxazine monomers from “lignin-like” naturally occurring phenolic derivatives. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4894–4903. [Google Scholar] [CrossRef]
- Rodríguez, R.B.; Iguchi, D.; Erra-Balsells, R.; Salum, M.L.; Froimowicz, P. Design and Effects of the Cinnamic Acids Chemical Structures as Organocatalyst on the Polymerization of Benzoxazines. Polymers 2020, 12, 1527. [Google Scholar] [CrossRef]
- Kirubakaran, R.; Sharma, P.; Manisekaran, A.; Bijwe, J.; Nebhani, L. Phloretic acid: A smart choice to develop low-temperature polymerizable bio-based benzoxazine thermosets. J. Therm. Anal. Calorim. 2020, 142, 1233–1242. [Google Scholar] [CrossRef]
- Trejo-Machin, A.; Verge, P.; Puchot, L.; Quintana, R. Phloretic acid as an alternative to the phenolation of aliphatic hydroxyls for the elaboration of polybenzoxazine. Green Chem. 2017, 19, 5065–5073. [Google Scholar] [CrossRef]
- Teng, N.; Yang, S.; Dai, J.; Wang, S.; Zhao, J.; Zhu, J.; Liu, X. Making Benzoxazine Greener and Stronger: Renewable Resource, Microwave Irradiation, Green Solvent and Excellent Thermal Properties. ACS Sustain. Chem. Eng. 2019, 7, 8715–8723. [Google Scholar] [CrossRef]
- Zhang, K.; Han, M.; Liu, Y.; Froimowicz, P. Design and Synthesis of Bio-Based High-Performance Trioxazine Benzoxazine Resin via Natural Renewable Resources. ACS Sustain. Chem. Eng. 2019, 7, 9399–9407. [Google Scholar] [CrossRef]
- İnan, T.Y.; Karaca, B.Y.; Dogan, H. Synthesis and characterizations of polybenzoxazines from coal-based products via microwave and conventional heat treatments. J. Appl. Polym. Sci. 2013, 128, 2046–2055. [Google Scholar] [CrossRef]
- Dai, J.; Teng, N.; Peng, Y.; Liu, Y.; Cao, L.; Zhu, J.; Liu, X. Biobased Benzoxazine Derived from Daidzein and Furfurylamine: Microwave-Assisted Synthesis and Thermal Properties Investigation. ChemSusChem 2018, 11, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, Y.; Han, M.; Froimowicz, P. Smart and Sustainable Design of Latent Catalyst-Containing Benzoxazine-Bio-Resins and Application Studies. Green Chem. 2020, 22, 1209–1219. [Google Scholar] [CrossRef]
- Dumas, L.; Bonnaud, L.; Olivier, M.; Poorteman, M.; Dubois, P. Arbutin-based benzoxazine: En route to an intrinsic water soluble biobased resin. Green Chem. 2016, 18, 4954–4960. [Google Scholar] [CrossRef]
- Zúñiga, C.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Self-foaming diphenolic acid benzoxazine. Polymer 2012, 53, 3089–3095. [Google Scholar] [CrossRef]
- Zúñiga, C.; Larrechi, M.; Lligadas, G.; Ronda, J.; Galià, M.; Cádiz, V. Polybenzoxazines from renewable diphenolic acid. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1219–1227. [Google Scholar] [CrossRef]
- Salum, M.a.L.; Iguchi, D.; Arza, C.R.; Han, L.; Ishida, H.; Froimowicz, P. Making benzoxazines greener: Design, synthesis, and polymerization of a biobased benzoxazine fulfilling two principles of green chemistry. ACS Sustain. Chem. Eng. 2018, 6, 13096–13106. [Google Scholar] [CrossRef]
- Higginson, C.J.; Malollari, K.G.; Xu, Y.; Kelleghan, A.V.; Ricapito, N.G.; Messersmith, P.B. Bioinspired Design Provides High-Strength Benzoxazine Structural Adhesives. Angew. Chem. Int. Ed. 2019, 58, 12271–12279. [Google Scholar] [CrossRef]
- Shen, X.; Dai, J.; Liu, Y.; Liu, X.; Zhu, J. Synthesis of high performance polybenzoxazine networks from bio-based furfurylamine: Furan vs benzene ring. Polymer 2017, 122, 258–269. [Google Scholar] [CrossRef]
- Sharma, P.; Lochab, B.; Kumar, D.; Roy, P.K. Sustainable bis-benzoxazines from cardanol and PET-derived terephthalamides. ACS Sustain. Chem. Eng. 2016, 4, 1085–1093. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Fadlallah, S.; Roy, P.S.; Garnier, G.; Saito, K.; Allais, F. Are lignin-derived monomers and polymers truly sustainable? An in-depth green metrics calculations approach. Green Chem. 2021, 23, 1495–1535. [Google Scholar] [CrossRef]
- Chirachanchai, S.; Phongtamrug, S.; Laobuthee, A.; Tashiro, K. Mono-Substituted Phenol-Based Benzoxazines: Inevitable Dimerization via Self-Termination and Its Metal Complexation. In Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 111–126. [Google Scholar]
- Ribeiro, F.W.M.; Rodrigues-Oliveira, A.F.; Correra, T.C. Benzoxazine Formation Mechanism Evaluation by Direct Observation of Reaction Intermediates. J. Phys. Chem. A 2019, 123, 8179–8187. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Salum, M.L.; Zhang, K.; Froimowicz, P.; Ishida, H. Intrinsic self-initiating thermal ring-opening polymerization of 1, 3-benzoxazines without the influence of impurities using very high purity crystals. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3434–3445. [Google Scholar] [CrossRef]
- Audebert, P.; Roche, M.; Pagetti, J. Electrochemical properties of some benzoxazines: Conditions for electropolymerization in alkaline medium. J. Electroanal. Chem. 1995, 383, 139–143. [Google Scholar] [CrossRef]
- Li, L.; He, J.; Wan, X.; Zhou, D.; Xue, G.; Wang, Y.; Choi, S.-W.; Ishida, H. Electrochemical synthesis and properties of polybenzoxazine. J. Adhes. 2003, 79, 351–360. [Google Scholar] [CrossRef]
- Chen, W.; He, J.; Li, L. Characterization of polybenzoxazine and its electrochemical polymerization mechanism. Front. Chem. China 2009, 4, 390. [Google Scholar] [CrossRef]
- Dunkers, J.; Zarate, E.; Ishida, H. Crystal structure and hydrogen-bonding characteristics of N, N-bis (3, 5-dimethyl-2-hydroxybenzyl) methylamine, a benzoxazine dimer. J. Phys. Chem. 1996, 100, 13514–13520. [Google Scholar] [CrossRef]
- Kim, H.J.; Brunovska, Z.; Ishida, H. Molecular characterization of the polymerization of acetylene-functional benzoxazine resins. Polymer 1999, 40, 1815–1822. [Google Scholar]
- Kim, H.J.; Brunovska, Z.; Ishida, H. Synthesis and thermal characterization of polybenzoxazines based on acetylene-functional monomers. Polymer 1999, 40, 6565–6573. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Novel benzoxazine monomers containing p-phenyl propargyl ether: Polymerization of monomers and properties of polybenzoxazines. Macromolecules 2001, 34, 7257–7263. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yu, J.M.; Chou, C.I. Preparation and properties of novel benzoxazine and polybenzoxazine with maleimide groups. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5954–5963. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chou, C.I. High performance benzoxazine monomers and polymers containing furan groups. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5267–5282. [Google Scholar] [CrossRef]
- Shukla, S.; Lochab, B. Role of higher aromatic content in modulating properties of cardanol based benzoxazines. Polymer 2016, 99, 684–694. [Google Scholar] [CrossRef]
- Chirachanchai, S.; Laobuthee, A.; Phongtamrug, S. Self termination of ring opening reaction of p-substituted phenol-based benzoxazines: An obstructive effect via intramolecular hydrogen bond. J. Heterocycl. Chem. 2009, 46, 714–721. [Google Scholar] [CrossRef]
- Zhen, H.; Yang, H.; Wang, M.; Lu, G.; Liu, Y.; Run, M. Cyclo-oligomerization of hydroxyl-containing mono-functional benzoxazines: A mechanism for oligomer formation. Polym. Chem. 2020, 11, 2325–2331. [Google Scholar] [CrossRef]
- Ning, X.; Ishida, H. Solid state dynamic analysis of phenolic compounds prepared by ring opening polymerization. Polym. Sci. Chem. Phys. 1994, 32, 921–927. [Google Scholar] [CrossRef]
- Ishida, H.; Rodriguez, Y. Curing kinetics of a new benzoxazine-based phenolic resin by differential scanning calorimetry. Polymer 1995, 36, 3151–3158. [Google Scholar] [CrossRef]
- Zeng, K.; Huang, J.; Ren, J.; Ran, Q. Curing reaction of benzoxazine under high pressure and the effect on thermal resistance of polybenzoxazine. Macromol. Chem. Phys. 2019, 220, 1800340. [Google Scholar] [CrossRef]
- Allen, D.J.; Ishida, H. Effect of phenol substitution on the network structure and properties of linear aliphatic diamine-based benzoxazines. Polymer 2009, 50, 613–626. [Google Scholar] [CrossRef]
- Andreu, R.; Reina, J.; Ronda, J. Studies on the thermal polymerization of substituted benzoxazine monomers: Electronic effects. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3353–3366. [Google Scholar] [CrossRef]
- Lyu, Y.; Rachita, E.; Pogharian, N.; Froimowicz, P.; Ishida, H. Electronic effects of asymmetric and meta-alkoxy substituents on the polymerization behavior of bis-benzoxazines. Polym. Chem. 2020, 11, 800–809. [Google Scholar] [CrossRef]
- Gârea, S.-A.; Iovu, H.; Nicolescu, A.; Deleanu, C. Thermal polymerization of benzoxazine monomers followed by GPC, FTIR and DETA. Polym. Test. 2007, 26, 162–171. [Google Scholar] [CrossRef]
- Ishida, H.; Sanders, D.P. Regioselectivity and network structure of difunctional alkyl-substituted aromatic amine-based polybenzoxazines. Macromolecules 2000, 33, 8149–8157. [Google Scholar] [CrossRef]
- Ishida, H.; Sanders, D. Regioselectivity of the ring-opening polymerization of monofunctional alkyl-substituted aromatic amine-based benzoxazines. Polymer 2001, 42, 3115–3125. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Lu, Y.; Gai, P.; Zhong, H. Synthesis of poly (amido amine)-derived dendrimers with pendant benzoxazine groups and their thermal behavior. J. Appl. Polym. Sci. 2012, 127, 282–288. [Google Scholar] [CrossRef]
- Soto, M.; Hiller, M.; Oschkinat, H.; Koschek, K. Multifunctional benzoxazines feature low polymerization temperature and diverse polymer structures. Polymers 2016, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Nalakathu Kolanadiyil, S.; Azechi, M.; Endo, T. Synthesis of novel tri-benzoxazine and effect of phenolic nucleophiles on its ring-opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2811–2819. [Google Scholar] [CrossRef]
- Schäfer, H.; Arnebold, A.; Stelten, J.; Marquet, J.; María Sebastián, R.; Hartwig, A.; Koschek, K. Bifunctional benzoxazines: Synthesis and polymerization of resorcinol based single isomers. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1243–1251. [Google Scholar] [CrossRef]
- Sini, N.; Endo, T. Toward elucidating the role of number of oxazine rings and intermediates in the benzoxazine backbone on their thermal characteristics. Macromolecules 2016, 49, 8466–8478. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, Z.; Ma, H.; Qiu, J.; Liu, C. Dendritic organic–inorganic hybrid polyphenol and branched benzoxazine monomers with low curing temperature. RSC Adv. 2014, 4, 53505–53513. [Google Scholar] [CrossRef]
- Lin, R.C.; Mohamed, M.G.; Kuo, S.W. Benzoxazine/Triphenylamine-Based Dendrimers Prepared through Facile One-Pot Mannich Condensations. Macromol. Rapid Commun. 2017, 38, 1700251. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-C.; Kuo, S.-W. Well-defined benzoxazine/triphenylamine-based hyperbranched polymers with controlled degree of branching. RSC Adv. 2018, 8, 13592–13611. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.-a.; Zegaoui, A.; Zhang, L.-l.; Dayo, A.Q.; Ghouti, H.A.; Wang, J.; Liu, W.-b.; Tang, T. One-pot synthesis, characterization and polymerization of hyperbranched benzoxazine resins derived from A2+ B3 monomers. Mater. Today Commun. 2019, 21, 100638. [Google Scholar] [CrossRef]
- Liu, J.; Ishida, H. Anomalous isomeric effect on the properties of bisphenol f-based benzoxazines: Toward the molecular design for higher performance. Macromolecules 2014, 47, 5682–5690. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chang, C.Y.; Hsu, C.Y.; Tseng, M.C.; Chou, C.I. Preparation, characterization, and properties of fluorene-containing benzoxazine and its corresponding cross-linked polymer. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4020–4026. [Google Scholar] [CrossRef]
- Lu, Y.; Li, M.; Zhang, Y.; Hu, D.; Ke, L.; Xu, W. Synthesis and curing kinetics of benzoxazine containing fluorene and furan groups. Thermochim. Acta 2011, 515, 32–37. [Google Scholar] [CrossRef]
- Lu, Y.; Li, M.; Ke, L.; Hu, D.; Xu, W. Curing kinetics of fluorene containing benzoxazine investigated by nonisothermal differential scanning calorimetry. J. Appl. Polym. Sci. 2011, 121, 2481–2487. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, H.; Cai, H.; Liu, X.; Ying, G.; Xu, K.; Chen, M. Synthesis, thermal polymerization, and properties of benzoxazine resins containing fluorenyl moiety. Polym. Eng. Sci. 2012, 52, 2473–2481. [Google Scholar] [CrossRef]
- Wang, J.; He, X.Y.; Liu, J.T.; Liu, W.B.; Yang, L. Investigation of the Polymerization Behavior and Regioselectivity of Fluorene Diamine-Based Benzoxazines. Macromol. Chem. Phys. 2013, 214, 617–628. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.-q.; Liu, W.-b.; Yang, S.-w.; Bai, J.-w.; Ding, Q.-q.; Li, Y. Synthesis, curing behavior and thermal properties of fluorene containing benzoxazines. Eur. Polym. J. 2010, 46, 1024–1031. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Liu, J.-t.; Liu, W.-b. Synthesis, curing kinetics and thermal properties of novel difunctional chiral and achiral benzoxazines with double chiral centers. J. Therm. Anal. Calorim. 2013, 114, 1255–1264. [Google Scholar] [CrossRef]
- Ejfler, J.; Krauzy-Dziedzic, K.; Szafert, S.; Lis, T.; Sobota, P. Novel chiral and achiral benzoxazine monomers and their thermal polymerization. Macromolecules 2009, 42, 4008–4015. [Google Scholar] [CrossRef]
- Ishida, H.; Sanders, D.P. Improved thermal and mechanical properties of polybenzoxazines based on alkyl-substituted aromatic amines. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 3289–3301. [Google Scholar] [CrossRef]
- Russell, V.M.; Koenig, J.L.; Low, H.Y.; Ishida, H. Study of the characterization and curing of benzoxazines using 13C solid-state nuclear magnetic resonance. J. Appl. Polym. Sci. 1998, 70, 1413–1425. [Google Scholar] [CrossRef]
- Low, H.Y.; Ishida, H. Structural effects of phenols on the thermal and thermo-oxidative degradation of polybenzoxazines. Polymer 1999, 40, 4365–4376. [Google Scholar] [CrossRef]
- Low, H.Y.; Ishida, H. Mechanistic study on the thermal decomposition of polybenzoxazines: Effects of aliphatic amines. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1935–1946. [Google Scholar] [CrossRef]
- Jubsilp, C.; Damrongsakkul, S.; Takeichi, T.; Rimdusit, S. Curing kinetics of arylamine-based polyfunctional benzoxazine resins by dynamic differential scanning calorimetry. Thermochim. Acta 2006, 447, 131–140. [Google Scholar] [CrossRef]
- Allen, D.J.; Ishida, H. Polymerization of linear aliphatic diamine-based benzoxazine resins under inert and oxidative environments. Polymer 2007, 48, 6763–6772. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Fu, R.; Chen, Y.; Liu, X. Contribution of blocking positions on the curing behaviors, networks and thermal properties of aromatic diamine-based benzoxazines. Thermochim. Acta 2018, 668, 65–72. [Google Scholar] [CrossRef]
- Jubsilp, C.; Takeichi, T.; Rimdusit, S. Property enhancement of polybenzoxazine modified with dianhydride. Polym. Degrad. Stab. 2011, 96, 1047–1053. [Google Scholar] [CrossRef]
- Fu, M.-C.; Uno, T.; Ueda, M.; Higashihara, T. Investigation of polycyanurate/benzoxazine curing system. Microsyst. Technol. 2018, 24, 597–604. [Google Scholar] [CrossRef]
- Singh, A.S.; Shukla, S.K.; Pandey, A.K.; Tripathi, D.; Prasad, N.E. Synthesis and evaluation of catalytic curing behavior of novel nitrile-functionalized benzoxazine for phthalonitrile resins. Polym. Bull. 2018, 75, 3781–3800. [Google Scholar] [CrossRef]
- Xu, M.; Ren, D.; Chen, L.; Li, K.; Liu, X. Understanding of the polymerization mechanism of the phthalonitrile-based resins containing benzoxazine and their thermal stability. Polymer 2018, 143, 28–39. [Google Scholar] [CrossRef]
- Yang, X.; Li, K.; Xu, M.; Jia, K.; Liu, X. Designing a low-temperature curable phenolic/benzoxazine-functionalized phthalonitrile copolymers for high performance composite laminates. J. Polym. Res. 2017, 24, 195. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Z.; Li, R.; Liu, Y.; Hu, J.; Zeng, K.; Yang, G. Curing kinetic of self-promoted alicyclic-based bisphthalonitrile monomer. Thermochim. Acta 2020, 683, 178446. [Google Scholar] [CrossRef]
- Kumar, K.S.; Nair, C.R.; Ninan, K. Investigations on the cure chemistry and polymer properties of benzoxazine–cyanate ester blends. Eur. Polym. J. 2009, 45, 494–502. [Google Scholar] [CrossRef]
- Kimura, H.; Ohtsuka, K.; Matsumoto, A. Curing reaction of bisphenol-A based benzoxazine with cyanate ester resin and the properties of the cured thermosetting resin. Express Polym. Lett. 2011, 5, 1113–1122. [Google Scholar] [CrossRef]
- Li, X.; Gu, Y. The co-curing process of a benzoxazine-cyanate system and the thermal properties of the copolymers. Polym. Chem. 2011, 2, 2778–2781. [Google Scholar] [CrossRef]
- Lin, C.H.; Huang, S.J.; Wang, P.J.; Lin, H.T.; Dai, S.A. Miscibility, microstructure, and thermal and dielectric properties of reactive blends of dicyanate ester and diamine-based benzoxazine. Macromolecules 2012, 45, 7461–7466. [Google Scholar] [CrossRef]
- Wang, M.W.; Jeng, R.J.; Lin, C.H. Origin of the Rapid Trimerization of Cyanate Ester in a Benzoxazine/Cyanate Ester Blend. Macromolecules 2015, 48, 2417–2421. [Google Scholar] [CrossRef]
- Hamerton, I. Properties of unreinforced cyanate ester matrix resins. In Chemistry and Technology of Cyanate Ester Resins; Springer: Berlin, Germany, 1994; pp. 193–229. [Google Scholar]
- Nair, C.R.; Mathew, D.; Ninan, K. Cyanate ester resins, recent developments. In New Polymerization Techniques and Synthetic Methodologies; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–99. [Google Scholar]
- Ohashi, S.; Kilbane, J.; Heyl, T.; Ishida, H. Synthesis and characterization of cyanate ester functional benzoxazine and its polymer. Macromolecules 2015, 48, 8412–8417. [Google Scholar] [CrossRef]
- Ohashi, S.; Pandey, V.; Arza, C.R.; Froimowicz, P.; Ishida, H. Simple and low energy consuming synthesis of cyanate ester functional naphthoxazines and their properties. Polym. Chem. 2016, 7, 2245–2252. [Google Scholar] [CrossRef]
- Agag, T. Preparation and properties of some thermosets derived from allyl-functional naphthoxazines. J. Appl. Polym. Sci. 2006, 100, 3769–3777. [Google Scholar] [CrossRef]
- Uyar, T.; Koyuncu, Z.; Ishida, H.; Hacaloglu, J. Polymerisation and degradation of an aromatic amine-based naphthoxazine. Polym. Degrad. Stab. 2008, 93, 2096–2103. [Google Scholar] [CrossRef]
- Ergin, M.; Kiskan, B.; Gacal, B.; Yagci, Y. Thermally curable polystyrene via click chemistry. Macromolecules 2007, 40, 4724–4727. [Google Scholar] [CrossRef]
- Kiskan, B.; Demiray, G.; Yagci, Y. Thermally curable polyvinylchloride via click chemistry. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3512–3518. [Google Scholar] [CrossRef]
- Nagai, A.; Kamei, Y.; Wang, X.S.; Omura, M.; Sudo, A.; Nishida, H.; Kawamoto, E.; Endo, T. Synthesis and crosslinking behavior of a novel linear polymer bearing 1, 2, 3-triazol and benzoxazine groups in the main chain by a step-growth click-coupling reaction. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2316–2325. [Google Scholar] [CrossRef]
- Andreu, R.; Espinosa, M.; Galia, M.; Cadiz, V.; Ronda, J.; Reina, J. Synthesis of novel benzoxazines containing glycidyl groups: A study of the crosslinking behavior. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1529–1540. [Google Scholar] [CrossRef]
- Kudoh, R.; Sudo, A.; Endo, T. A highly reactive benzoxazine monomer, 1-(2-hydroxyethyl)-1, 3-benzoxazine: Activation of benzoxazine by neighboring group participation of hydroxyl group. Macromolecules 2010, 43, 1185–1187. [Google Scholar] [CrossRef]
- Kiskan, B.; Koz, B.; Yagci, Y. Synthesis and characterization of fluid 1, 3-benzoxazine monomers and their thermally activated curing. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 6955–6961. [Google Scholar] [CrossRef]
- Yei, D.R.; Fu, H.K.; Chen, W.Y.; Chang, F.C. Synthesis of a novel benzoxazine monomer-intercalated montmorillonite and the curing kinetics of polybenzoxazine/clay hybrid nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, Q.-Q.; Tian, L.; Wang, L.-Y.; Xu, K.; Chen, Q.-X.; Ou, B.-L. Structural effects of highly π-conjugated mesogenic Schiff-base moiety on the cationic polymerization of benzoxazine and formation of ordered morphologies. React. Funct. Polym. 2018, 124, 139–148. [Google Scholar] [CrossRef]
- Agag, T.; Liu, J.; Graf, R.; Spiess, H.W.; Ishida, H. Benzoxazole resin: A novel class of thermoset polymer via smart benzoxazine resin. Macromolecules 2012, 45, 8991–8997. [Google Scholar] [CrossRef]
- Liu, J.; Cao, L.; Dai, J.; Xia, D.; Peng, Y.; Wang, S.; Liu, Y.; Liu, X. Regulating the performance of polybenzoxazine via the regiochemistry of amide substituents. Polymer 2019, 181, 121807. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Ohashi, S.; Liu, X.; Han, Z.; Ishida, H. Synthesis of high thermal stability polybenzoxazoles via ortho-imide-functional benzoxazine monomers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1330–1338. [Google Scholar] [CrossRef]
- Zhang, K.; Ishida, H. Thermally stable polybenzoxazines via ortho-norbornene functional benzoxazine monomers: Unique advantages in monomer synthesis, processing and polymer properties. Polymer 2015, 66, 240–248. [Google Scholar] [CrossRef]
- Zhang, K.; Ishida, H. An anomalous trade-off effect on the properties of smart ortho-functional benzoxazines. Polym. Chem. 2015, 6, 2541–2550. [Google Scholar] [CrossRef]
- Zhang, K.; Ishida, H. Smart Synthesis of High-Performance Thermosets Based on ortho-Amide–Imide Functional Benzoxazines. Front. Mater. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Liu, Y.; Han, L.; Wang, J.; Ishida, H. Synthesis and thermally induced structural transformation of phthalimide and nitrile-functionalized benzoxazine: Toward smart ortho-benzoxazine chemistry for low flammability thermosets. RSC Adv. 2019, 9, 1526–1535. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Shang, Z.; Evans, C.J.; Han, L.; Ishida, H.; Yang, S. Benzoxazine atropisomers: Intrinsic atropisomerization mechanism and conversion to high performance thermosets. Macromolecules 2018, 51, 7574–7585. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Shang, Z.; Evans, C.J.; Yang, S. An investigation of atropisomerism in ortho-imide substituted 1, 3-benzoxazine by experimental NMR and DFT calculations. J. Phys. Org. Chem. 2019, 32, e3926. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Shang, Z.; Evans, C.J.; Yang, S. Effects of End-Caps on the Atropisomerization, Polymerization, and the Thermal Properties of ortho-Imide Functional Benzoxazines. Polymers 2019, 11, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Liu, Y.; Evans, C.J.; Yang, S. Easily Processable Thermosets with Outstanding Performance via Smart Twisted Small-Molecule Benzoxazines. Macromol. Rapid Commun. 2020, 41, 1900625. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Ran, Q.; Zhu, R.; Gu, Y. Research on curing mechanism and thermal property of bis-allyl benzoxazine and N, N′-(2, 2, 4-trimethylhexane-1, 6-diyl) dimaleimide blend. React. Funct. Polym. 2013, 73, 668–673. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, C.; Hao, Z.; Luo, X.; Jing, S.; Run, M. The polymerization behavior and thermal properties of benzoxazine based on o-allylphenol and 4, 4′-diaminodiphenyl methane. React. Funct. Polym. 2014, 75, 9–15. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, Z.; Lv, S.; Huang, J.; Liao, C.; Run, M. Structural effects of diamines on synthesis, polymerization, and properties of benzoxazines based on o-allylphenol. Polymer 2015, 57, 29–38. [Google Scholar] [CrossRef]
- Sha, X.-L.; Yuan, L.; Liang, G.; Gu, A. Development and Mechanism of High-Performance Fully Biobased Shape Memory Benzoxazine Resins with a Green Strategy. ACS Sustain. Chem. Eng. 2020, 8, 18696–18705. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Thermally curable benzoxazine monomer with a photodimerizable coumarin group. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1670–1676. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Self-healing of poly (propylene oxide)-polybenzoxazine thermosets by photoinduced coumarine dimerization. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2911–2918. [Google Scholar] [CrossRef]
- Froimowicz, P.; Rodriguez Arza, C.; Ohashi, S.; Ishida, H. Tailor-made and chemically designed synthesis of coumarin-containing benzoxazines and their reactivity study toward their thermosets. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1428–1435. [Google Scholar] [CrossRef]
- Lin, R.-C.; Mohamed, M.G.; Hsu, K.-C.; Wu, J.-Y.; Jheng, Y.-R.; Kuo, S.-W. Multivalent photo-crosslinkable coumarin-containing polybenzoxazines exhibiting enhanced thermal and hydrophobic surface properties. RSC Adv. 2016, 6, 10683–10696. [Google Scholar] [CrossRef]
- Lin, C.H.; Chien, C.K.; Chen, C.H.; Juang, T.Y. Photo-sensitive benzoxazine II: Chalcone-containing benzoxazine and its photo and thermal-cured thermoset. RSC Adv. 2017, 7, 37844–37851. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Chen, Z.J.; Chen, C.H.; Wang, M.W.; Juang, T.Y. Synthesis of a Bisbenzylideneacetone-Containing Benzoxazine and Its Photo-and Thermally Cured Thermoset. ACS Omega 2017, 2, 3432–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthukaruppan, A.; Arumugam, H.; Krishnan, S.; Kannan, K.; Chavali, M. A low cure thermo active polymerization of chalcone based benzoxazine and cross linkable olefin blends. J. Polym. Res. 2018, 25, 163. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Ishida, H. Polymerization of an AB-Type Benzoxazine Monomer toward Different Polybenzoxazine Networks: When Diels–Alder Reaction Meets Benzoxazine Chemistry in a Single-Component Resin. Macromolecules 2019, 52, 7386–7395. [Google Scholar] [CrossRef]
- Zhang, K.; Tan, X.; Wang, Y.; Ishida, H. Unique self-catalyzed cationic ring-opening polymerization of a high performance deoxybenzoin-based 1, 3-benzoxazine monomer. Polymer 2019, 168, 8–15. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y.; Ishida, H. Synthesis, characterization, and properties of new thermally curable polyetheresters containing benzoxazine moieties in the main chain. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 414–420. [Google Scholar] [CrossRef]
- Tuzun, A.; Kiskan, B.; Alemdar, N.; Erciyes, A.T.; Yagci, Y. Benzoxazine containing polyester thermosets with improved adhesion and flexibility. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4279–4284. [Google Scholar] [CrossRef]
- Baqar, M.; Agag, T.; Ishida, H.; Qutubuddin, S. Poly (benzoxazine-co-urethane) s: A new concept for phenolic/urethane copolymers via one-pot method. Polymer 2011, 52, 307–317. [Google Scholar] [CrossRef]
- Bagherifam, S.; Kiskan, B.; Aydogan, B.; Yagci, Y.; Hacaloglu, J. Thermal degradation of polysiloxane and polyetherester containing benzoxazine moieties in the main chain. J. Anal. Appl. Pyrolysis 2011, 90, 155–163. [Google Scholar] [CrossRef]
- Chernykh, A.; Liu, J.; Ishida, H. Synthesis and properties of a new crosslinkable polymer containing benzoxazine moiety in the main chain. Polymer 2006, 47, 7664–7669. [Google Scholar] [CrossRef]
- Ishida, H.; Lee, Y.-H. Dynamic mechanical and thermal analysis of reactive poly (butadiene-co-acrylonitrile) rubber-modified polybenzoxazine resin. Polym. Polym. Compos. 2001, 9, 121–134. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Ishida, H. Probing the properties of particle-matrix interphase in reactive rubber-grafted polybenzoxazine resins by atomic force microscopy. Compos. Interfaces 2005, 12, 481–499. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, S. Morphology and thermomechanical properties of main-chain polybenzoxazine-block-polydimethylsiloxane multiblock copolymers. Polymer 2010, 51, 1124–1132. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, D.; Roy, P.K. Poly (benzoxazine–co–urea): A Solventless Approach Towards The Introduction of Alternating Urea Linkages In Polybenzoxazine. ChemistrySelect 2017, 2, 5372–5377. [Google Scholar] [CrossRef]
- Wang, M.W.; Jeng, R.J.; Lin, C.H. Study on the ring-opening polymerization of benzoxazine through multisubstituted polybenzoxazine precursors. Macromolecules 2015, 48, 530–535. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Synthetic strategies to combine high performance benzoxazine thermosets with polymers. In Macromolecular Symposia; Wiley: Weinheim, Germany, 2010; Volume 298, pp. 145–153. [Google Scholar]
- Jin, L.; Agag, T.; Yagci, Y.; Ishida, H. Methacryloyl-functional benzoxazine: Photopolymerization and thermally activated polymerization. Macromolecules 2011, 44, 767–772. [Google Scholar] [CrossRef]
- Koz, B.; Kiskan, B.; Yagci, Y. A novel benzoxazine monomer with methacrylate functionality and its thermally curable (co) polymers. Polym. Bull. 2011, 66, 165–174. [Google Scholar] [CrossRef]
- Takeichi, T.; Thongpradith, S.; Kawauchi, T. Copolymers of vinyl-containing benzoxazine with vinyl monomers as precursors for high performance thermosets. Molecules 2015, 20, 6488–6503. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-X.; Ma, H.-M.; Liu, Y.; Qiu, J.-J.; Liu, C.-M. A well-defined poly (vinyl benzoxazine) obtained by selective free radical polymerization of vinyl group in bifunctional benzoxazine monomer. Polymer 2016, 82, 32–39. [Google Scholar] [CrossRef]
- Li, S.F. Synthesis of benzoxazine-based phenolic resin containing furan groups. Chin. Chem. Lett. 2010, 21, 868–871. [Google Scholar] [CrossRef]
- Kukut, M.; Kiskan, B.; Yagci, Y. Self-curable benzoxazine functional polybutadienes synthesized by click chemistry. Des. Monomers Polym. 2009, 12, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Vietmeier, A.C.K.; Agag, T.; Ishida, H. Synthesis and properties of benzoxazine-functional cellulose via click chemistry in: The First International Symposium on Polybenzoxazines. PMSE Div. ACS San Fr. 2010, 609–610. [Google Scholar]
- Agag, T.; Vietmeier, K.; Chernykh, A.; Ishida, H. Side-chain type benzoxazine-functional cellulose via click chemistry. J. Appl. Polym. Sci. 2012, 125, 1346–1351. [Google Scholar] [CrossRef]
- Temel, G.; Esen, D.S.; Arsu, N. One-component benzoxazine type photoinitiator for free radical polymerization. Polym. Eng. Sci. 2012, 52, 133–138. [Google Scholar] [CrossRef]
- Bai, J.; Shi, Z. Novel benzoxazine resins as photoinitiator comprising benzophenone and coinitiator amine for photopolymerization. J. Appl. Polym. Sci. 2012, 128, 1785–1791. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, D.; Roy, P.K. Microwave-Assisted Sustainable Synthesis of Telechelic Poly (ethylene glycol) s with Benzoxazine End Groups. ChemistrySelect 2016, 1, 6941–6947. [Google Scholar] [CrossRef]
- Nakamura, M.; Ishida, H. Synthesis and properties of new crosslinkable telechelics with benzoxazine moiety at the chain end. Polymer 2009, 50, 2688–2695. [Google Scholar] [CrossRef]
- Qi, H.; Pan, G.; Zhuang, Y.; Huang, F.; Du, L. Synthesis and characterization of acetylene-terminated polybenzoxazines based on polyaralkyl-phenolic prepolymer. Polym. Eng. Sci. 2010, 50, 1751–1757. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Ishida, H. An ultrahigh performance cross-linked polybenzoxazole via thermal conversion from poly (benzoxazine amic acid) based on smart o-benzoxazine chemistry. Macromolecules 2014, 47, 8674–8681. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Ishida, H. High performance crosslinked polyimide based on main-chain type polybenzoxazine. RSC Adv. 2014, 4, 62550–62556. [Google Scholar] [CrossRef]
- Han, L.; Zhang, K.; Ishida, H.; Froimowicz, P. Study of the effects of intramolecular and intermolecular hydrogen-bonding systems on the polymerization of amide-containing benzoxazines. Macromol. Chem. Phys. 2017, 218, 1600562. [Google Scholar] [CrossRef] [Green Version]
- El-Mahdy, A.F.; Kuo, S.-W. Direct synthesis of poly (benzoxazine imide) from an ortho-benzoxazine: Its thermal conversion to highly cross-linked polybenzoxazole and blending with poly (4-vinylphenol). Polym. Chem. 2018, 9, 1815–1826. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Wang, Y.; Sun, L. A high performance polybenzoxazine via smart ortho-norbornene functional benzoxazine monomer based on ring-opening metathesis polymerization. High Perform. Polym. 2018, 31, 513–520. [Google Scholar] [CrossRef]
- Han, L.; Iguchi, D.; Gil, P.; Heyl, T.R.; Sedwick, V.M.; Arza, C.R.; Ohashi, S.; Lacks, D.J.; Ishida, H. Oxazine ring-related vibrational modes of benzoxazine monomers using fully aromatically substituted, deuterated, 15N isotope exchanged, and oxazine-ring-substituted compounds and theoretical calculations. J. Phys. Chem. A 2017, 121, 6269–6282. [Google Scholar] [CrossRef]
- Ohashi, S.; Cassidy, F.; Huang, S.; Chiou, K.; Ishida, H. Synthesis and ring-opening polymerization of 2-substituted 1, 3-benzoxazine: The first observation of the polymerization of oxazine ring-substituted benzoxazines. Polym. Chem. 2016, 7, 7177–7184. [Google Scholar] [CrossRef]
- De Oliveira, D.R.; Mazzetto, S.E.; Lomonaco, D. Synthesis and Polymerization of Naphthoxazines Containing Furan Groups: An Approach to Novel Biobased and Flame-Resistant Thermosets. Int. J. Polym. Sci. 2018, 2018. [Google Scholar] [CrossRef]
- Tavernier, R.; Granado, L.; Foyer, G.; David, G.; Caillol, S. Formaldehyde-free polybenzoxazines for high performance thermosets. Macromolecules 2020. [Google Scholar] [CrossRef]
- Heydenreich, M.; Koch, A.; Szatmári, I.; Fülöp, F.; Kleinpeter, E. Synthesis and conformational analysis of naphth [1, 2-e][1, 3] oxazino [4, 3-a][1, 3] isoquinoline and naphth [2, 1-e][1, 3] oxazino [4, 3-a] isoquinoline derivatives. Tetrahedron 2008, 64, 7378–7385. [Google Scholar] [CrossRef]
- Deb, M.L.; Dey, S.S.; Bento, I.; Barros, M.T.; Maycock, C.D. Copper-Catalyzed Regioselective Intramolecular Oxidative α-Functionalization of Tertiary Amines: An Efficient Synthesis of Dihydro-1, 3-Oxazines. Angew. Chem. Int. Ed. 2013, 52, 9791–9795. [Google Scholar] [CrossRef]
- Mahato, S.; Haldar, S.; Jana, C.K. Diastereoselective α-C–H functionalization of aliphatic N-heterocycles: An efficient route to ring fused oxazines. Chem. Commun. 2014, 50, 332–334. [Google Scholar] [CrossRef]
- Cimarelli, C.; Fratoni, D.; Mazzanti, A.; Palmieri, G. Betti reaction of cyclic imines with naphthols and phenols–preparation of new derivatives of Betti’s bases. Eur. J. Org. Chem. 2011, 2011, 2094–2100. [Google Scholar] [CrossRef]
- Arza, C.R.; Froimowicz, P.; Han, L.; Graf, R.; Ishida, H. Design, synthesis, characterization, and polymerization of fused-ring naphthoxazine resins. Macromolecules 2017, 50, 9249–9256. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Ishida, H. Cationic ring-opening polymerization of benzoxazines. Polymer 1999, 40, 4563–4570. [Google Scholar] [CrossRef]
- Dunkers, J.; Ishida, H. Reaction of benzoxazine-based phenolic resins with strong and weak carboxylic acids and phenols as catalysts. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1913–1921. [Google Scholar] [CrossRef]
- Cid, J.A.; Wang, Y.-X.; Ishida, H. Cationic polymerization of benzoxazine monomers by boron trifluoride complex. Polym. Polym. Compos. 1999, 7, 409–420. [Google Scholar]
- Wang, Y.; Ishida, H. Methyl p-toluenesulfonate-initiated cationic polymerization of a benzoxazine resin. Polym. Mater. Sci. Eng. 1999, 81, 114–115. [Google Scholar]
- Sudo, A.; Hirayama, S.; Endo, T. Highly efficient catalysts-acetylacetonato complexes of transition metals in the 4th period for ring-opening polymerization of 1, 3-benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 479–484. [Google Scholar] [CrossRef]
- Burke, W.; Stephens, C.W. Monomeric products from the condensation of phenol with formaldehyde and primary amines. J. Am. Chem. Soc. 1952, 74, 1518–1520. [Google Scholar] [CrossRef]
- Riess, G.; Schwob, J.; Guth, G.; Roche, M.; Lande, B.; Culbertson, B. Advances in polymer synthesis; Plenum: New York, NY, USA, 1985; Volume 27. [Google Scholar]
- McDonagh, A.F.; Smith, H.E. Ring-chain tautomerism of derivatives of o-hydroxybenzylamine with aldehydes and ketones. The nuclear magnetic resonance spectra of immonium ions. J. Org. Chem. 1968, 33, 8–12. [Google Scholar] [CrossRef]
- Ishida, H.; Ohba, S. Synthesis and characterization of maleimide and norbornene functionalized benzoxazines. Polymer 2005, 46, 5588–5595. [Google Scholar] [CrossRef]
- Wirasate, S.; Dhumrongvaraporn, S.; Allen, D.J.; Ishida, H. Molecular origin of unusual physical and mechanical properties in novel phenolic materials based on benzoxazine chemistry. J. Appl. Polym. Sci. 1998, 70, 1299–1306. [Google Scholar] [CrossRef]
- Kim, H.-D.; Ishida, H. A study on hydrogen-bonded network structure of polybenzoxazines. J. Phys. Chem. A 2002, 106, 3271–3280. [Google Scholar] [CrossRef]
- Ishida, H.; Rodriguez, Y. Catalyzing the curing reaction of a new benzoxazine-based phenolic resin. J. Appl. Polym. Sci. 1995, 58, 1751–1760. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Ishida, H. Synthesis and properties of new thermoplastic polymers from substituted 3, 4-dihydro-2 H-1, 3-benzoxazines. Macromolecules 2000, 33, 2839–2847. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Q.-Y. Catalytic Accelerated Polymerization of Benzoxazines and Their Mechanistic Considerations. In Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 9–21. [Google Scholar]
- Sudo, A.; Kudoh, R.; Nakayama, H.; Arima, K.; Endo, T. Selective formation of poly (N, O-acetal) by polymerization of 1, 3-benzoxazine and its main chain rearrangement. Macromolecules 2008, 41, 9030–9034. [Google Scholar] [CrossRef]
- Ohashi, S.; Zhang, K.; Ran, Q.; Arza, C.R.; Froimowicz, P.; Ishida, H. Preparation of high purity samples, effect of purity on properties, and FT-IR, Raman, 1H and 13C NMR, and SDC data of highly purified benzoxazine monomers. In Advanced and Emerging Polybenzoxazine Science and Technology; Ishida, H., Froimowicz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 49; pp. 1053–1082. [Google Scholar]
- Chutayothin, P.; Ishida, H. Cationic ring-opening polymerization of 1, 3-benzoxazines: Mechanistic study using model compounds. Macromolecules 2010, 43, 4562–4572. [Google Scholar] [CrossRef]
- Hayakawa, T.; Osanai, Y.; Niizeki, K.; Haba, O.; Ueda, M. The curing reaction of 3-aryl substituted benzoxazine. High Perform. Polym. 2000, 12, 237–246. [Google Scholar] [CrossRef]
- Hariharan, A.; Srinivasan, K.; Murthy, C.; Alagar, M. Synthesis and characterization of a novel class of low temperature cure Benzoxazines. J. Polym. Res. 2018, 25, 20. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Hiziroglu, S.; Rimdusit, S. Effect of cashew nut shell liquid on gelation, cure kinetics, and thermomechanical properties of benzoxazine resin. Thermochim. Acta 2011, 520, 84–92. [Google Scholar] [CrossRef]
- Hamerton, I.; McNamara, L.T.; Howlin, B.J.; Smith, P.A.; Cross, P.; Ward, S. Examining the initiation of the polymerization mechanism and network development in aromatic polybenzoxazines. Macromolecules 2013, 46, 5117–5132. [Google Scholar] [CrossRef] [PubMed]
- Gorodisher, I.; DeVoe, R.; Webb, R.; Hatsuo, I. Handbook of Benzoxazine Resins; Hatsuo, I., Tarek, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Kawaguchi, A.W.; Sudo, A.; Endo, T. Polymerization–depolymerization system based on reversible addition-dissociation reaction of 1, 3-benzoxazine with thiol. ACS Macro Lett. 2013, 2, 1–4. [Google Scholar] [CrossRef]
- William Kawaguchi, A.; Sudo, A.; Endo, T. Promoting effect of thiophenols on the ring-opening polymerization of 1, 3-benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2523–2527. [Google Scholar] [CrossRef]
- Semerci, E.; Kiskan, B.; Yagci, Y. Thiol reactive polybenzoxazine precursors: A novel route to functional polymers by thiol-oxazine chemistry. Eur. Polym. J. 2015, 69, 636–641. [Google Scholar] [CrossRef]
- Kawaguchi, A.W.; Sudo, A.; Endo, T. Thiol-functionalized 1, 3-benzoxazine: Preparation and its use as a precursor for highly polymerizable benzoxazine monomers bearing sulfide moiety. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1448–1457. [Google Scholar] [CrossRef]
- Urbaniak, T.; Soto, M.; Liebeke, M.; Koschek, K. Insight into the Mechanism of Reversible Ring-Opening of 1, 3-Benzoxazine with Thiols. J. Org. Chem. 2017, 82, 4050–4055. [Google Scholar] [CrossRef]
- Kawaguchi, A.W.; Sudo, A.; Endo, T. Synthesis of highly polymerizable 1, 3-benzoxazine assisted by phenyl thio ether and hydroxyl moieties. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1457–1461. [Google Scholar] [CrossRef]
- Beyazkilic, Z.; Kahveci, M.U.; Aydogan, B.; Kiskan, B.; Yagci, Y. Synthesis of polybenzoxazine precursors using thiols: Simultaneous thiol–ene and ring-opening reactions. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4029–4036. [Google Scholar] [CrossRef]
- Narayanan, J.; Jungman, M.J.; Patton, D.L. Hybrid dual-cure polymer networks via sequential thiol–ene photopolymerization and thermal ring-opening polymerization of benzoxazines. React. Funct. Polym. 2012, 72, 799–806. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Combining elemental sulfur with polybenzoxazines via inverse vulcanization. Macromolecules 2016, 49, 767–773. [Google Scholar] [CrossRef]
- Arza, C.R.; Froimowicz, P.; Ishida, H. Triggering effect caused by elemental sulfur as a mean to reduce the polymerization temperature of benzoxazine monomers. RSC Adv. 2016, 6, 35144–35151. [Google Scholar] [CrossRef]
- Bayram, O.; Kiskan, B.; Demir, E.; Demir-Cakan, R.; Yagci, Y. Advanced Thermosets from Sulfur and Renewable Benzoxazine and Ionones via Inverse Vulcanization. ACS Sustain. Chem. Eng. 2020, 8, 9145–9155. [Google Scholar] [CrossRef]
- Lin, H.K.; Liu, Y.L. Sulfur radical transfer and coupling reaction to benzoxazine groups: A new reaction route for preparation of polymeric materials using elemental sulfur as a feedstock. Macromol. Rapid Commun. 2018, 39, 1700832. [Google Scholar] [CrossRef]
- Lee, S.-D.; Takata, T.; Endo, T. Arenesulfonates as non-salt-type latent thermal initiators for cationic polymerization. Macromolecules 1996, 29, 3317–3319. [Google Scholar] [CrossRef]
- Sudo, A.; Yamashita, H.; Endo, T. Ring-opening polymerization of 1, 3-benzoxazines by p-toluenesulfonates as thermally latent initiators. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3631–3636. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, W.; Liu, Q.; Chen, T.; Zhou, X.; Yang, C.; Zhang, K.; Xie, Z. Atom-economical, room-temperature, and high-efficiency synthesis of polyamides via a three-component polymerization involving benzoxazines, odorless isocyanides, and water. Polym. Chem. 2018, 9, 5566–5571. [Google Scholar] [CrossRef]
- Andreu, R.; Reina, J.; Ronda, J. Carboxylic acid-containing benzoxazines as efficient catalysts in the thermal polymerization of benzoxazines. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6091–6101. [Google Scholar] [CrossRef]
- Zou, T.; Li, S.; Huang, W.; Liu, X. Comparison of two bisbenzoxazines containing carboxylic groups and their thermal polymerization. Des. Monomers Polym. 2013, 16, 25–30. [Google Scholar] [CrossRef]
- Hassan, W.A.W.; Liu, J.; Howlin, B.J.; Ishida, H.; Hamerton, I. Examining the influence of bisphenol a on the polymerisation and network properties of an aromatic benzoxazine. Polymer 2016, 88, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Hemvichian, K.; Ishida, H. Thermal decomposition processes in aromatic amine-based polybenzoxazines investigated by TGA and GC–MS. Polymer 2002, 43, 4391–4402. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, L.K.; Chiang, S.J.; Liu, Y.L. A cocatalytic effect between meldrum’s acid and benzoxazine compounds in preparation of high performance thermosetting resins. Macromol. Rapid Commun. 2017, 38, 1600616. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, C.; Liu, L.; Ding, J.; Li, Y. Catalytic effect of sulfonated poly (styrene-divinylbenzene) microspheres on the thermal curing behavior of benzoxazine. Thermochim. Acta 2018, 661, 106–115. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, C.; Wu, M.; Li, Y.; Li, H.; Xiang, D.; Guo, C. Curing kinetics of phenolphthalein based polyphosphazene towards thermal stability and flame retardancy of polybenzoxazine. RSC Adv. 2019, 9, 31583–31593. [Google Scholar] [CrossRef] [Green Version]
- Kasapoglu, F.; Cianga, I.; Yagci, Y.; Takeichi, T. Photoinitiated cationic polymerization of monofunctional benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3320–3328. [Google Scholar] [CrossRef]
- Zhang, D.; Yue, J.; Li, H.; Li, Y.; Zhao, C. Curing kinetics study of benzoxazine using diaryliodonium salts as thermal initiators. Thermochim. Acta 2016, 643, 13–22. [Google Scholar] [CrossRef]
- Rimdusit, S.; Jiraprawatthagool, V.; Tiptipakorn, S.; Covavisaruch, S.; Kitano, T. Characterization of SiC Whisker-Filled Polybenzoxazine Cured by Microwave Radiation and Heat. Int. J. Polym. Anal. Charact. 2006, 11, 441–453. [Google Scholar] [CrossRef]
- Shi, Z.; Yu, D.; Wang, Y.; Xu, R. Nonisothermal cure kinetics in the synthesis of polybenzoxazine–clay nanocomposites. J. Appl. Polym. Sci. 2003, 88, 194–200. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kuo, S.-W.; Su, Y.-C.; Chen, J.-K.; Tu, C.-W.; Chang, F.-C. Syntheses, thermal properties, and phase morphologies of novel benzoxazines functionalized with polyhedral oligomeric silsesquioxane (POSS) nanocomposites. Polymer 2004, 45, 6321–6331. [Google Scholar] [CrossRef]
- Agag, T.; Tsuchiya, H.; Takeichi, T. Novel organic–inorganic hybrids prepared from polybenzoxazine and titania using sol–gel process. Polymer 2004, 45, 7903–7910. [Google Scholar] [CrossRef]
- Xin, C.; Yang, X.; Yu, D. Non-isothermal cure kinetics of polybenzoxazine/carbon fiber composites by phase change theory. Polym. Polym. Compos. 2005, 13, 599–605. [Google Scholar] [CrossRef]
- Zhang, S.; Ran, Q.; Fu, Q.; Gu, Y. Controlled polymerization of 3, 4-dihydro-2H-1, 3-benzoxazine and its properties tailored by Lewis acids. React. Funct. Polym. 2019, 139, 75–84. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Mechanistic studies on ring-opening polymerization of benzoxazines: A mechanistically based catalyst design. Macromolecules 2011, 44, 4616–4622. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Pei, L.; Zhao, S.; Zhang, C.; Wang, Z. In (NO3) 3 catalyzed curing reaction of benzoxazine. High Perform. Polym. 2020, 32, 702–709. [Google Scholar] [CrossRef]
- Zhang, T.; Bonnaud, L.; Raquez, J.-M.; Poorteman, M.; Olivier, M.; Dubois, P. Cerium Salts: An Efficient Curing Catalyst for Benzoxazine Based Coatings. Polymers 2020, 12, 415. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Srivastava, M.; Lochab, B.; Kumar, D.; Ramanan, A.; Roy, P.K. Metal-Organic Frameworks as curing accelerators for benzoxazines. ChemistrySelect 2016, 1, 3924–3932. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, D.; Roy, P.K. Enhancing the processibility of high temperature polymerizing cardanol derived benzoxazines using eco-friendly curing accelerators. Polymer 2018, 138, 343–351. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, R.; Yu, D. Multiwalled carbon nanotube/polybenzoxazine nanocomposites: Preparation, characterization and properties. Polymer 2006, 47, 7711–7719. [Google Scholar] [CrossRef]
- Monisha, M.; Yadav, N.; Srivastava, S.B.; Singh, S.P.; Lochab, B. Sustainable one-step strategy towards low temperature curable superparamagnetic composite based on smartly designed iron nanoparticles and cardanol benzoxazine. J. Mater. Chem. A 2018, 6, 2555–2567. [Google Scholar] [CrossRef]
- Andreu, R.; Galià, M.; Cádiz, V.; Lligadas, G.; Reina, J.A.; Ronda, J.C. BF3· OEt2 in alcoholic media, an efficient initiator in the cationic polymerization of phenyl-1, 3-benzoxazines. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 5075–5084. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Ring-opening polymerization of 1, 3-benzoxazines via borane catalyst. Polymers 2018, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Ran, Q.; Li, C.; Zhu, R.; Gu, Y. Study on the catalytic prepolymerization of an acetylene-functional benzoxazine and the thermal degradation of its cured product. RSC Adv. 2015, 5, 82429–82437. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H.; Cheng, J.; Fang, Z.; Wang, H. Effect of iron acetylacetonate on the crosslink structure, thermal and flammability properties of novel aromatic diamine-based benzoxazines containing cyano group. RSC Adv. 2015, 5, 18538–18545. [Google Scholar] [CrossRef]
- Du, W.; Shan, J.; Wu, Y.; Xu, R.; Yu, D. Preparation and characterization of polybenzoxazine/trisilanol polyhedral oligomeric silsesquioxanes composites. Mater. Des. 2010, 31, 1720–1725. [Google Scholar] [CrossRef]
- Wang, S.; Jia, Q.; Liu, Y.; Jing, X. An investigation on the effect of phenylboronic acid on the processibilities and thermal properties of bis-benzoxazine resins. React. Funct. Polym. 2015, 93, 111–119. [Google Scholar] [CrossRef]
- Wang, Y.n.; Niu, X.; Xing, X.; Wang, S.; Jing, X. Curing behaviour and properties of a novel benzoxazine resin via catalysis of 2-phenyl-1, 3, 2-benzodioxaborole. React. Funct. Polym. 2017, 117, 60–69. [Google Scholar] [CrossRef]
- Wang, S.; Bian, C.; Jia, B.; Wang, Y.; Jing, X. Structure and thermal pyrolysis mechanism of poly (resorcinol borate) with high char yield. Polym. Degrad. Stab. 2016, 130, 328–337. [Google Scholar] [CrossRef]
- Agag, T.; Arza, C.R.; Maurer, F.H.; Ishida, H. Primary amine-functional benzoxazine monomers and their use for amide-containing monomeric benzoxazines. Macromolecules 2010, 43, 2748–2758. [Google Scholar] [CrossRef]
- Sun, J.; Wei, W.; Xu, Y.; Qu, J.; Liu, X.; Endo, T. A curing system of benzoxazine with amine: Reactivity, reaction mechanism and material properties. RSC Adv. 2015, 5, 19048–19057. [Google Scholar] [CrossRef]
- Zong, J.; Ran, Q. Ring Opening Reaction of 3, 4-Dihydro-2H-1, 3-Benzoxazine with Amines at Room Temperature. ChemistrySelect 2019, 4, 6687–6696. [Google Scholar] [CrossRef]
- Prabunathan, P.; Vasanthakumar, A.; Manoj, M.; Hariharan, A.; Alagar, M. Polypyrrole inter-layered low temperature curing benzoxazine matrices with enhanced thermal and dielectric properties. J. Polym. Res. 2020, 27, 48. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Ling, H.; Ran, Q.; Gu, Y. The effect of curing cycles on curing reactions and properties of a ternary system based on benzoxazine, epoxy resin, and imidazole. J. Appl. Polym. Sci. 2013, 127, 2169–2175. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Ran, Q.; Zhu, R.; Gu, Y. Reaction induced phase separation in a bisphenol A-aniline benzoxazine/N,N′-(2,2,4-trimethylhexane-1,6-diyl)bis(maleimide)/imidazole blend: The effect of initial curing temperature on morphology and properties. RSC Adv. 2013, 3, 14029–14036. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, Q.; Deng, Y.Y.; Zhu, R.Q.; Gu, Y. Reaction induced phase separation in thermosetting/thermosetting blends: Effects of imidazole content on the phase separation of benzoxazine/epoxy blends. RSC Adv. 2014, 4, 61634–61642. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, N.; Miao, Y.; Gu, Y. Influence of curing sequence on phase structure and properties of bisphenol A-aniline benzoxazine/N, N′-(2, 2, 4-trimethylhexane-1, 6-diyl) bis (maleimide)/imidazole blend. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, R.; Yang, P.; Gu, Y. A study on the chain propagation of benzoxazine. Polym. Chem. 2016, 7, 860–866. [Google Scholar] [CrossRef]
- Akkus, B.; Kiskan, B.; Yagci, Y. Counterion Effect of Amine Salts on Ring-Opening Polymerization of 1, 3-Benzoxazines. Macromol. Chem. Phys. 2019, 220, 1800268. [Google Scholar] [CrossRef]
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw Hill Professional: Boston, MA, USA, 2005. [Google Scholar]
- Zhang, W.; Froimowicz, P.; Arza, C.R.; Ohashi, S.; Xin, Z.; Ishida, H. Latent catalyst-containing naphthoxazine: Synthesis and effects on ring-opening polymerization. Macromolecules 2016, 49, 7129–7140. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Sun, J.; Huang, S.; Liu, X.; Endo, T. Synthesis and thermal properties of a bio-based polybenzoxazine with curing promoter. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2016–2023. [Google Scholar] [CrossRef]
- Kimura, H.; Matsumoto, A.; Ohtsuka, K. New type of phenolic resin—the curing reaction of bisphenol A based benzoxazine with bisoxazoline and the properties of the cured resin. III. The cure reactivity of benzoxazine with a latent curing agent. J. Appl. Polym. Sci. 2008, 107, 710–718. [Google Scholar] [CrossRef]
- Kocaarslan, A.; Kiskan, B.; Yagci, Y. Ammonium salt catalyzed ring-opening polymerization of 1,3-benzoxazines. Polymer 2017, 122, 340–346. [Google Scholar] [CrossRef]
- Kimura, H.; Matsumoto, A.; Ohtsuka, K. Studies on new type of phenolic resin—Curing reaction of bisphenol-A-based benzoxazine with epoxy resin using latent curing agent and the properties of the cured resin. J. Appl. Polym. Sci. 2008, 109, 1248–1256. [Google Scholar] [CrossRef]
- Kimura, H.; Matsumoto, A.; Ohtsuka, K. New type of phenolic resin: Curing reaction of phenol-novolac based benzoxazine with bisoxazoline or epoxy resin using latent curing agent and the properties of the cured resin. J. Appl. Polym. Sci. 2009, 112, 1762–1770. [Google Scholar] [CrossRef]
- Akkus, B.; Kiskan, B.; Yagci, Y. Cyanuric chloride as a potent catalyst for the reduction of curing temperature of benzoxazines. Polym. Chem. 2020, 11, 1025–1032. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.Z.; Fu, Y.F.; Liu, X.D. Latent curing systems stabilized by reaction equilibrium in homogeneous mixtures of benzoxazine and amine. Sci. Rep. 2016, 6, 38584. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Froimowicz, P.; Ishida, H. A smart latent catalyst containing o-trifluoroacetamide functional benzoxazine: Precursor for low temperature formation of very high Performance polybenzoxazole with low dielectric constant and high thermal stability. Macromolecules 2017, 50, 6552–6560. [Google Scholar] [CrossRef]
- Kaya, G.; Kiskan, B.; Yagci, Y. Phenolic naphthoxazines as curing promoters for benzoxazines. Macromolecules 2018, 51, 1688–1695. [Google Scholar] [CrossRef]
- Ren, S.; Miao, X.; Zhao, W.; Zhang, S.; Wang, W. A fully bio-based benzoxazine as latent catalyst for bisphenol A/aniline-based benzoxazine. Mater. Today Commun. 2019, 20, 100568. [Google Scholar] [CrossRef]
- Arslan, M. Synthesis and characterization of novel bio-based benzoxazines from gallic acid with latent catalytic characteristics. React. Funct. Polym. 2019, 139, 9–16. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, C.; Pilla, S.; Gong, S. Polybenzoxazine-core shell rubber–carbon nanotube nanocomposites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1653–1659. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Li, R.; Liu, J.; Chen, W.; Xu, Q.; Gu, Y. The curing behavior and thermal property of graphene oxide/benzoxazine nanocomposites. Polymer 2013, 54, 3107–3116. [Google Scholar] [CrossRef]
- Alhassan, S.M.; Qutubuddin, S.; Schiraldi, D.A.; Agag, T.; Ishida, H. Preparation and thermal properties of graphene oxide/main chain benzoxazine polymer. Eur. Polym. J. 2013, 49, 3825–3833. [Google Scholar] [CrossRef]
- Meng, F.; Ishida, H.; Liu, X. Introduction of benzoxazine onto the graphene oxide surface by click chemistry and the properties of graphene oxide reinforced polybenzoxazine nanohybrids. RSC Adv. 2014, 4, 9471–9475. [Google Scholar] [CrossRef]
- García-Martínez, V.; Gude, M.; Calvo, S.; Martínez-Miranda, M.; Ureña, A. Influence of graphene nanoplatelets on curing kinetics and rheological properties of a benzoxazine resin. Mater. Today Commun. 2020, 24, 100990. [Google Scholar] [CrossRef]
- Xu, R.; Schreiber, H.P.; Huang, M.; Ishida, H. Polybenzoxazine resins: Aspects of interaction and adsorption behavior. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1441–1447. [Google Scholar] [CrossRef]
- Yue, J.; Zhao, C.; Dai, Y.; Li, H.; Li, Y. Catalytic effect of exfoliated zirconium phosphate on the curing behavior of benzoxazine. Thermochim. Acta 2017, 650, 18–25. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhou, J.; Zhang, D.; Zhang, A. Dramatic toughness enhancement of benzoxazine/epoxy thermosets with a novel hyperbranched polymeric ionic liquid. Chem. Eng. J. 2018, 334, 1371–1382. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Froimowicz, P.; Ishida, H. Synthesis, polymerization kinetics and thermal properties of para-methylol functional benzoxazine. React. Funct. Polym. 2018, 129, 23–28. [Google Scholar] [CrossRef]
- Ishida, H.; Lee, Y.-H. Synergism observed in polybenzoxazine and poly (ε-caprolactone) blends by dynamic mechanical and thermogravimetric analysis. Polymer 2001, 42, 6971–6979. [Google Scholar] [CrossRef]
- Ishida, H.; Lee, Y.H. Study of hydrogen bonding and thermal properties of polybenzoxazine and poly-(ε-caprolactone) blends. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 736–749. [Google Scholar] [CrossRef]
- Zheng, S.; Lü, H.; Guo, Q. Thermosetting blends of polybenzoxazine and poly (ε-caprolactone): Phase behavior and intermolecular specific interactions. Macromol. Chem. Phys. 2004, 205, 1547–1558. [Google Scholar] [CrossRef]
- Huang, J.M.; Kuo, S.W.; Lee, Y.J.; Chang, F.C. Synthesis and characterization of a vinyl-terminated benzoxazine monomer and its blends with poly (ethylene oxide). J. Polym. Sci. Part B Polym. Phys. 2007, 45, 644–653. [Google Scholar] [CrossRef]
- Su, W.-C.; Tsai, F.-C.; Huang, C.-F.; Dai, L.; Kuo, S.-W. Flexible epoxy resins formed by blending with the diblock copolymer PEO-b-PCL and using a hydrogen-bonding benzoxazine as the curing agent. Polymers 2019, 11, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan/polybenzoxazine cross-linked films: Preparation in aqueous media and synergistic improvements in thermal and mechanical properties. Biomacromolecules 2013, 14, 1806–1815. [Google Scholar] [CrossRef]
- Ramdani, N.; Chrigui, M.; Wang, J.; Feng, T.-t.; He, X.-y.; Liu, W.-b.; Zheng, X.-s. Preparation and properties of chitosan particle-reinforced polybenzoxazine blends. J. Compos. Mater. 2015, 49, 2449–2458. [Google Scholar] [CrossRef]
- Monisha, M.; Yadav, N.; Lochab, B. Sustainable framework of chitosan–benzoxazine with mutual benefits: Low curing temperature and improved thermal and mechanical properties. ACS Sustain. Chem. Eng. 2019, 7, 4473–4485. [Google Scholar] [CrossRef]
- Yadav, N.; Monisha, M.; Niranjan, R.; Dubey, A.; Patil, S.; Priyadarshini, R.; Lochab, B. Antibacterial performance of fully biobased chitosan-grafted-polybenzoxazine films: Elaboration and properties of released material. Carbohydr. Polym. 2021, 254, 117296. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Wang, Y.; Huang, Y.-H.; Bian, J.; Li, M.-F.; Peng, F.; Sun, R.-C. Benzoxazine Enhanced Amino Cellulose-based Composite Films: Preparation, Proposed Mechanism, and Improved Performance. Carbohydr. Polym. 2019, 222, 115008. [Google Scholar] [CrossRef]
- Takeichi, T.; Guo, Y.; Agag, T. Synthesis and characterization of poly (urethane-benzoxazine) films as novel type of polyurethane/phenolic resin composites. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4165–4176. [Google Scholar] [CrossRef]
- Takeichi, T.; Guo, Y. Preparation and properties of poly (urethane-benzoxazine) s based on monofunctional benzoxazine monomer. Polym. J. 2001, 33, 437. [Google Scholar] [CrossRef] [Green Version]
- Rimdusit, S.; Pirstpindvong, S.; Tanthapanichakoon, W.; Damrongsakkul, S. Toughening of polybenzoxazine by alloying with urethane prepolymer and flexible epoxy: A comparative study. Polym. Eng. Sci. 2005, 45, 288–296. [Google Scholar] [CrossRef]
- Kirschbaum, S.; Landfester, K.; Taden, A. Synthesis and thermal curing of benzoxazine functionalized polyurethanes. Macromolecules 2015, 48, 3811–3816. [Google Scholar] [CrossRef]
- Su, Y.-C.; Chen, W.-C.; Ou, K.-l.; Chang, F.-C. Study of the morphologies and dielectric constants of nanoporous materials derived from benzoxazine-terminated poly (ε-caprolactone)/polybenzoxazine co-polymers. Polymer 2005, 46, 3758–3766. [Google Scholar] [CrossRef]
- Chiu, H.-J.; Tsai, R.-S.; Huang, J.-M. Miscibility and crystallization kinetics of poly (ε-caprolactone)/bezoxazine blends. e-Polymers 2010, 10, 75–86. [Google Scholar] [CrossRef]
- Huang, J.-M.; Yang, S.-J. Studying the miscibility and thermal behavior of polybenzoxazine/poly (ε-caprolactone) blends using DSC, DMA, and solid state 13C NMR spectroscopy. Polymer 2005, 46, 8068–8078. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Synthesis and characterization of naphthoxazine functional poly (ε-caprolactone). Polymer 2005, 46, 11690–11697. [Google Scholar] [CrossRef]
- Selvi, M.; Devaraju, S.; Sethuraman, K.; Revathi, R.; Alagar, M. Cyclotriphosphazene fibre reinforced poly (benzoxazine-co-ε-caprolactam) nanocomposites for flame retardant applications. Chin. J. Polym. Sci. 2014, 32, 1086–1098. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; Ji, Y.; Zhao, Z.; Li, W.; Jia, X. Sustainable Preparation of bio-based polybenzoxazine resins from amino acid and their application in CO2 adsorption. ACS Sustain. Chem. Eng. 2019, 7, 17313–17324. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, S.; Song, K.; Gong, X.; Zhang, S.; Gao, S.; Lu, Z. A bio-based benzoxazine surfactant from amino acids. Green Chem. 2020, 22, 3481–3488. [Google Scholar] [CrossRef]
- Ran, Q.C.; Gu, Y. Concerted reactions of aldehyde groups during polymerization of an aldehyde-functional benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1671–1677. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Deng, Y.; Zhang, C.; Ran, Q.; Gu, Y. A novel polybenzoxazine containing styrylpyridine structure via the Knoevenagel reaction. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Jerry, M. Advanced Organic Chemistry: Reactions, Mechanisms and Structure; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Rupainwar, R.; Pandey, J. The Importance and Applications of Knoevenagel Reaction (Brief Review). Orient. J. Chem. 2019, 35, 423–429. [Google Scholar]
- Velez-Herrera, P.; Ishida, H. Low temperature polymerization of novel, monotropic liquid crystalline benzoxazines. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5871–5881. [Google Scholar] [CrossRef]
- Chernykh, A.; Agag, T.; Ishida, H. Novel benzoxazine monomer containing diacetylene linkage: An approach to benzoxazine thermosets with low polymerization temperature without added initiators or catalysts. Polymer 2009, 50, 3153–3157. [Google Scholar] [CrossRef]
- Ito, M.; Kawauchi, T.; Sakajiri, K.; Takeichi, T. Synthesis of liquid–crystalline benzoxazines containing a biphenyl group in the mesogenic moiety. React. Funct. Polym. 2013, 73, 1223–1230. [Google Scholar] [CrossRef]
- Kawauchi, T.; Murai, Y.; Hashimoto, K.; Ito, M.; Sakajiri, K.; Takeichi, T. Synthesis and polymerization behavior of novel liquid-crystalline benzoxazines. Polymer 2011, 52, 2150–2156. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Qi, Y.; Gao, S.; Balaji, K.; Zhang, Y.; Xue, Q.; Lu, Z. Cross-linked liquid crystalline polybenzoxazines bearing cholesterol-based mesogen side groups. Polymer 2018, 145, 252–260. [Google Scholar] [CrossRef]
- Jang, J.; Shin, S. Cure studies of a benzoxazine-based phenolic resin by isothermal experiment. Polym. J. 1995, 27, 601. [Google Scholar] [CrossRef]
- Gillham, J.K. Formation and properties of thermosetting and high Tg polymeric materials. Polym. Eng. Sci. 1986, 26, 1429–1433. [Google Scholar] [CrossRef]
- Rajasekar, S.; Hari, N. Synthesis and polymerization of benzoxazine molecules with electron-withdrawing group substitution and ring-opening polymerization. High Perform. Polym. 2016, 29, 349–361. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Zhu, Z.; Gu, Y. Preparation and characterization of novel multi-branched polymers in situ cured from benzoxazine/epoxy resin/primary amines blends. RSC Adv. 2016, 6, 15271–15278. [Google Scholar] [CrossRef]
- Sudha; Sarojadevi. Synthesis, characterization, curing, and thermal properties of bifunctional phenol-based polybenzoxazines. High Perform. Polym. 2015, 28, 331–339. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, F.; Zhou, Y.; Du, L. Synthesis and characterization of a novel acetylene-and maleimide-terminated benzoxazine and its high-performance thermosets. J. Appl. Polym. Sci. 2013, 128, 340–346. [Google Scholar] [CrossRef]
- Martos, A.; Soto, M.; Schäfer, H.; Koschek, K.; Marquet, J.; Sebastián, R.M. Highly Crosslinked Polybenzoxazines from Monobenzoxazines: The Effect of Meta-Substitution in the Phenol Ring. Polymers 2020, 12, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agag, T.; Takeichi, T. Effect of hydroxyphenylmaleimide on the curing behaviour and thermomechanical properties of rubber-modified polybenzoxazine. High Perform. Polym. 2001, 13, S327–S342. [Google Scholar] [CrossRef]
- Men, W.; Lu, Z.; Zhan, Z. Synthesis of a novel benzoxazine precursor containing phenol hydroxyl groups and its polymer. J. Appl. Polym. Sci. 2008, 109, 2219–2223. [Google Scholar] [CrossRef]
- Nordin, R.; Nazir, M.M.; Thirmizir, M.A. Curing kinetic study of tri-hydroxyl benzoxazine using differential scanning calorimetry. Adv. Environ. Biol. 2014, 2650–2655. [Google Scholar]
- Nordin, R.; Nazir, M.M.; Thirmizir, M.A.; Jani, A.M. Synthesis and characterization trihydroxyl polybenzoxazine precursor for post-polymer modifications. Adv. Environ. Biol. 2014, 2709–2714. [Google Scholar]
- Gunasekaran, S.; Arivalagan, V.; Stephen, L.D.; Dharmendirakumar, M. Triaryl Pendant Pyridine Core Hydroxyl Terminal Benzoxazine Based Polybenzoxazine-Silica (PBZ-SiO2) Hybrid Nanocomposites. J. Nanosci. Nanotechnol. 2017, 17, 5271–5283. [Google Scholar] [CrossRef]
- Chiou, K.; Hollanger, E.; Agag, T.; Ishida, H. Highly Improved Thermal Properties of Hydroxyl-Containing Polymers via Modification by Benzoxazine Groups. Macromol. Chem. Phys. 2013, 214, 1629–1635. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Nie, Y.; Szigeti, M.L.; Ishida, H. Examining the effect of hydroxyl groups on the thermal properties of polybenzoxazines: Using molecular design and Monte Carlo simulation. RSC Adv. 2018, 8, 18038–18050. [Google Scholar] [CrossRef] [Green Version]
- Baqar, M.; Agag, T.; Ishida, H.; Qutubuddin, S. Methylol-functional benzoxazines as precursors for high-performance thermoset polymers: Unique simultaneous addition and condensation polymerization behavior. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2275–2285. [Google Scholar] [CrossRef]
- Lochab, B.; Varma, I.K.; Bijwe, J. Cardanol-based bisbenzoxazines. J. Therm. Anal. Calorim. 2012, 107, 661–668. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Gu, Y. Influence of electronic effects from bridging groups on synthetic reaction and thermally activated polymerization of bisphenol-based benzoxazines. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1443–1452. [Google Scholar] [CrossRef]
- Rishwana, S.S.; Mahendran, A.; Vijayakumar, C. Studies on structurally different benzoxazines: Curing characteristics and thermal degradation aspects. High Perform. Polym. 2014, 27, 802–812. [Google Scholar] [CrossRef]
- Arnebold, A.; Schorsch, O.; Stelten, J.; Hartwig, A. Resorcinol-based benzoxazine with low polymerization temperature. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1693–1699. [Google Scholar] [CrossRef]
- Baqar, M.; Agag, T.; Huang, R.; Maia, J.o.; Qutubuddin, S.; Ishida, H. Mechanistic pathways for the polymerization of methylol-functional benzoxazine monomers. Macromolecules 2012, 45, 8119–8125. [Google Scholar] [CrossRef]
- Sudo, A.; Kudoh, R.; Endo, T. Functional benzoxazines containing ammonium salt of carboxylic acid: Synthesis and highly activated thermally induced ring-opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1724–1729. [Google Scholar] [CrossRef]
- Sudo, A.; Du, L.C.; Hirayama, S.; Endo, T. Substituent effects of N-alkyl groups on thermally induced polymerization behavior of 1, 3-benzoxazines. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2777–2782. [Google Scholar] [CrossRef]
- Oie, H.; Sudo, A.; Endo, T. Acceleration effect of N-allyl group on thermally induced ring-opening polymerization of 1, 3-benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5357–5363. [Google Scholar] [CrossRef]
- Baqar, M.; Agag, T.; Ishida, H.; Qutubuddin, S. Polymerization behavior of methylol-functional benzoxazine monomer. React. Funct. Polym. 2013, 73, 360–368. [Google Scholar] [CrossRef]
- Lin, R.; Zhu, Y.; Zhang, Y.; Wang, L.; Yu, S. Pyrogallol-based benzoxazines with latent catalytic characteristics: The temperature-dependent effect of hydrogen bonds on ring-opening polymerization. Eur. Polym. J. 2018, 102, 141–150. [Google Scholar] [CrossRef]
- Goward, G.R.; Sebastiani, D.; Schnell, I.; Spiess, H.W.; Kim, H.-D.; Ishida, H. Benzoxazine oligomers: Evidence for a helical structure from solid-state NMR spectroscopy and DFT-based dynamics and chemical shift calculations. J. Am. Chem. Soc. 2003, 125, 5792–5800. [Google Scholar] [CrossRef]
- Kim, H.-D.; Ishida, H. Model compounds study on the network structure of polybenzoxazines. Macromolecules 2003, 36, 8320–8329. [Google Scholar] [CrossRef]
- Kim, H.D.; Ishida, H. A study on hydrogen bonding in controlled-structure benzoxazine model oligomers. Macromol. Symp. 2003, 195, 123–140. [Google Scholar] [CrossRef]
- Zhong, H.; Lu, Y.; Chen, J.; Xu, W.; Liu, X. Preparation, characterization, and polymerization of novel maleimidobenzoxazine containing carboxylic moiety and its cocuring behaviors with epoxy resin. J. Appl. Polym. Sci. 2010, 118, 705–710. [Google Scholar] [CrossRef]
- Chernykh, A.; Agag, T.; Ishida, H. Effect of polymerizing diacetylene groups on the lowering of polymerization temperature of benzoxazine groups in the highly thermally stable, main-chain-type polybenzoxazines. Macromolecules 2009, 42, 5121–5127. [Google Scholar] [CrossRef]
- Agag, T.; Arza, C.; Ishida, H. Unique polymerization behavior of amide-containing benzoxazine monomers. In Proceedings of the Abstracts of papers of the American Chemical Society, 2010. [Google Scholar]
- Froimowicz, P.; Zhang, K.; Ishida, H. Intramolecular hydrogen bonding in benzoxazines: When structural design becomes functional. Chem. Eur. J. 2016, 22, 2691–2707. [Google Scholar] [CrossRef]
- Zhang, S.; Ran, Q.; Gu, Y. Polymerization mechanism of 1,3-benzoxazine catalyzed by PCl5 and rearrangement of chemical structures. Eur. Polym. J. 2021, 140, 110133. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y. Study on the volumetric expansion of benzoxazine curing with different catalysts. J. Appl. Polym. Sci. 2002, 84, 1107–1113. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Synthesis and characterization of thermally curable polyacetylenes by polymerization of propargyl benzoxazine using rhodium catalyst. Polymer 2008, 49, 2455–2460. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Synthesis and characterization of benzoxazine resin-SiO2 hybrids by sol-gel process: The role of benzoxazine-functional silane coupling agent. Polymer 2011, 52, 2757–2763. [Google Scholar] [CrossRef]
- Chow, W.; Grishchuk, S.; Burkhart, T.; Karger-Kocsis, J. Gelling and curing behaviors of benzoxazine/epoxy formulations containing 4, 4′-thiodiphenol accelerator. Thermochim. Acta 2012, 543, 172–177. [Google Scholar] [CrossRef]
- Haque, H.; Islam, Z.; Kawauchi, T.; Takeichi, T. Preparation and properties of polybenzoxazine/lignin alloy. Appl. Mech. Mater. 2012, 217–219, 571–577. [Google Scholar] [CrossRef]
- Oie, H.; Mori, A.; Sudo, A.; Endo, T. Synthesis of networked polymer based on ring-opening addition reaction of 1, 3-benzoxazine with resorcinol. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4756–4761. [Google Scholar] [CrossRef]
- Nour-Eddine, E.M.; Yuan, Q.; Huang, F. Investigation of curing and thermal behavior of benzoxazine and lignin mixtures. J. Appl. Polym. Sci. 2012, 125, 1773–1781. [Google Scholar] [CrossRef]
- Ran, Q.-C.; Zhang, D.-X.; Zhu, R.-Q.; Gu, Y. The structural transformation during polymerization of benzoxazine/FeCl3 and the effect on the thermal stability. Polymer 2012, 53, 4119–4127. [Google Scholar] [CrossRef]
- Bandeira, C.F.; Pereira, A.C.; Botelho, E.C.; Costa, M.L. Benzoxazine resin and their nanostructured composites cure kinetic by DSC. J. Mater. Res. 2013, 28, 3094–3099. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Catalyst effects on the ring-opening polymerization of 1,3-benzoxazine and on the polymer structure. Polymer 2013, 54, 2873–2878. [Google Scholar] [CrossRef]
- Wang, Z.; Ran, Q.; Zhu, R.; Gu, Y. A novel benzoxazine/bismaleimide blend resulting in bi-continuous phase separated morphology. RSC Adv. 2013, 3, 1350–1353. [Google Scholar] [CrossRef]
- Wang, Z.; Ran, Q.; Zhu, R.; Gu, Y. Curing behaviors and thermal properties of benzoxazine and N, N′-(2, 2, 4-trimethylhexane-1, 6-diyl) dimaleimide blend. J. Appl. Polym. Sci. 2013, 129, 1124–1130. [Google Scholar] [CrossRef]
- Wu, G.; Kou, K.; Zhuo, L.; Wang, Y.; Zhang, J. Preparation and characterization of novel dicyanate/benzoxazine/bismaleimide copolymer. Thermochim. Acta 2013, 559, 86–91. [Google Scholar] [CrossRef]
- Zhang, K.; Cai, R.; Zhuang, Q.; Liu, X.; Yang, G.; Han, Z. High performance crosslinked system based on reaction of benzoxazine with benzoxazole. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1514–1518. [Google Scholar] [CrossRef]
- Li, X.; Luo, X.; Liu, M.; Ran, Q.; Gu, Y. The catalytic mechanism of benzoxazine to the polymerization of cyanate ester. Mater. Chem. Phys. 2014, 148, 328–334. [Google Scholar] [CrossRef]
- Bektas, S.; Kiskan, B.; Orakdogen, N.; Yagci, Y. Synthesis and properties of organo-gels by thiol-benzoxazine chemistry. Polymer 2015, 75, 44–50. [Google Scholar] [CrossRef]
- Fan, M.J.; Zhou, Y.; Luan, Y.H.; Zang, Q.; Zhang, D.X.; Shang, C.Y.; Jiang, W.G. Non-Isothermal Curing Kinetics of a Modified Benzoxazine Resin System Suitable for Hot-Melt Prepreg Preparation. Solid State Phenom. 2017, 266, 128–134. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, J.; Wang, S.; Yang, Y.; Chen, Y.; Lu, H. Meta-phenylenediamine formaldehyde oligomer: A new accelerator for benzoxazine resin. React. Funct. Polym. 2017, 121, 51–57. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Ma, R.-k.; Kiran, S.; Zegaoui, A.; Cai, W.-a.; Shah, A.H.; Wang, J.; Derradji, M.; Liu, W.-b. Reinforcement of economical and environment friendly Acacia catechu particles for the reduction of brittleness and curing temperature of polybenzoxazine thermosets. Compos. Part A Appl. Sci. Manuf. 2018, 105, 258–264. [Google Scholar] [CrossRef]
- Wazarkar, K.; Sabnis, A. Effect of pendant functional groups on curing kinetics and final properties of cardanol-based benzoxazines. J. Coat. Technol. Res. 2018, 15, 555–569. [Google Scholar] [CrossRef]
- Xu, Q.; Zeng, M.; Chen, J.; Zeng, S.; Huang, Y.; Feng, Z.; Xu, Q.; Yan, C.; Gu, Y. Synthesis, polymerization kinetics, and high-frequency dielectric properties of novel main-chain benzoxazine copolymers. React. Funct. Polym. 2018, 122, 158–166. [Google Scholar] [CrossRef]
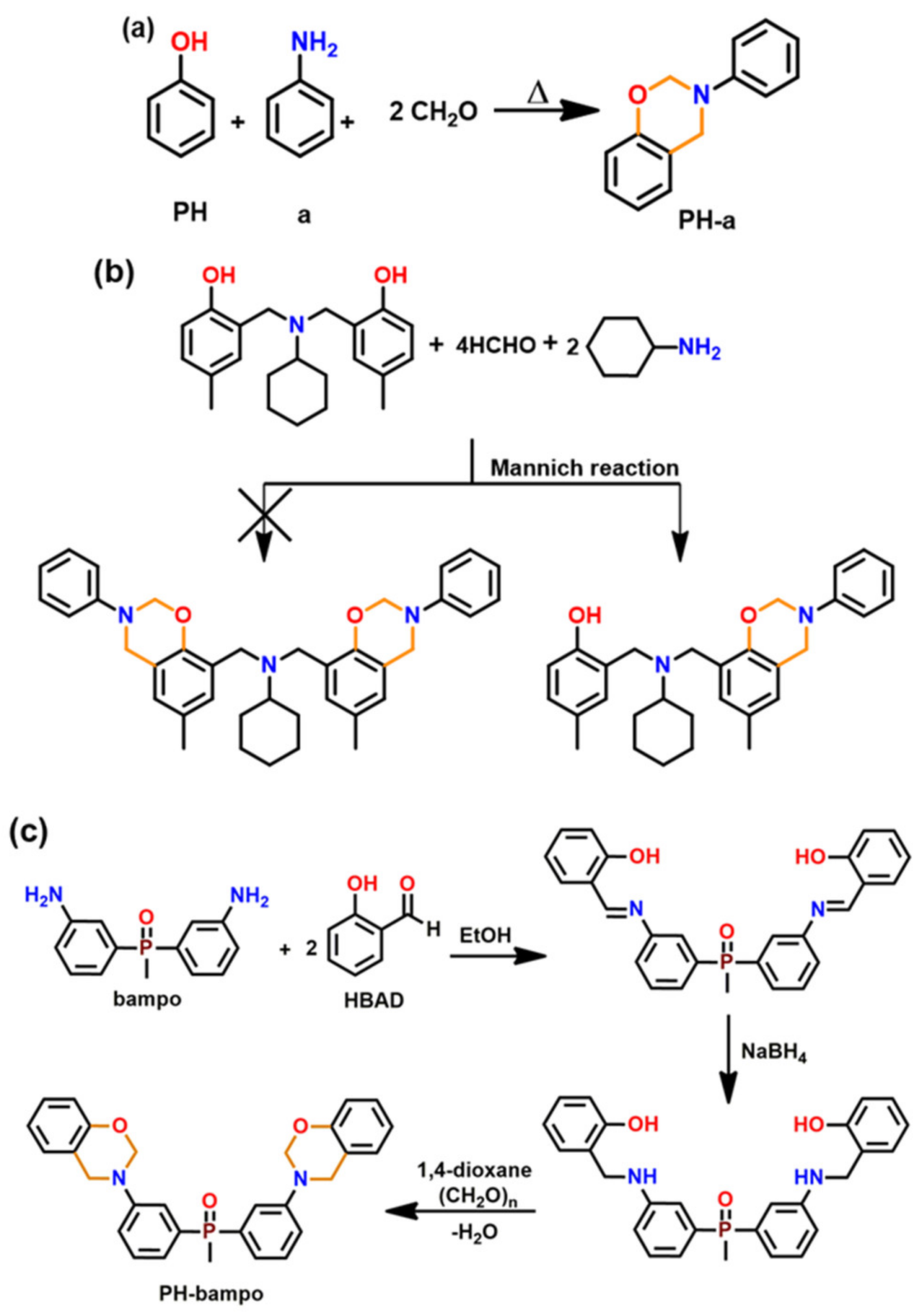

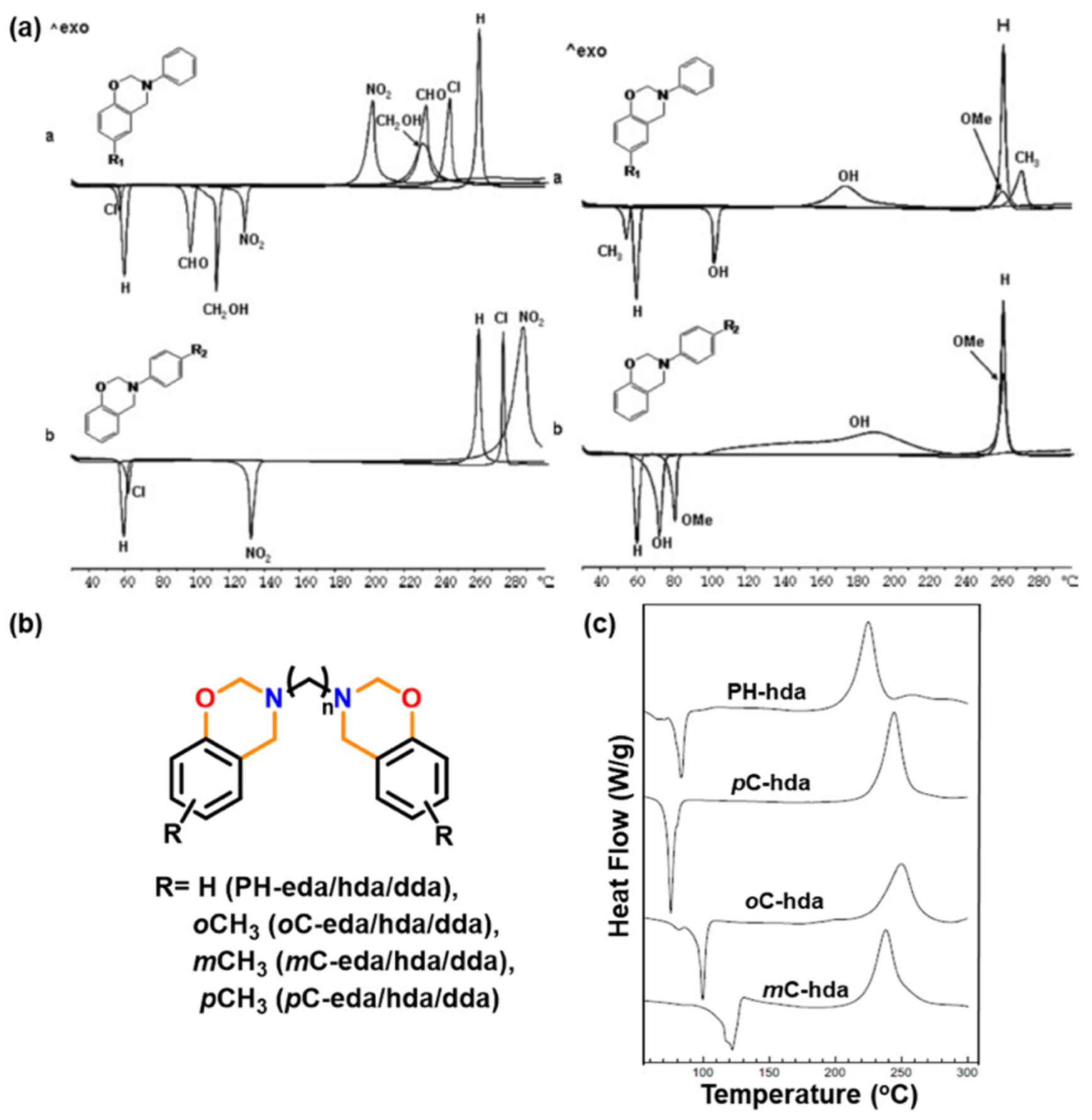
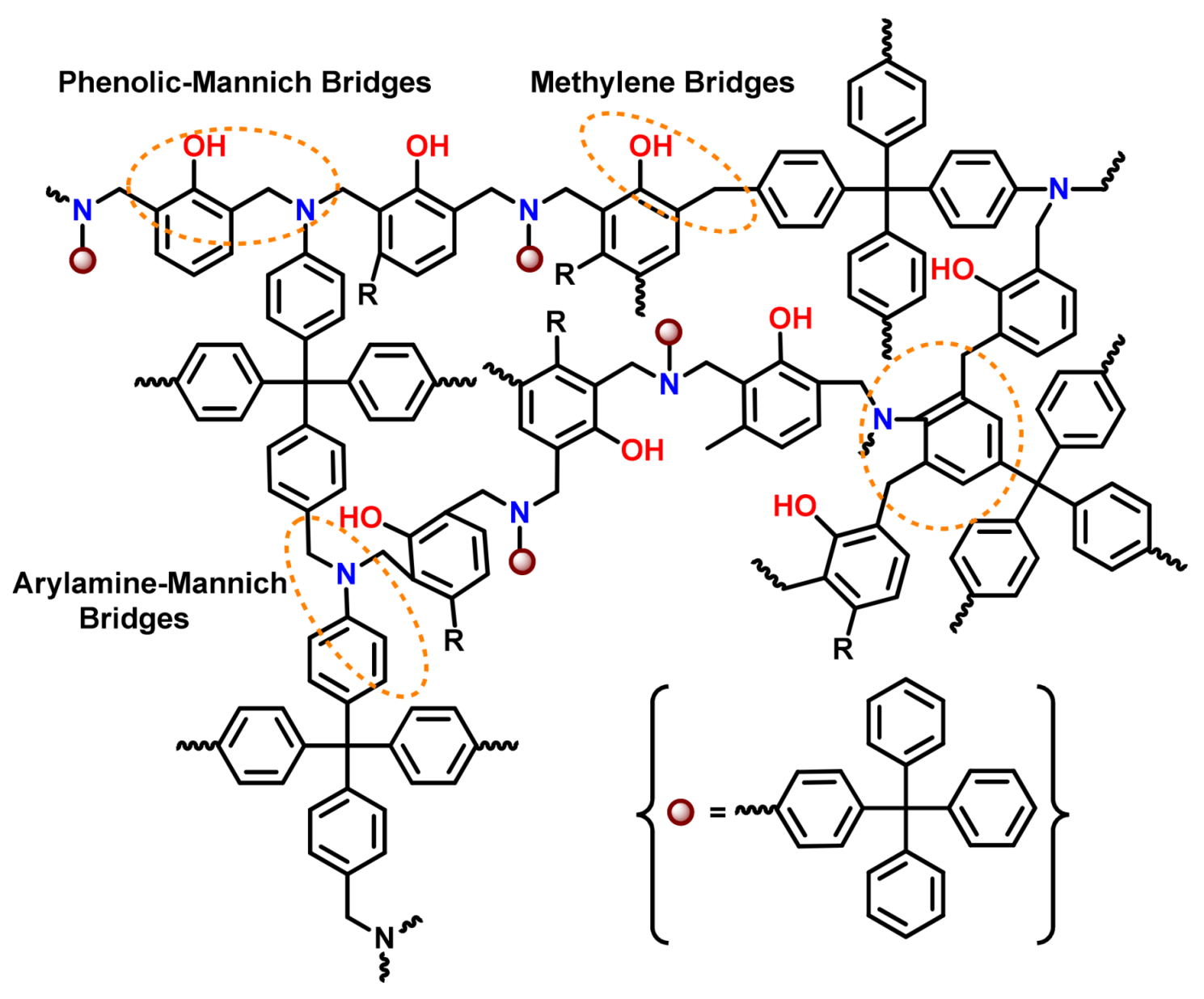
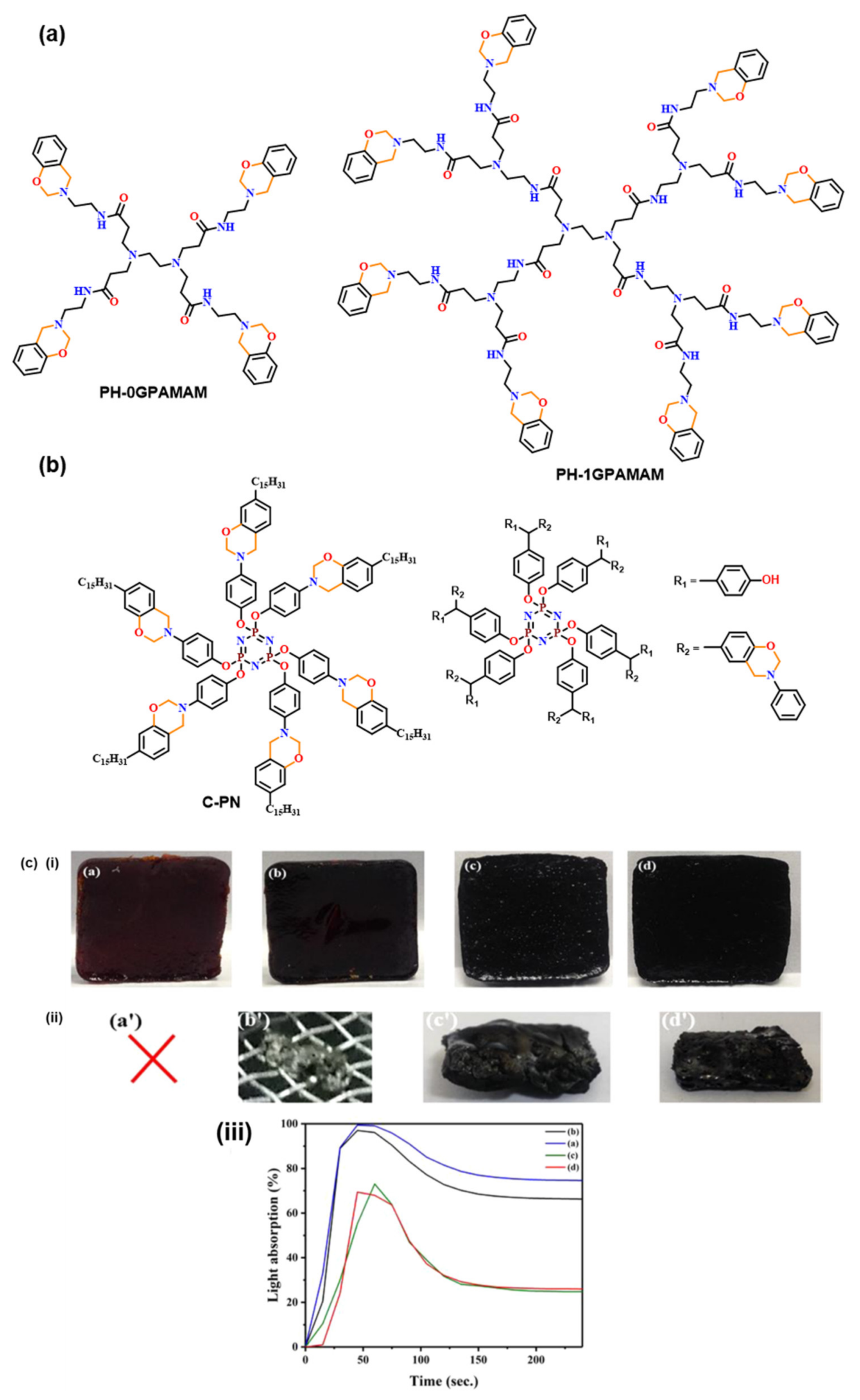
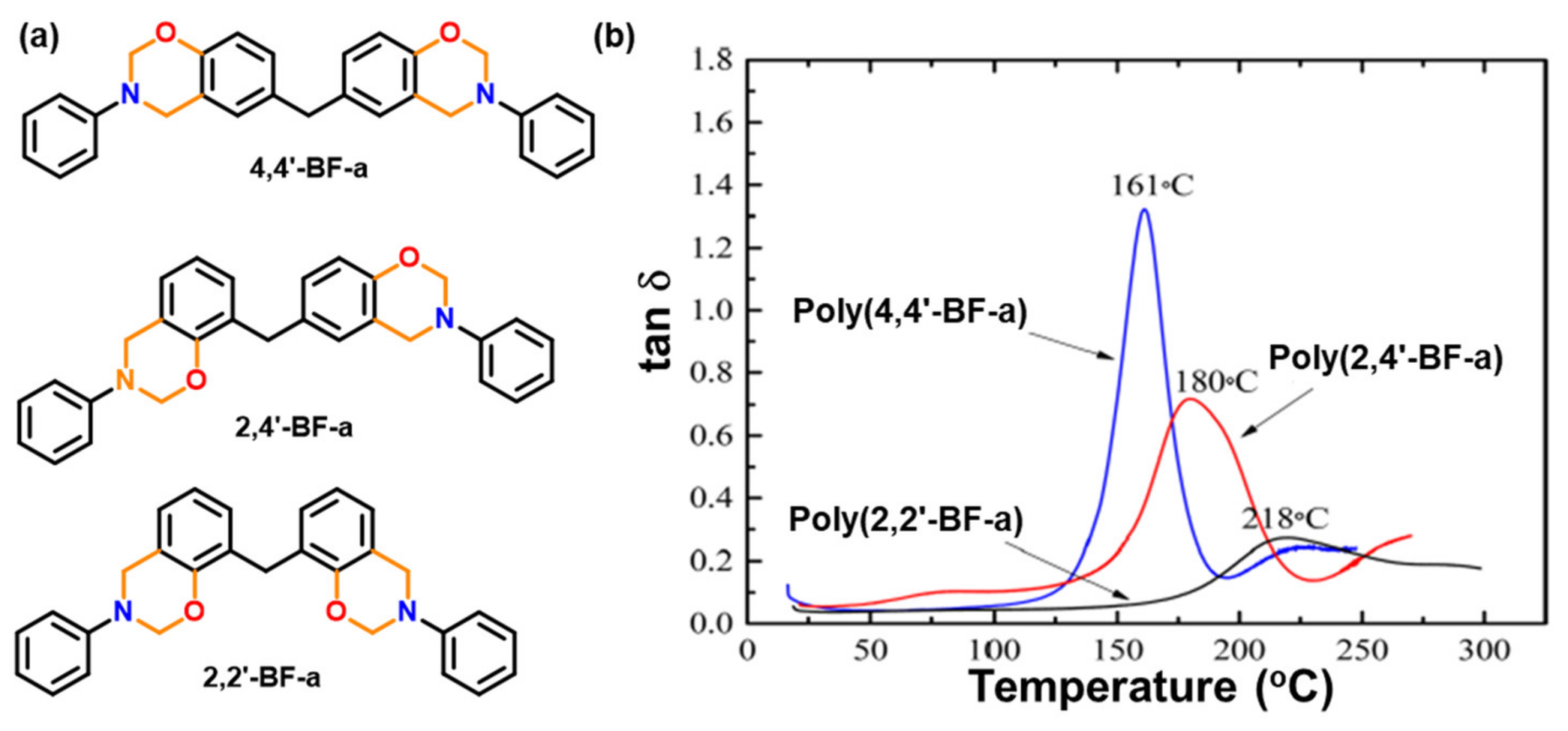

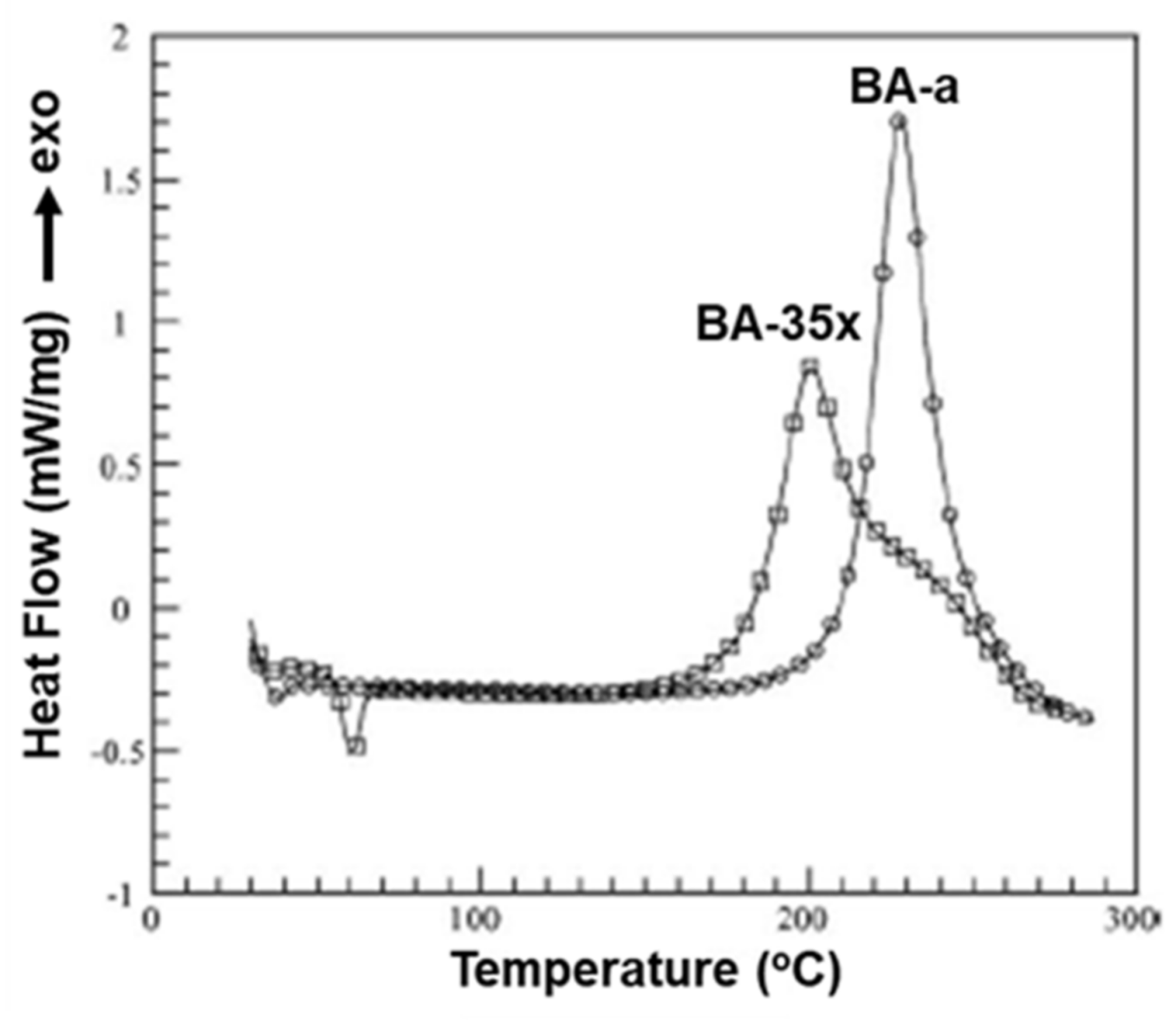
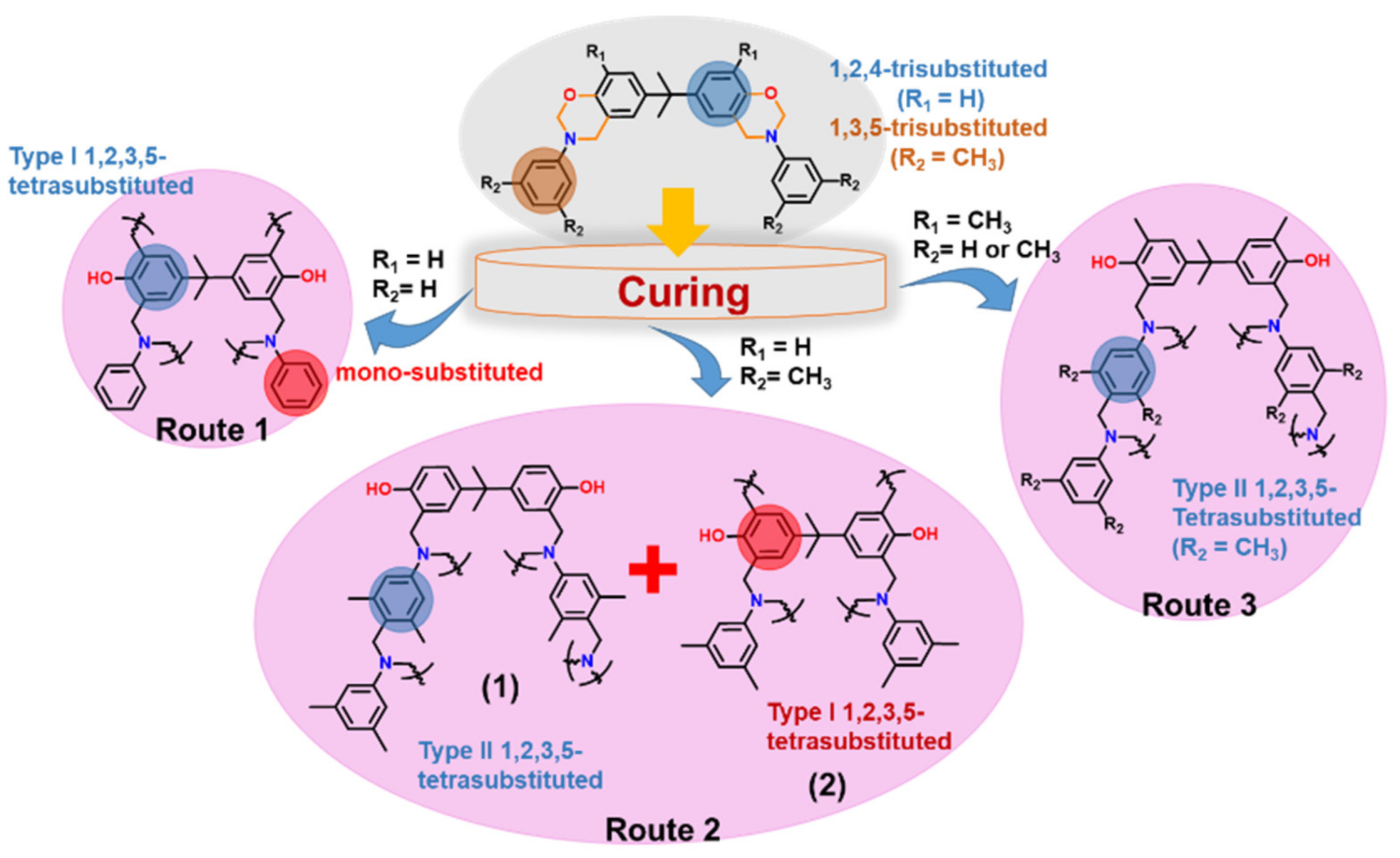


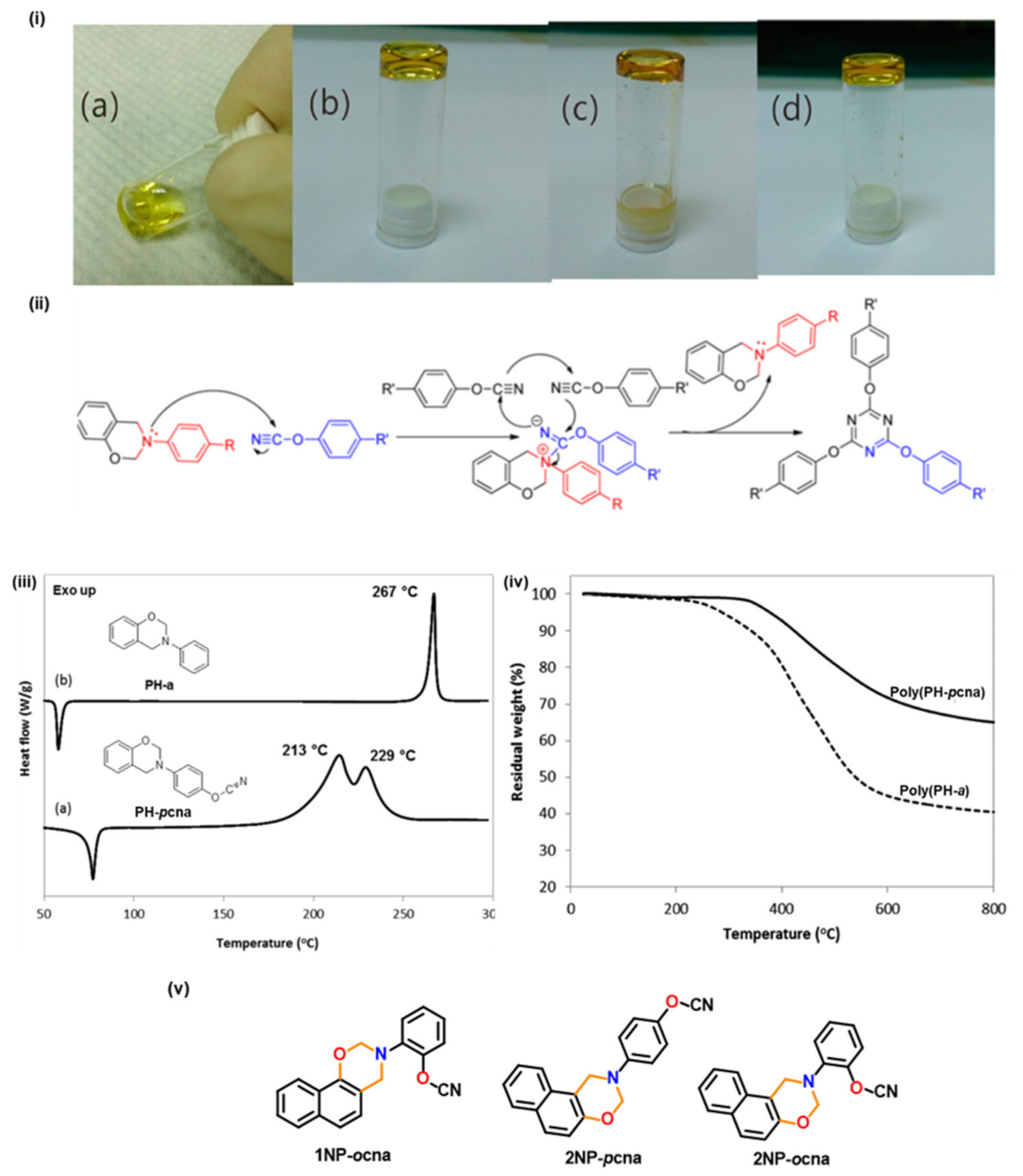
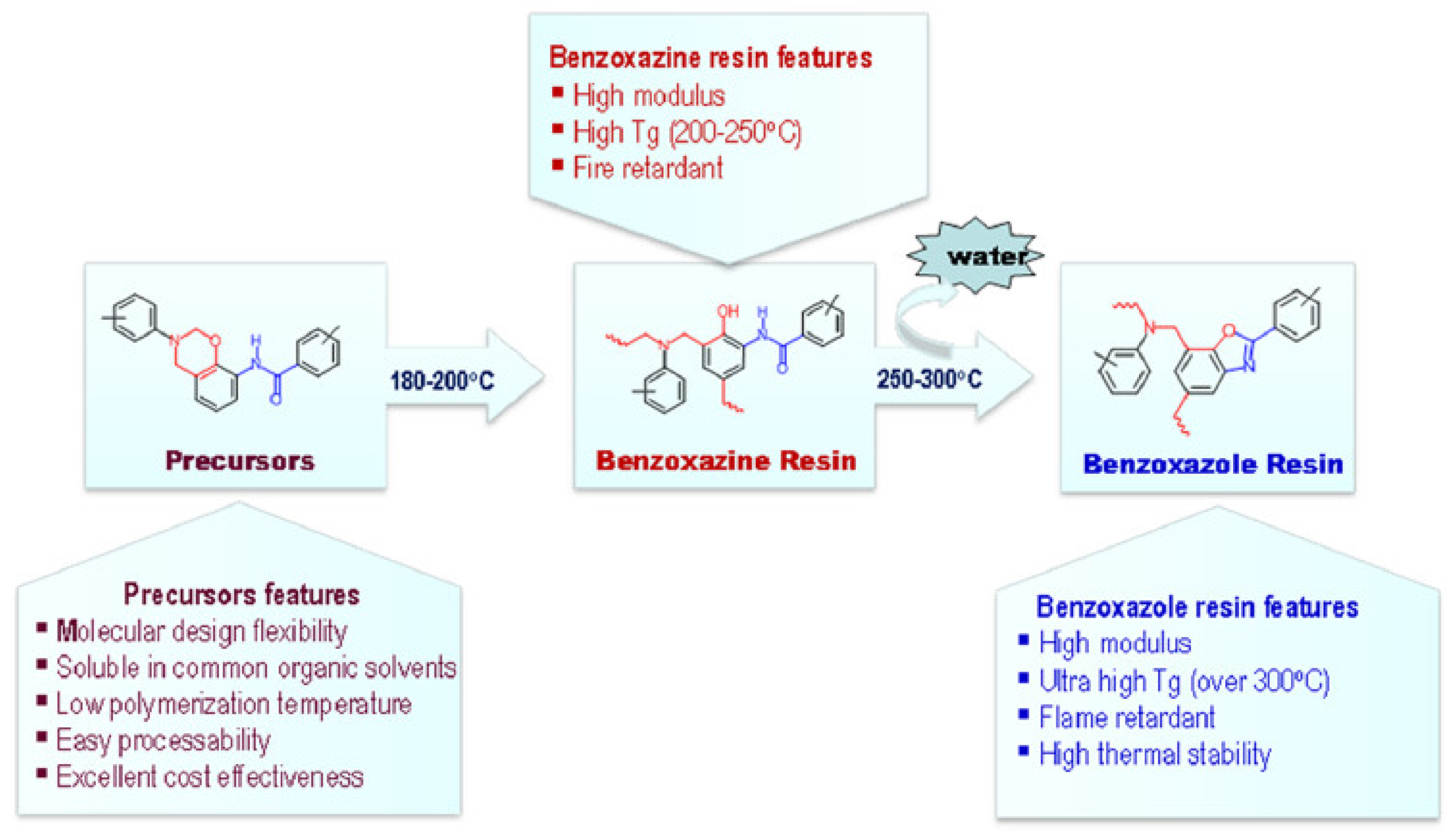


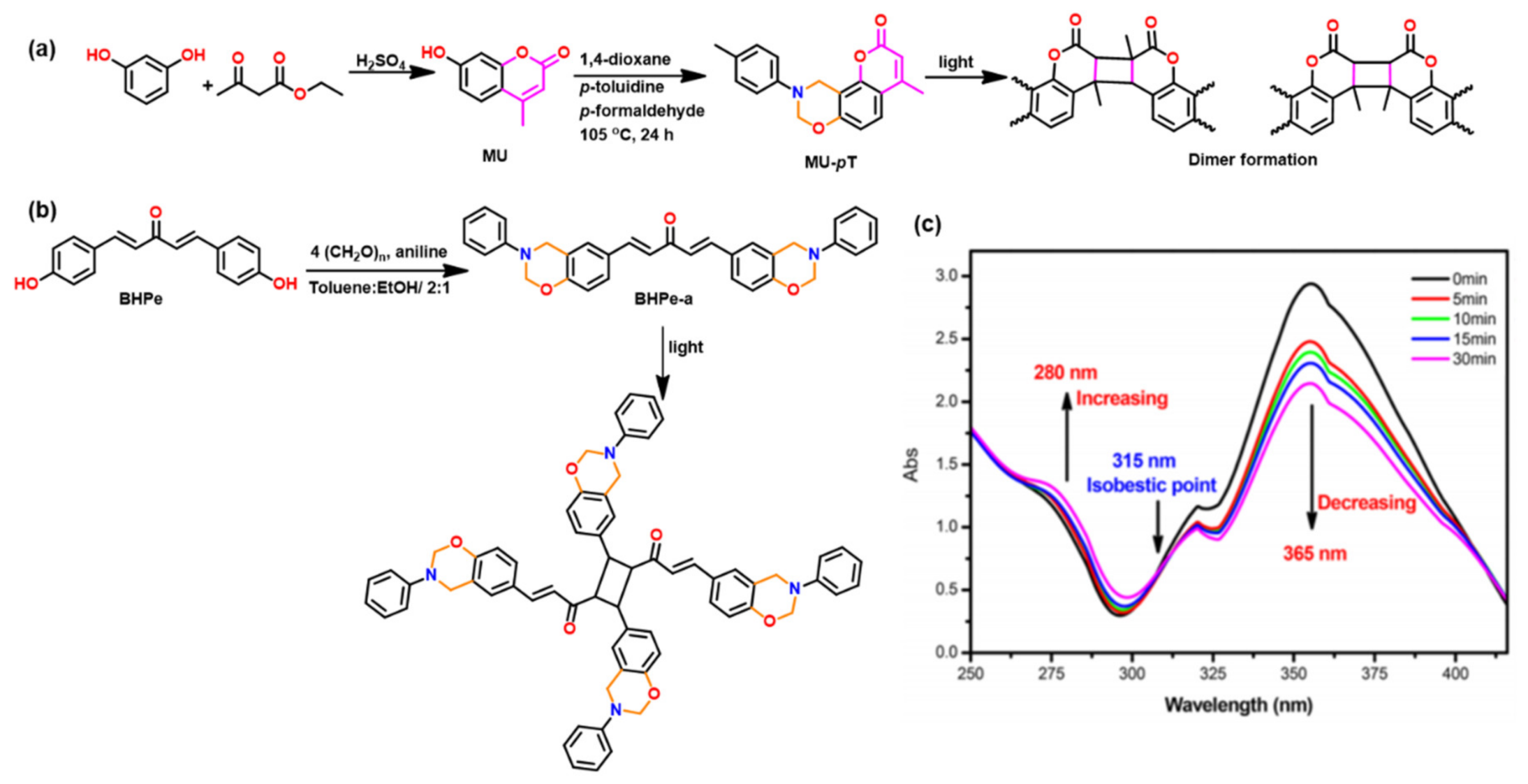
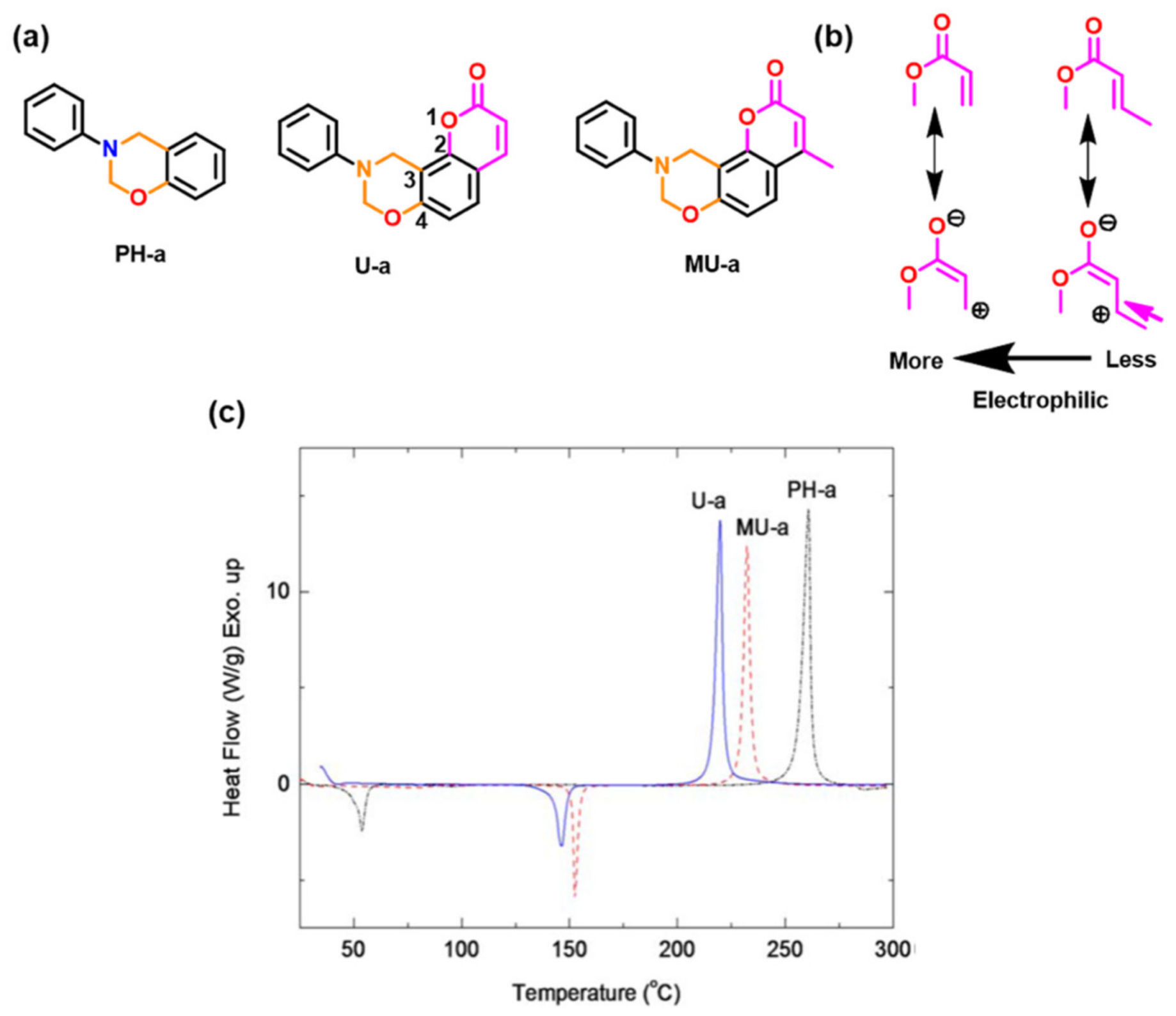


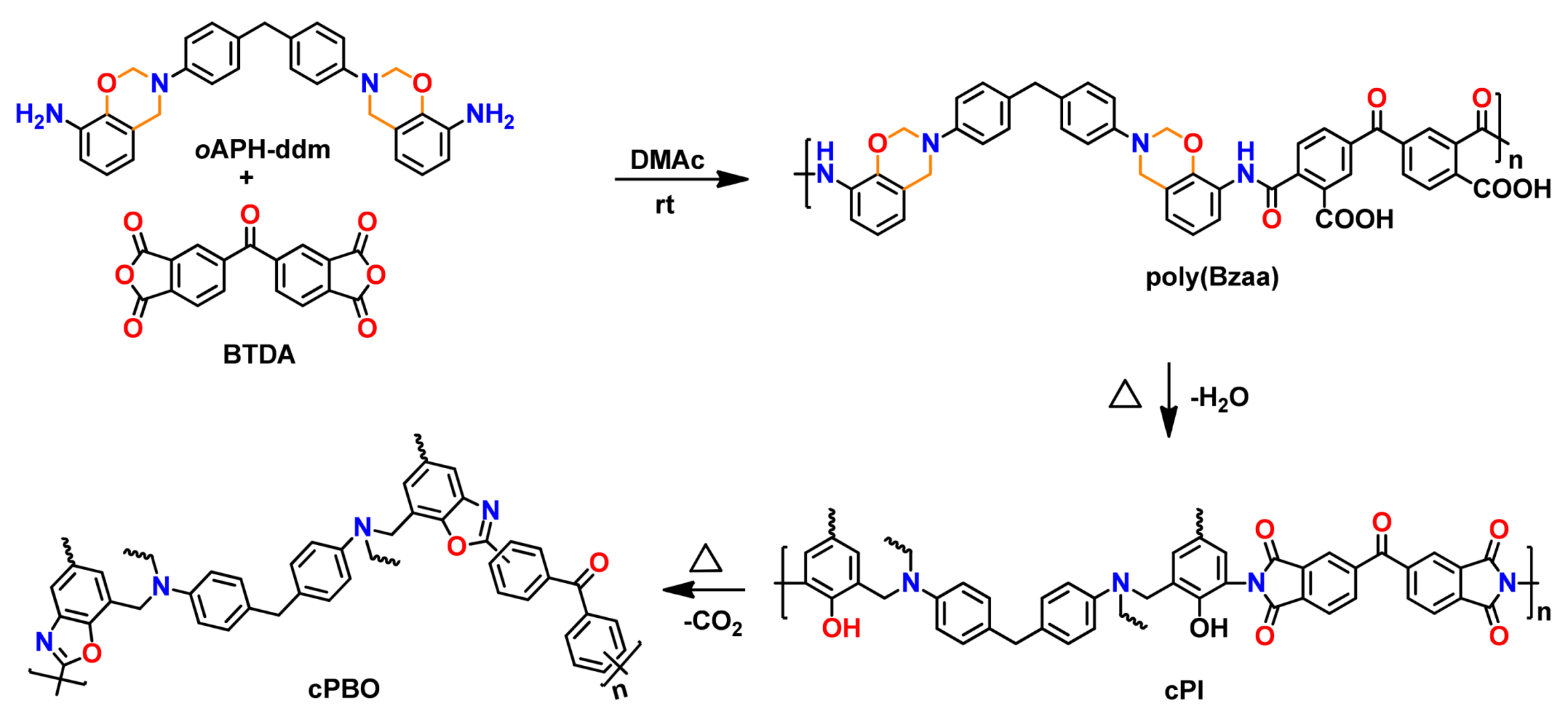
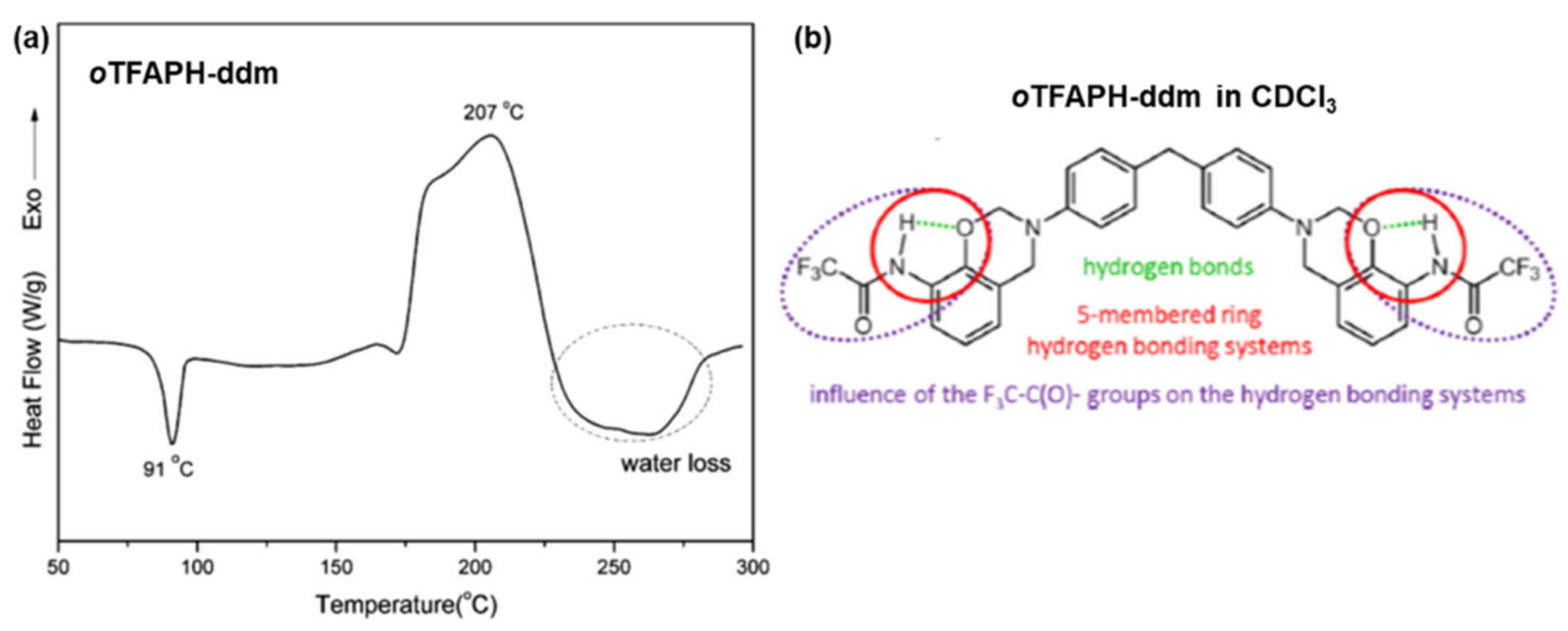



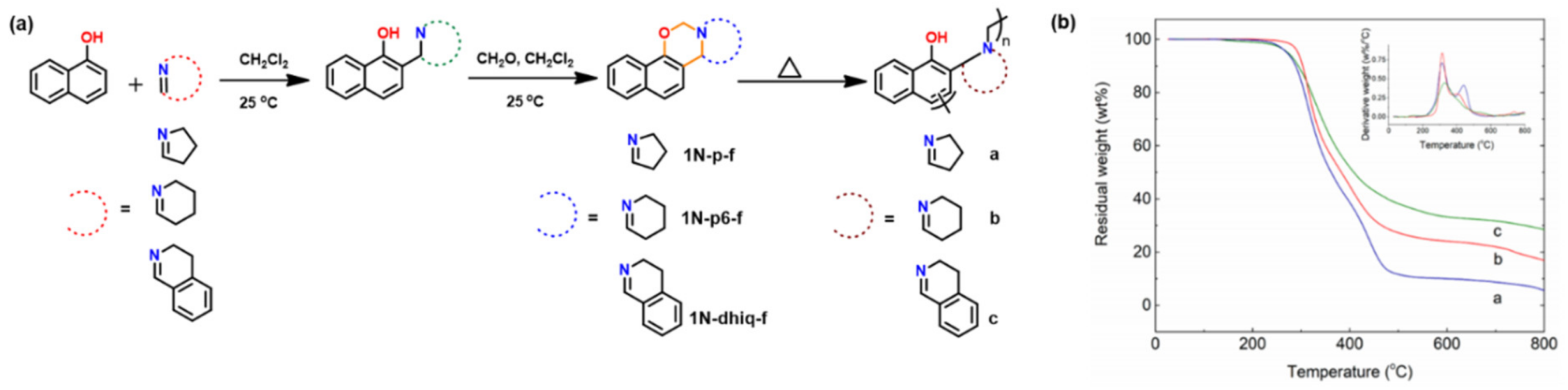
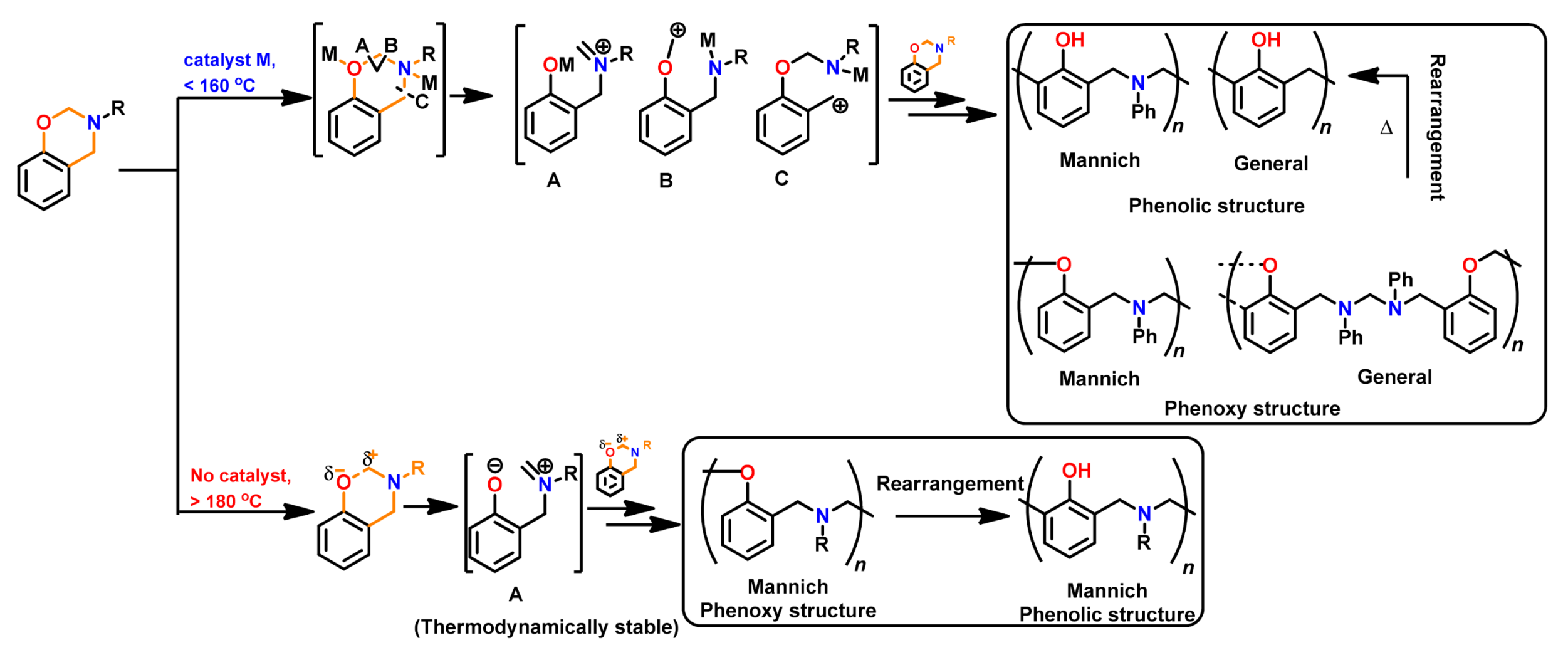
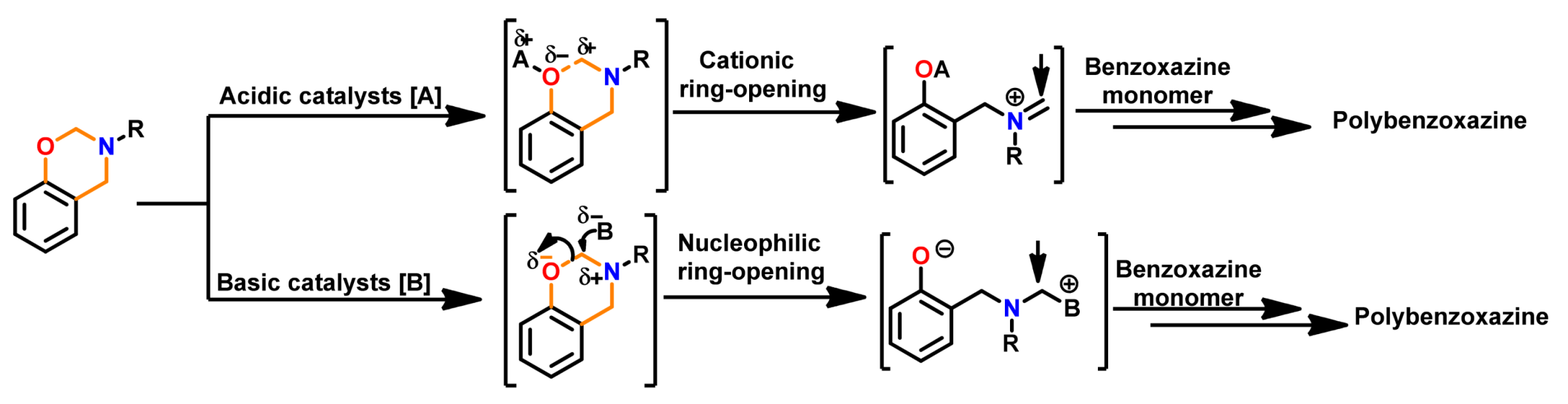

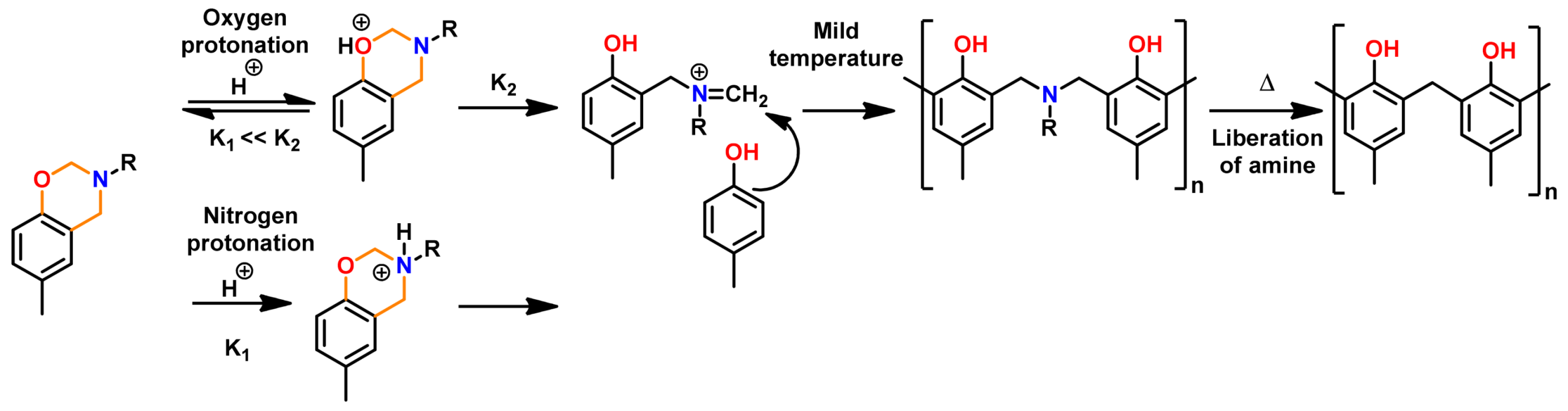

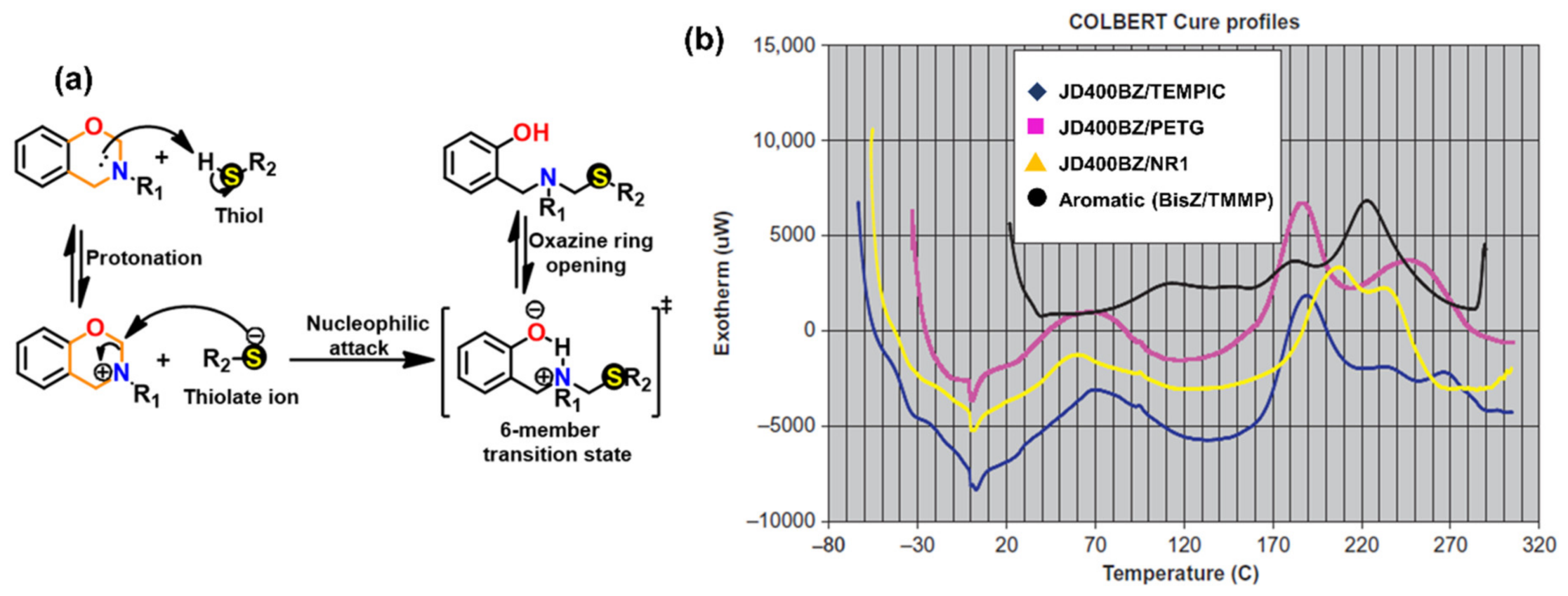

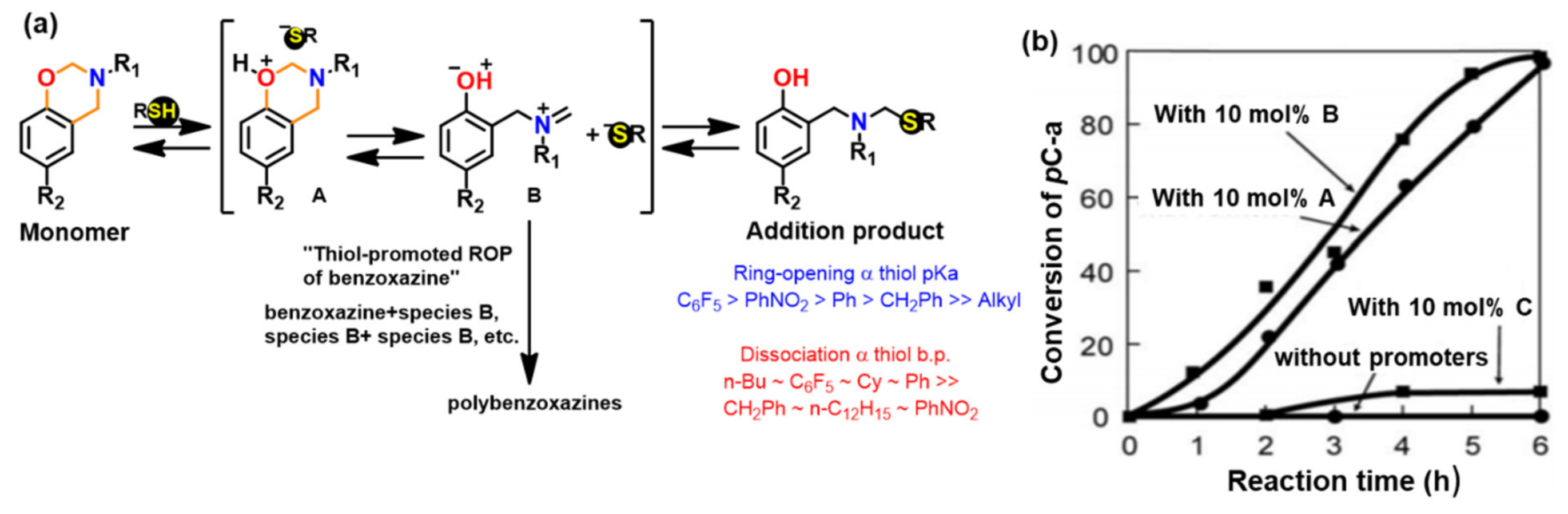
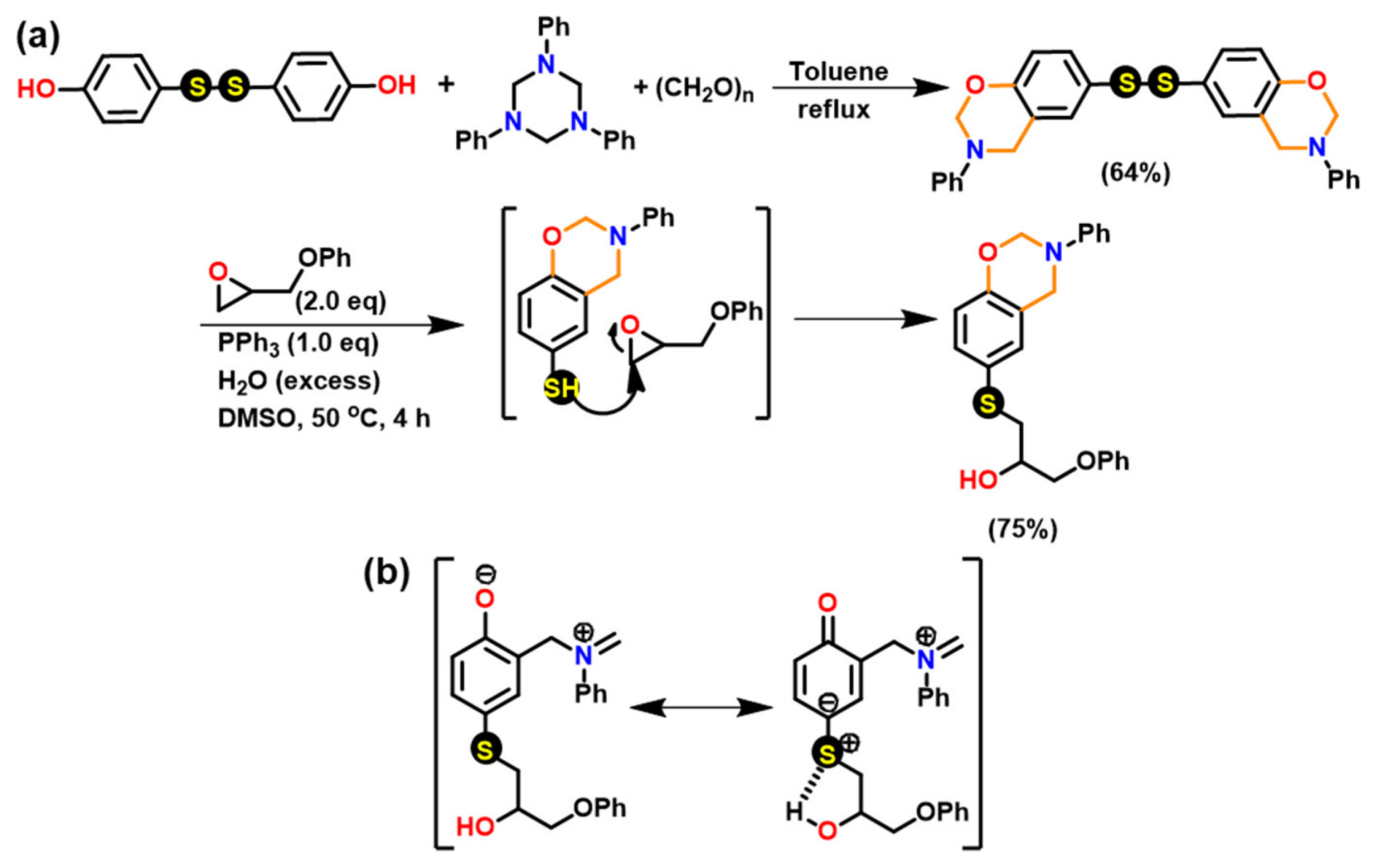

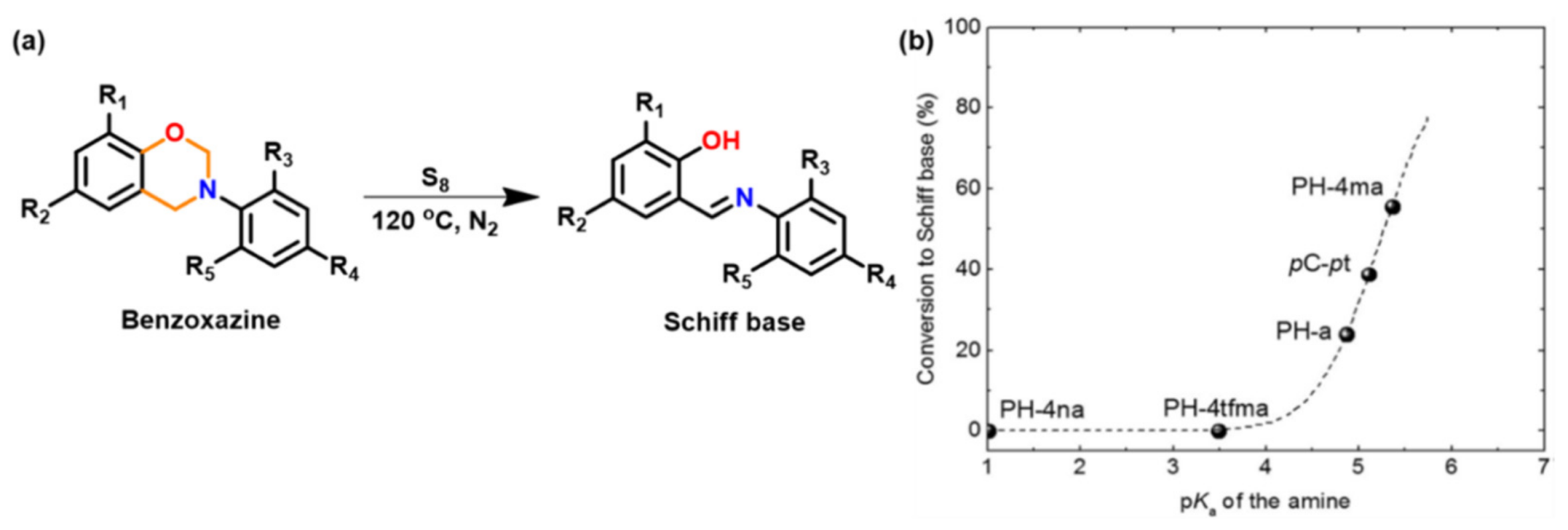

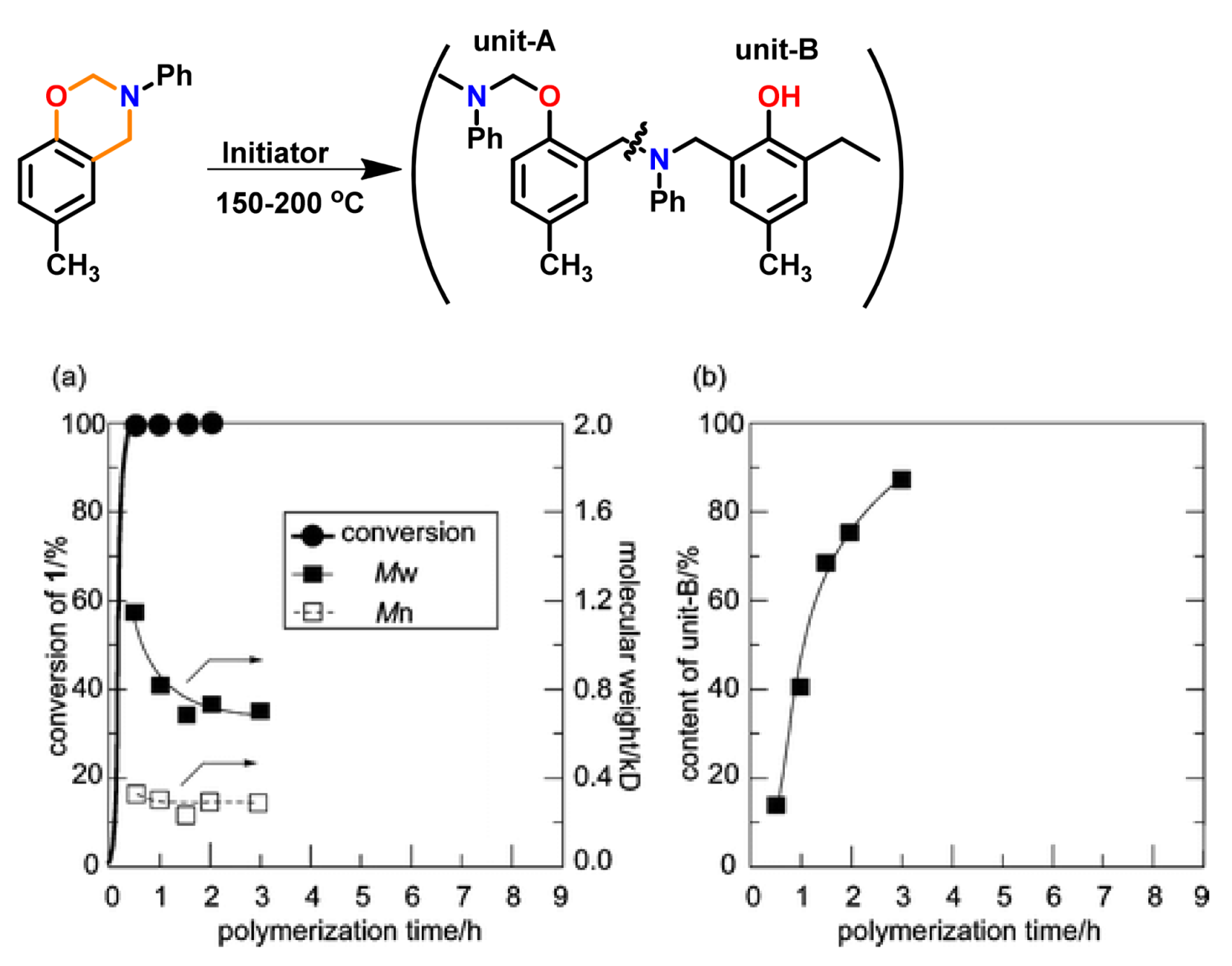
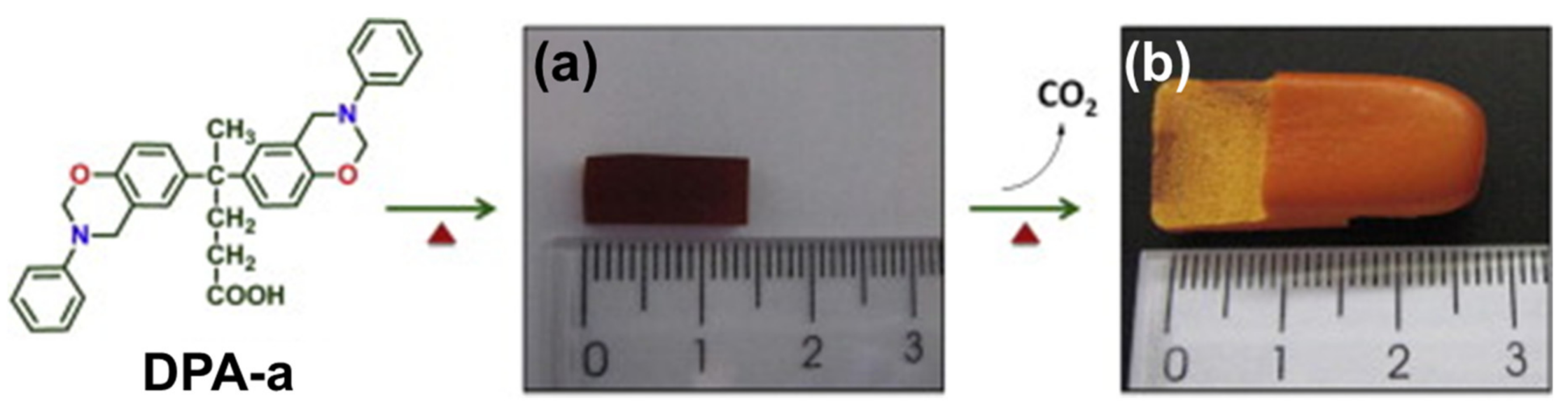

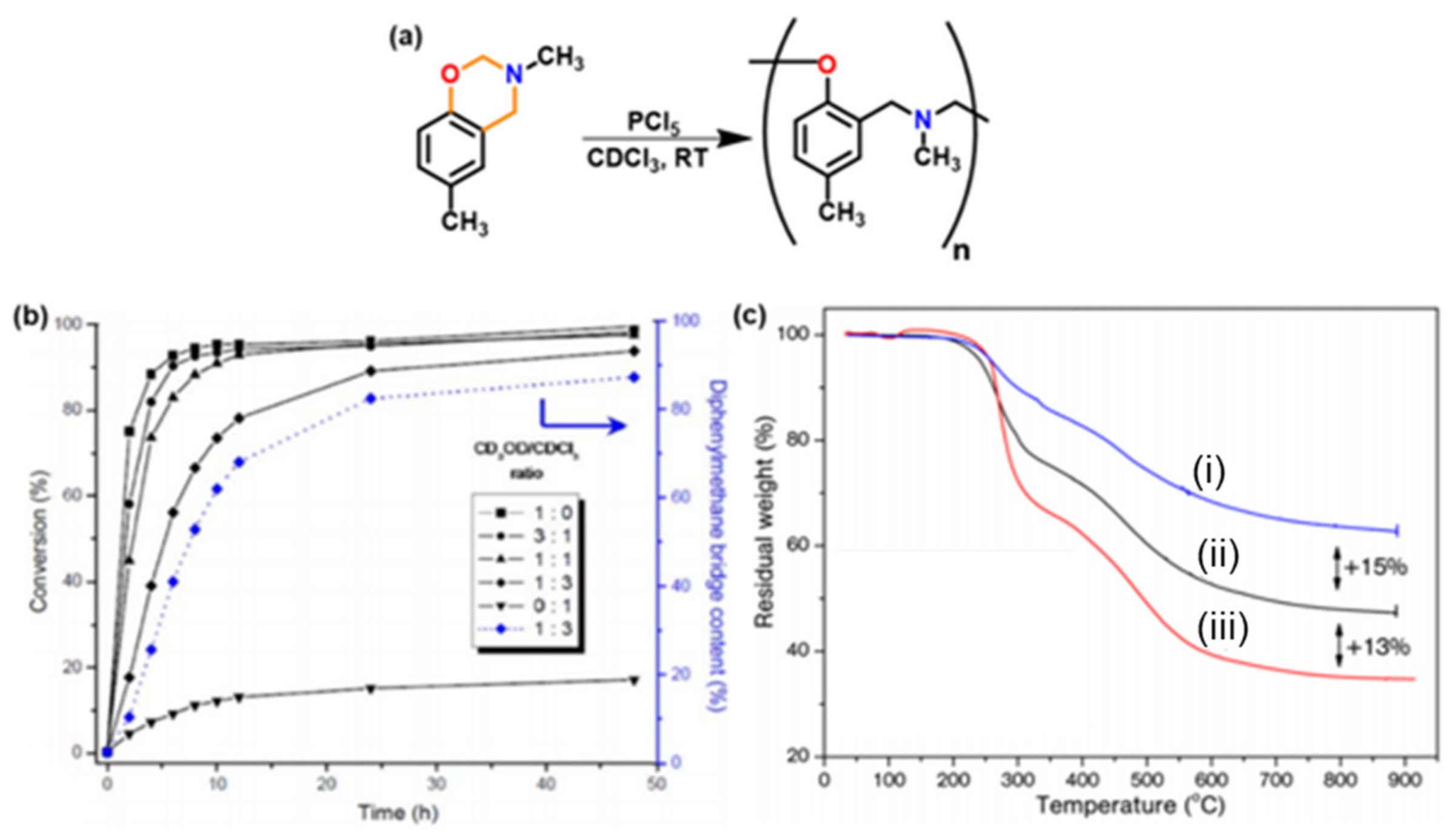

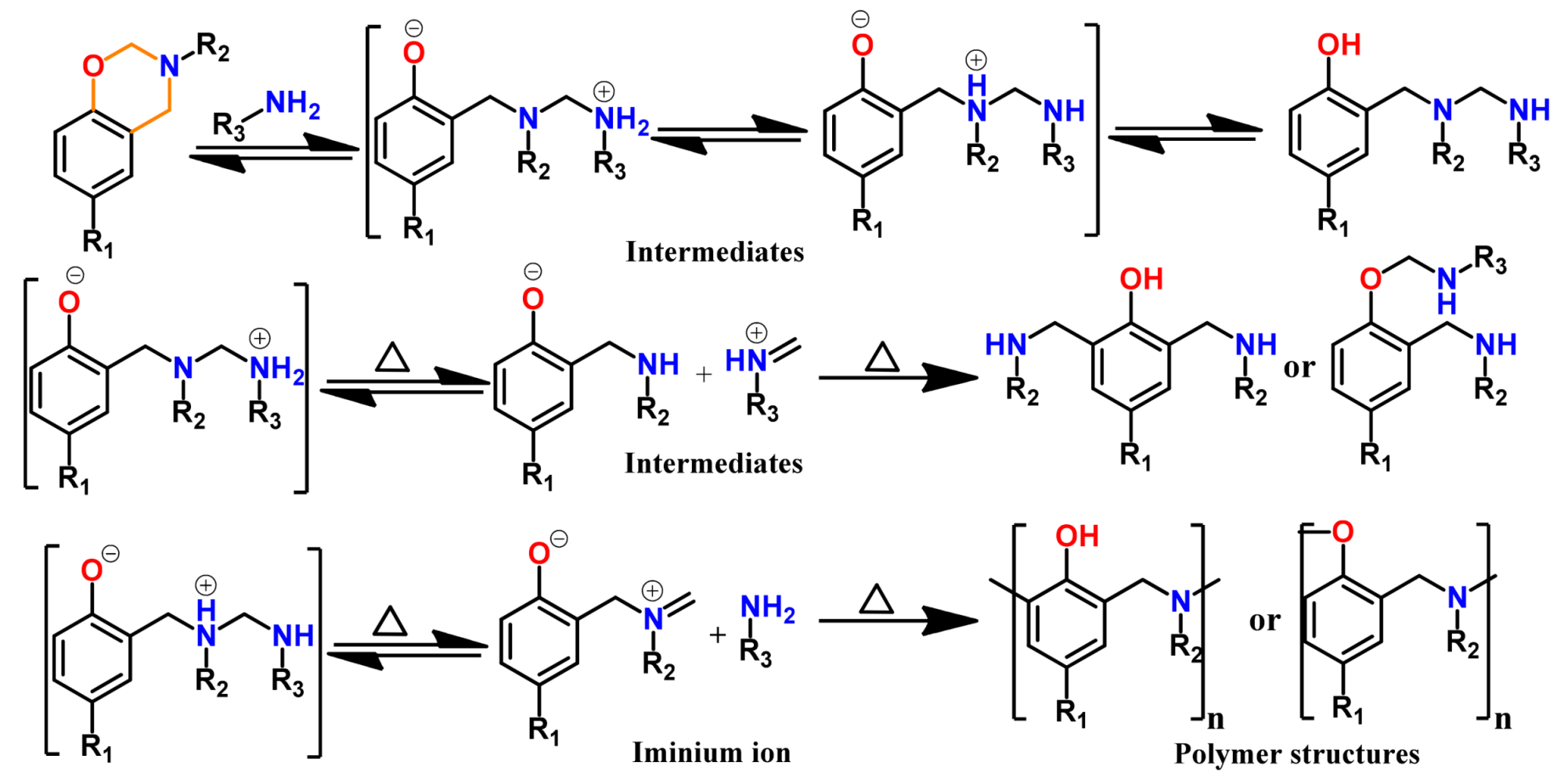
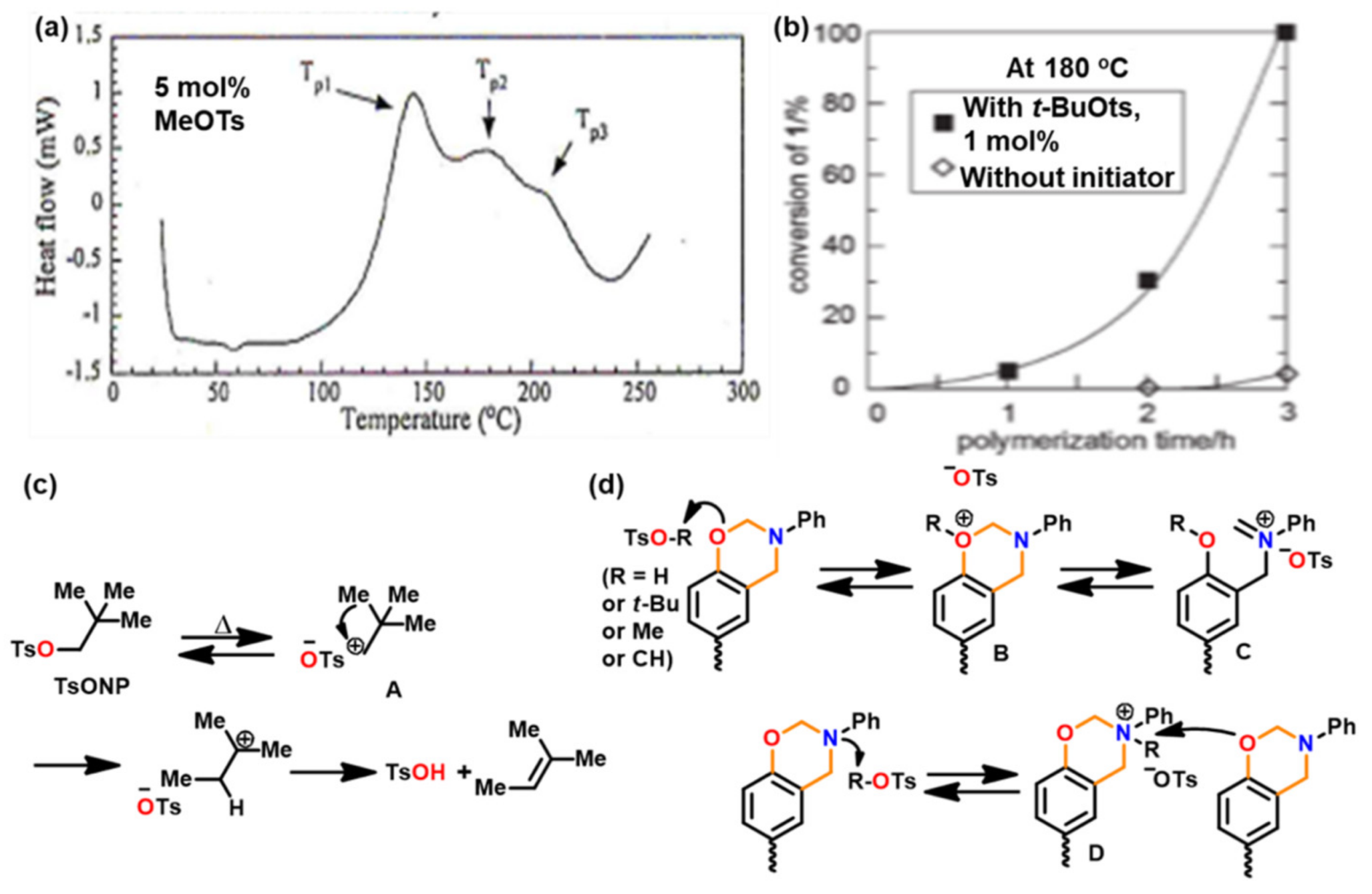
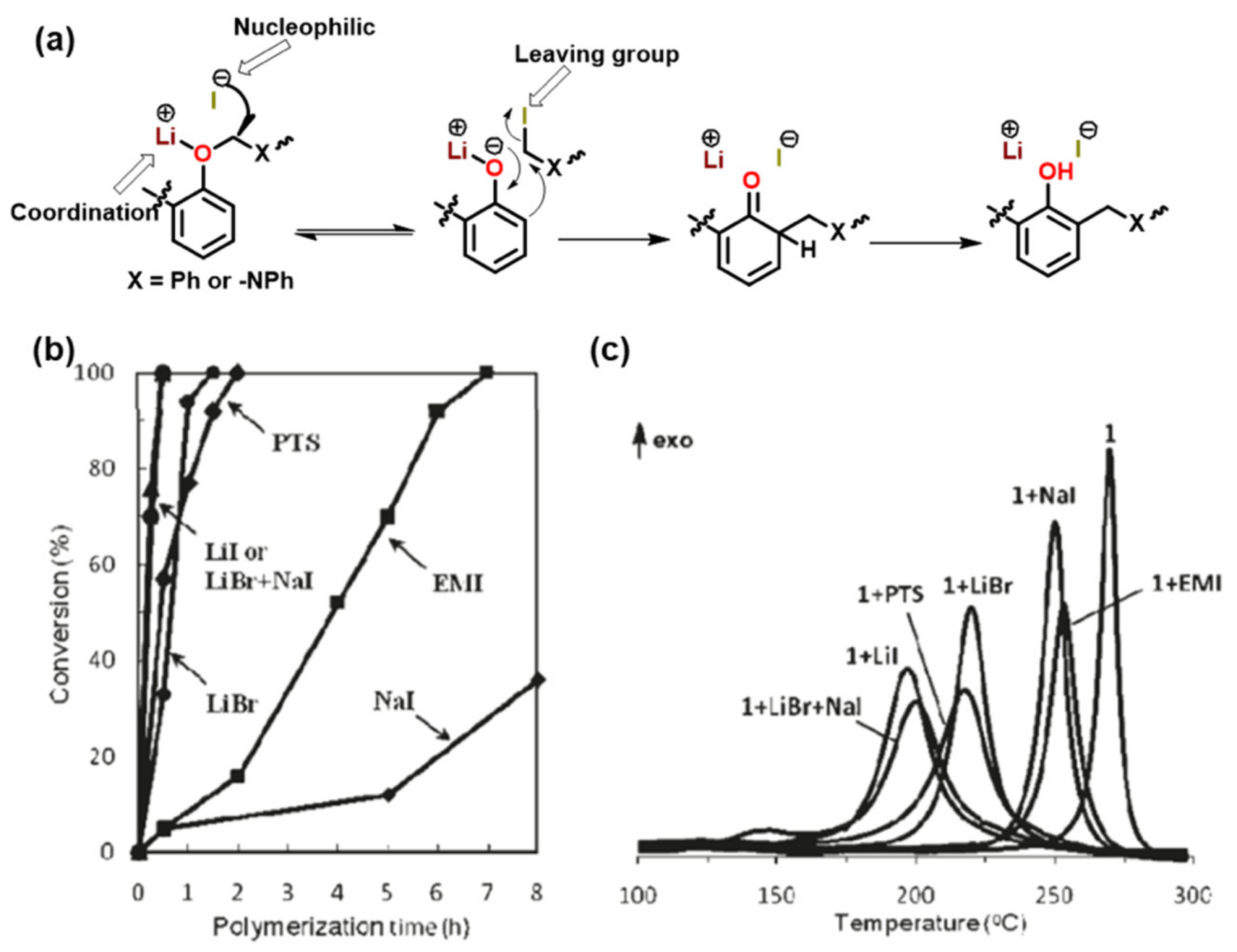
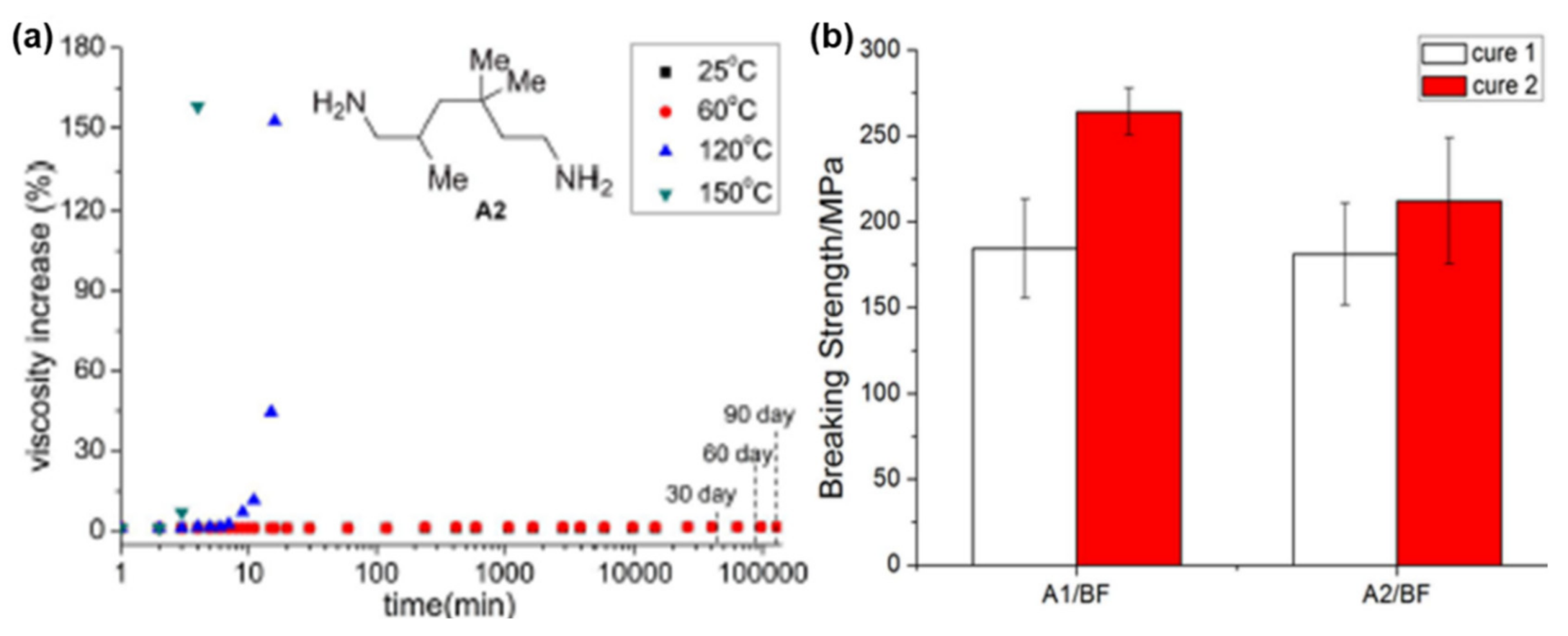
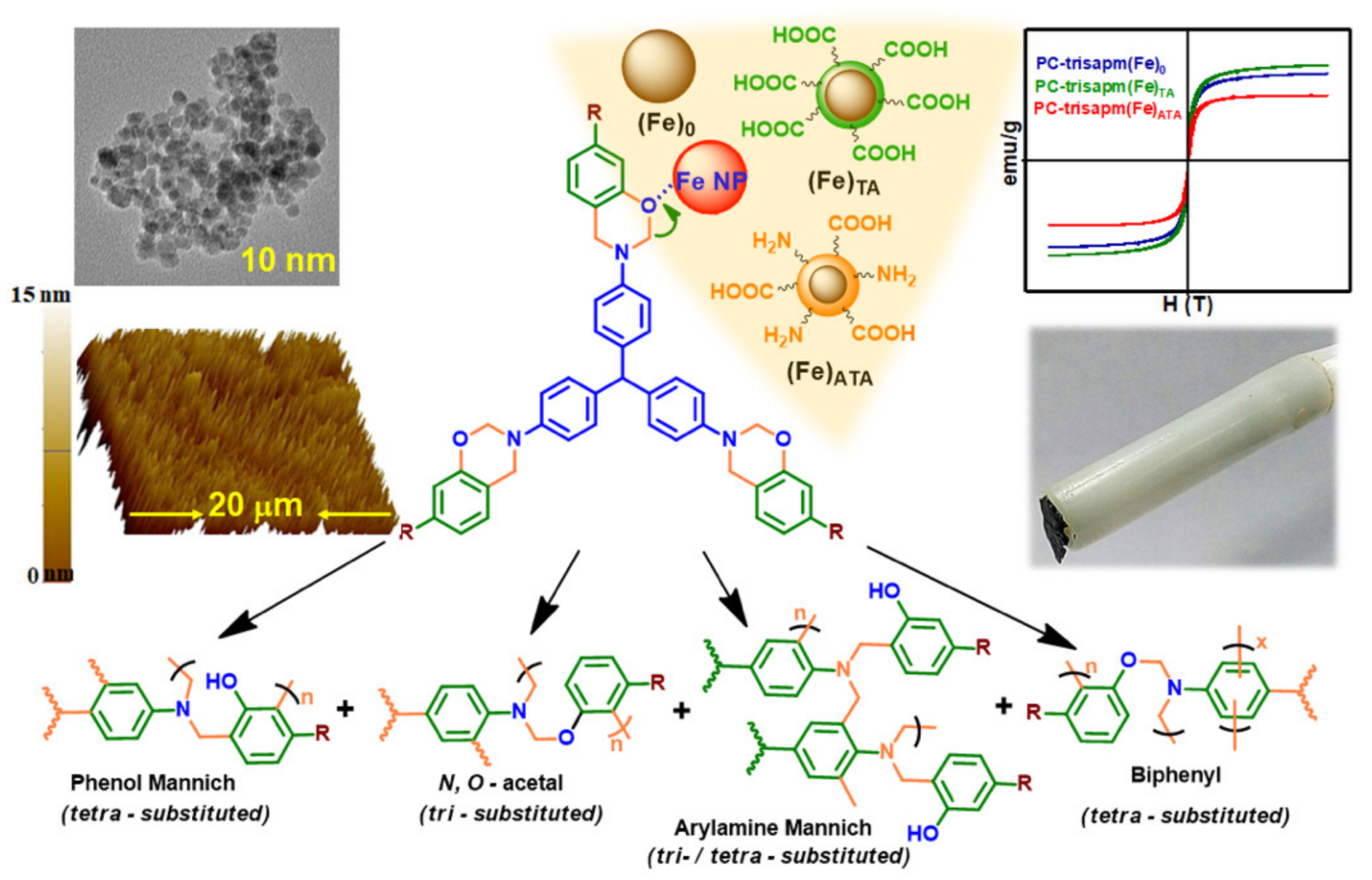

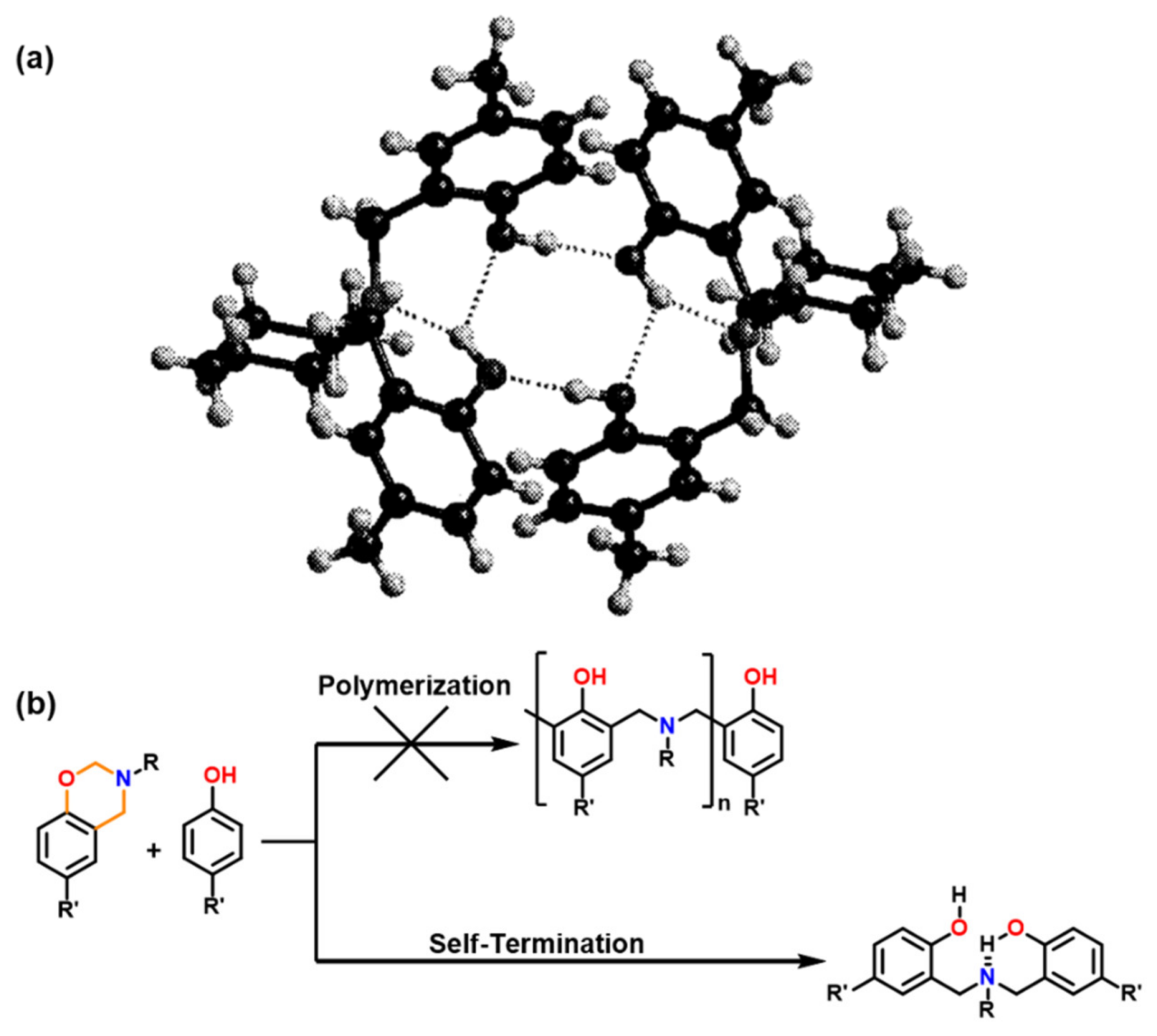
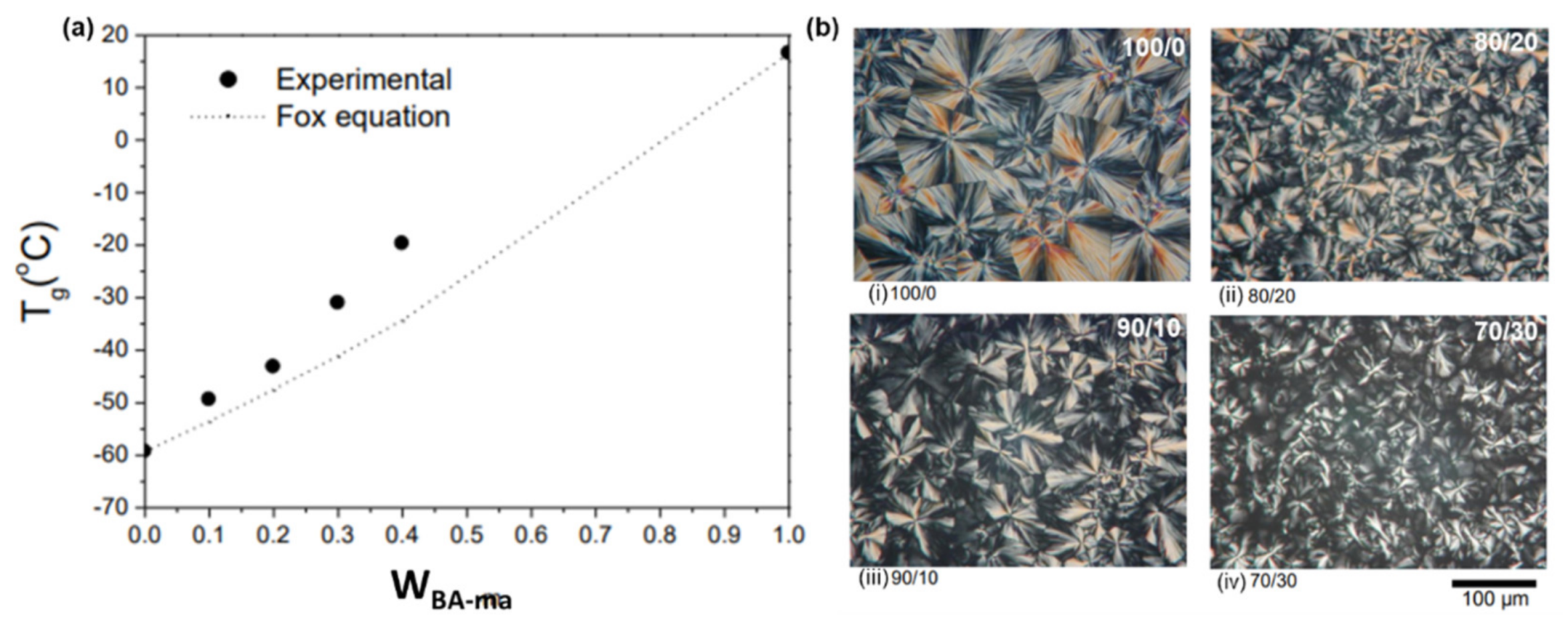



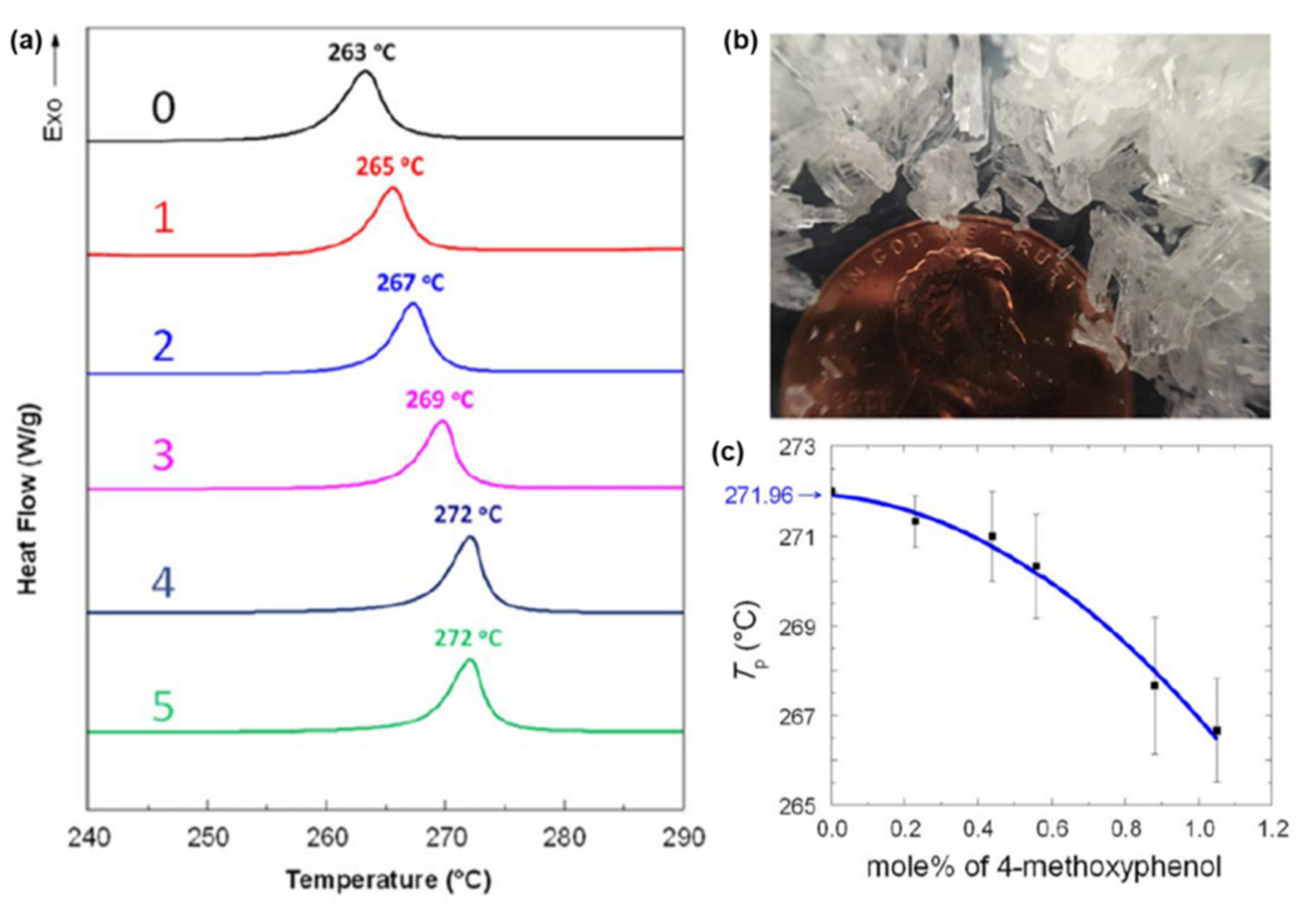

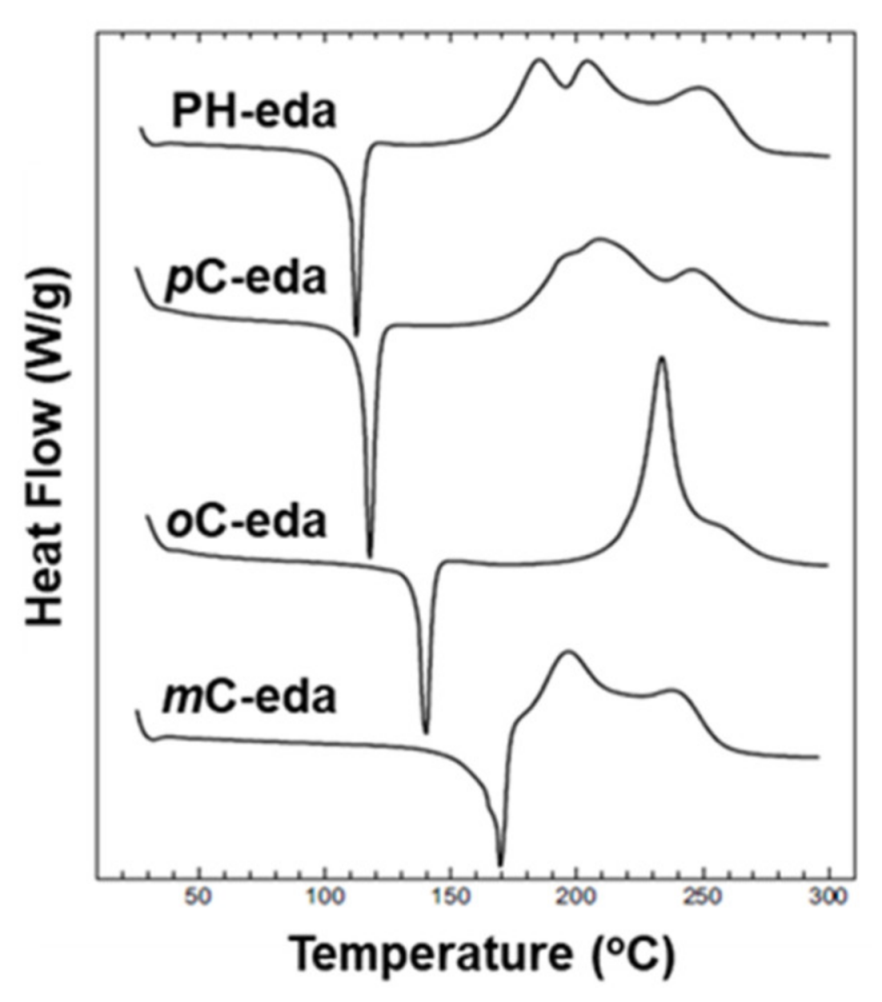
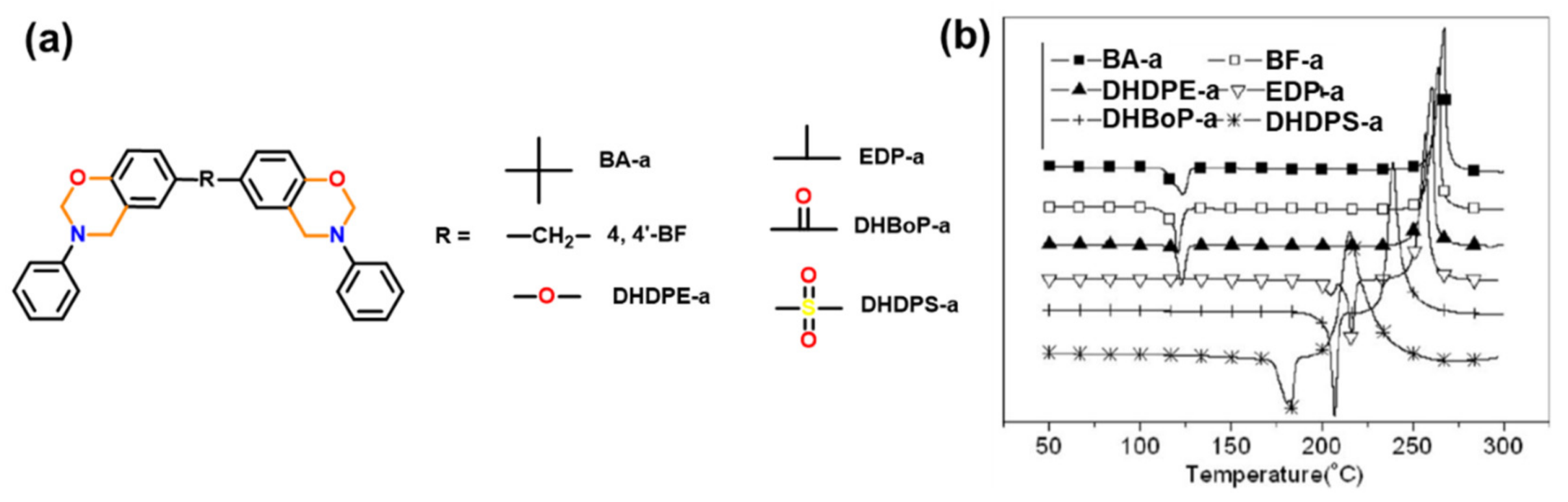
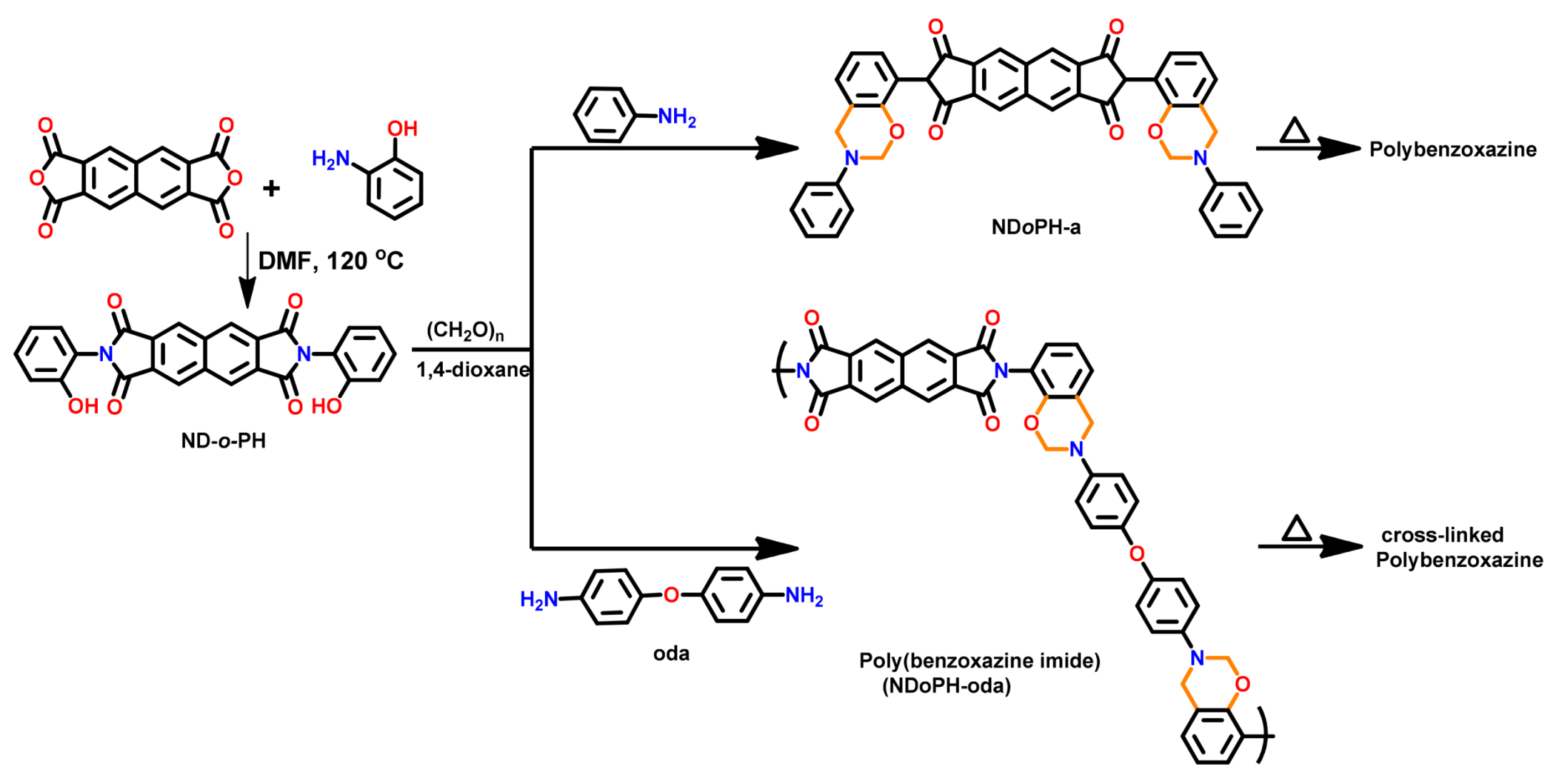
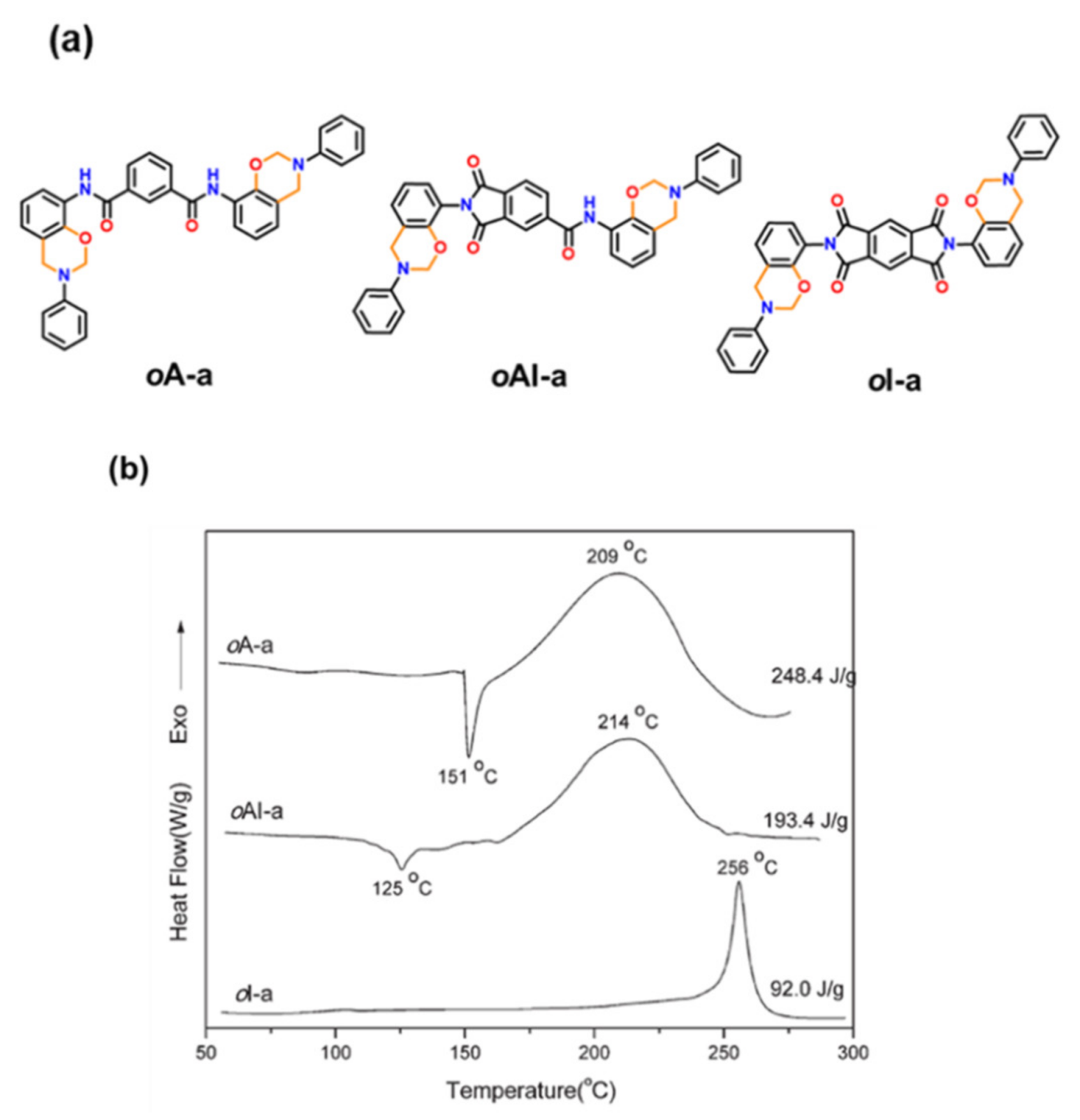

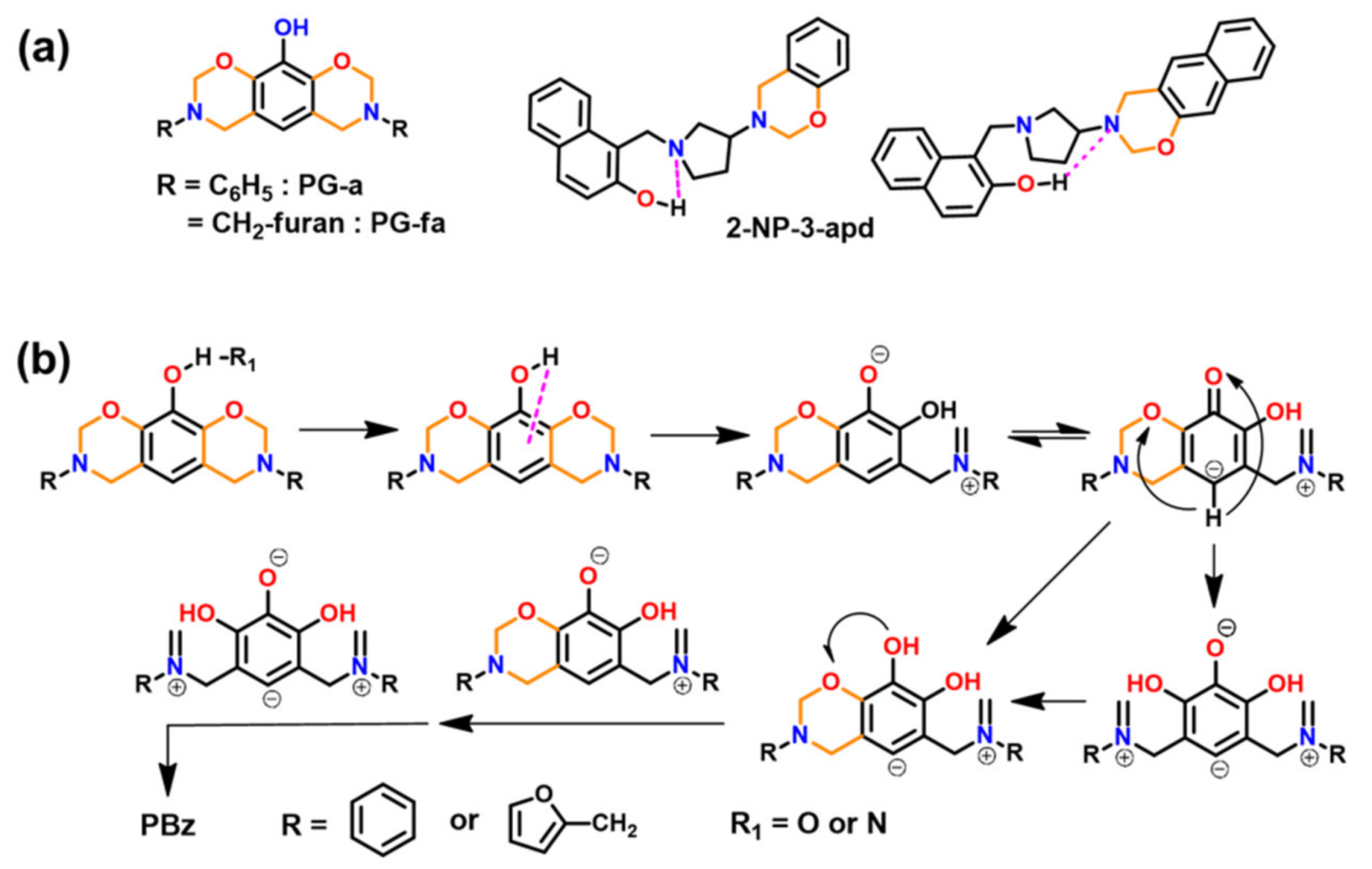

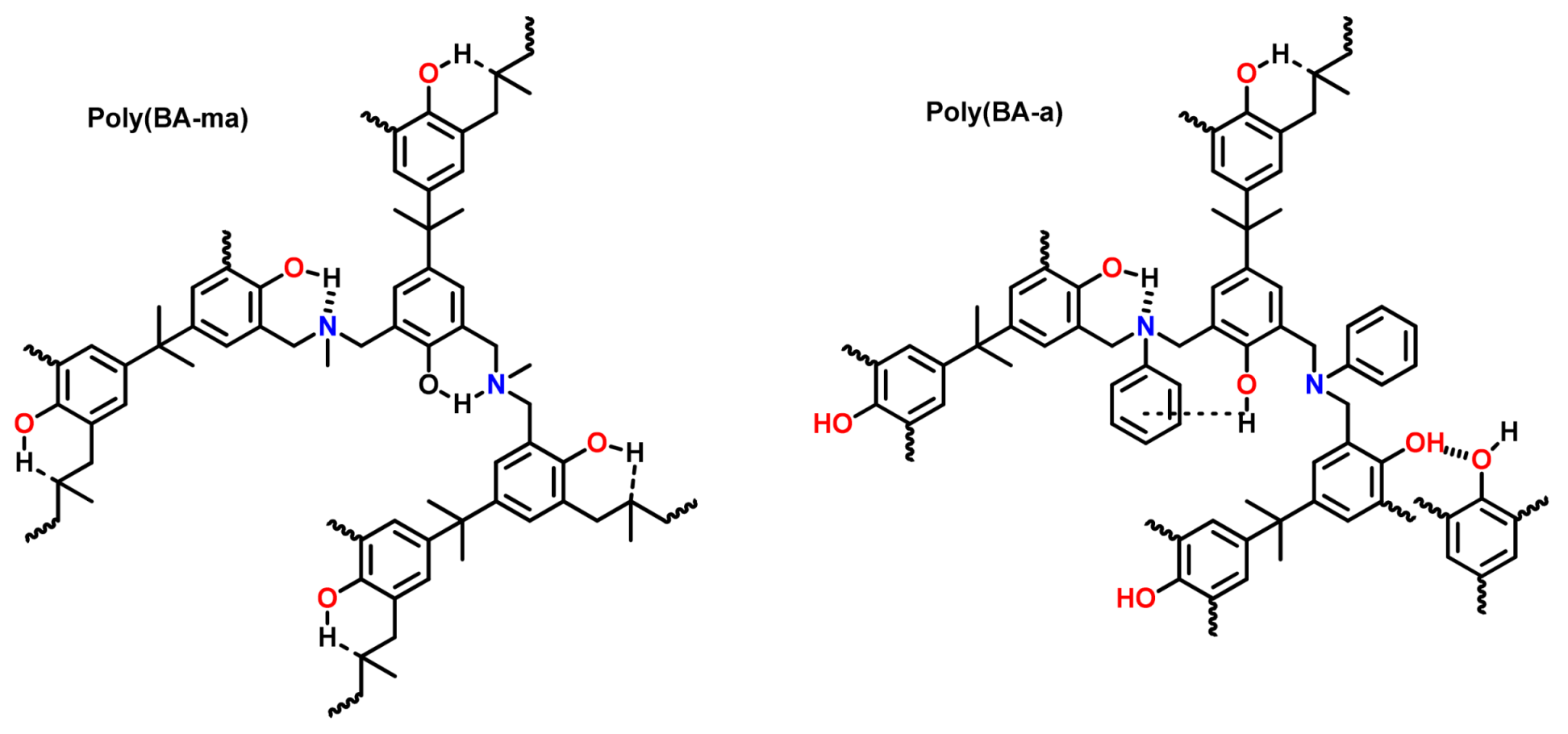
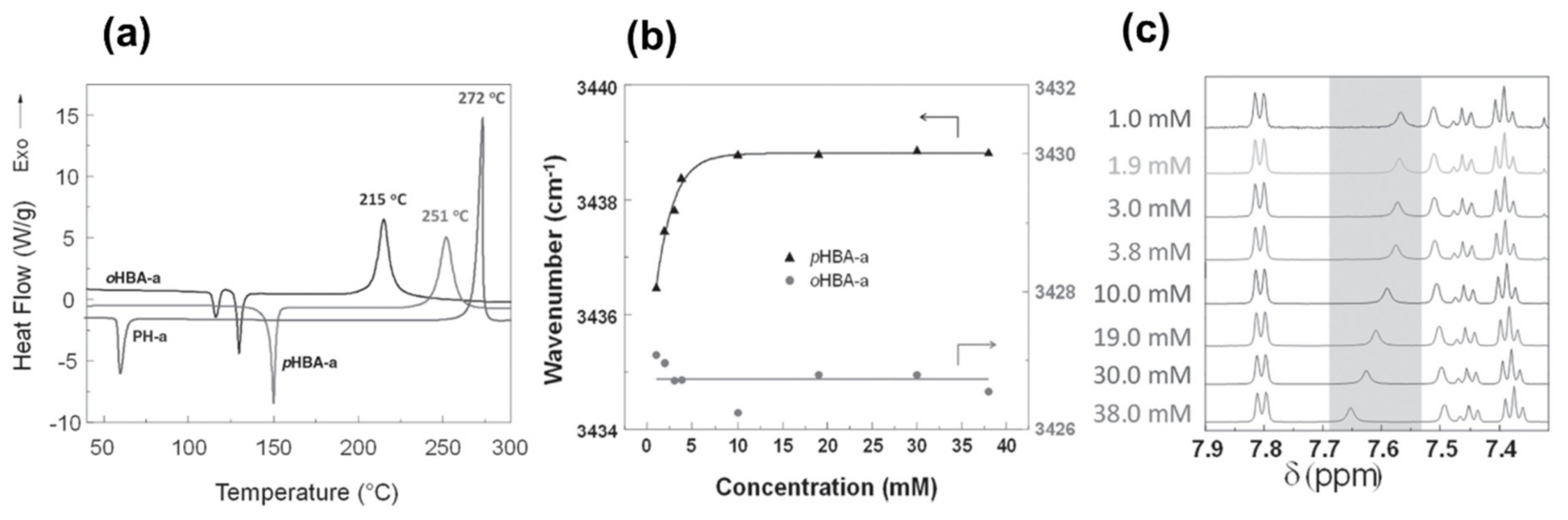
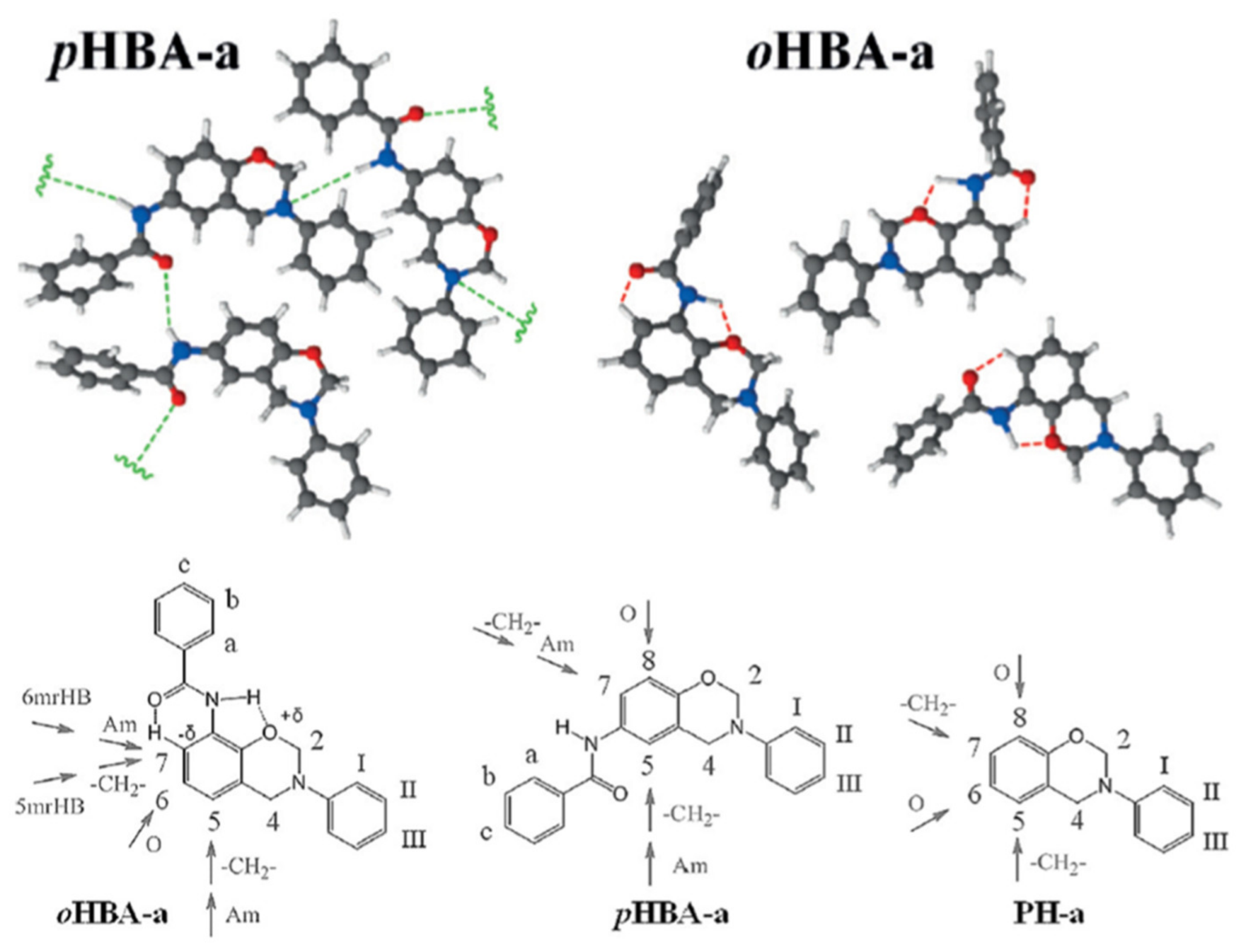
| Tga (°C) | Tp (°C) | H (J/g) | T5% (°C) | Yc | |
|---|---|---|---|---|---|
| BA-a | 168 | 251 | 340 | 315 | 30 |
| BA-ot | 114 | 247 | 289 | 228 | 32 |
| BA-mt | 209 | 231 | 325 | 350 | 31 |
| BA-pt | 158 | 259 | 310 | 305 | 32 |
| BA-35x | 243 | 217 | 298 | 350 | 28 |
| Monomer | Tp (°C) | T5% (°C) | T10% (°C) | Yc at 800 °C (%) |
|---|---|---|---|---|
| 1NP-pcna | 206 | 332 | 359 | 57 |
| 2NP-pcna | 215 | 311 | 330 | 47 |
| 1NP-ocna | 172, 186 | 302 | 323 | 51 |
| 2NP-ocna | 174,201 | 309 | 327 | 41 |
| 1NP-a [183] | 156 | 310 | 341 | 46 |
| 2NP-a [183] | 255 | 216 | 236 | 20 |
| PH-a [181] | 267 | 294 | 347 | 40 |
| Tgc (°C) | T5% (°C) | T10% (°C) | Yc (%) | |
|---|---|---|---|---|
| poly(BHPICA-a) a | 332 | 406 | 476 | 63 |
| poly(BHPICA-ddm) a | >400 | 410 | 473 | 52 |
| poly(BHPICA-a)-400 b | - | 536 | 589 | 71 |
| poly(BHPICA-ddm)-400 b | - | 503 | 555 | 60 |
| T5% (°C) | Ti (°C) | T50% (°C) | Tp (°C) | Yc (700 °C) | |
|---|---|---|---|---|---|
| BA/ECC | 310 | 310 | 382 | 373 | 20 |
| HBP-AMIM+PF6−-2 (1–7 wt%) | 332–341 | 352–359 | 404–415 | 417–422 | 27–30 |
| HBP-AMIM+PF6−-1 (3 wt%) | 332 | 359 | 413 | 416 | 25 |
| HBP-AMIM+PF6−-3 (3 wt%) | 345 | 362 | 417 | 425 | 29 |
| Free OH | OH-p Intra HB | OH-O Intra HB | OH-N Intra HB | OH-O Inter HB | |
|---|---|---|---|---|---|
| 3615 cm−1 | 3559 cm−1 | 3467 cm−1 | 3000 cm−1 | 3364 cm−1 | |
| Asym. Methyl-dimer | - | - | - | 1.00 | - |
| Methyl-dimer | 0.09 | 0.14 | 0.11 | 0.53 | 0.13 |
| Methyl-trimer | 0.04 | 0.11 | 0.09 | 0.67 | 0.10 |
| Methyl-tetramer | 0.01 | 0.04 | 0.02 | 0.88 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lochab, B.; Monisha, M.; Amarnath, N.; Sharma, P.; Mukherjee, S.; Ishida, H. Review on the Accelerated and Low-Temperature Polymerization of Benzoxazine Resins: Addition Polymerizable Sustainable Polymers. Polymers 2021, 13, 1260. https://doi.org/10.3390/polym13081260
Lochab B, Monisha M, Amarnath N, Sharma P, Mukherjee S, Ishida H. Review on the Accelerated and Low-Temperature Polymerization of Benzoxazine Resins: Addition Polymerizable Sustainable Polymers. Polymers. 2021; 13(8):1260. https://doi.org/10.3390/polym13081260
Chicago/Turabian StyleLochab, Bimlesh, Monisha Monisha, Nagarjuna Amarnath, Pratibha Sharma, Sourav Mukherjee, and Hatsuo Ishida. 2021. "Review on the Accelerated and Low-Temperature Polymerization of Benzoxazine Resins: Addition Polymerizable Sustainable Polymers" Polymers 13, no. 8: 1260. https://doi.org/10.3390/polym13081260
APA StyleLochab, B., Monisha, M., Amarnath, N., Sharma, P., Mukherjee, S., & Ishida, H. (2021). Review on the Accelerated and Low-Temperature Polymerization of Benzoxazine Resins: Addition Polymerizable Sustainable Polymers. Polymers, 13(8), 1260. https://doi.org/10.3390/polym13081260












