Synthesis and Properties of Copolyphenylene Sulphones with Cardo Fragments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization and Methods
2.3. Synthesis of Polymers
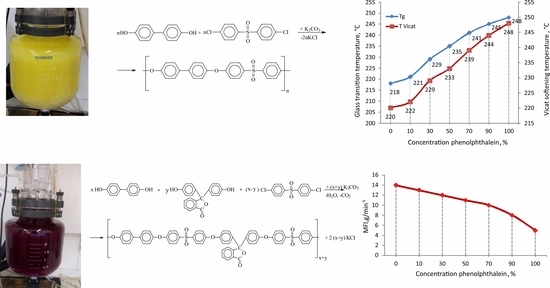
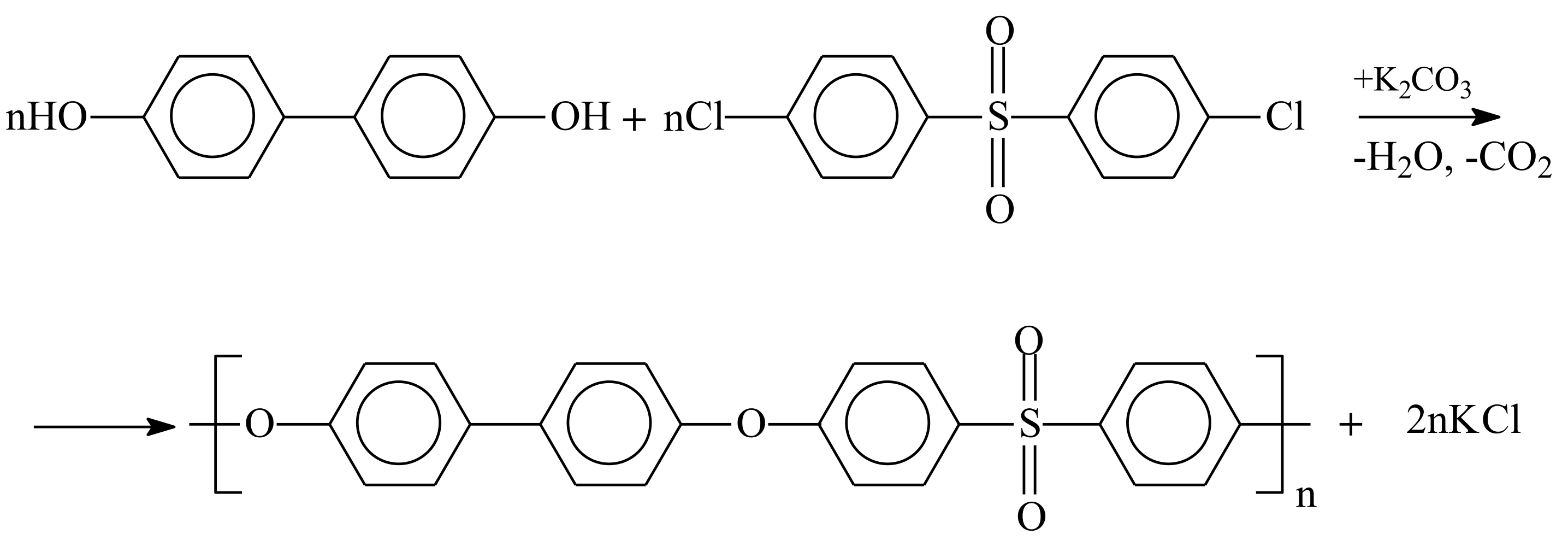
2.3.1. Synthesis of PPSU
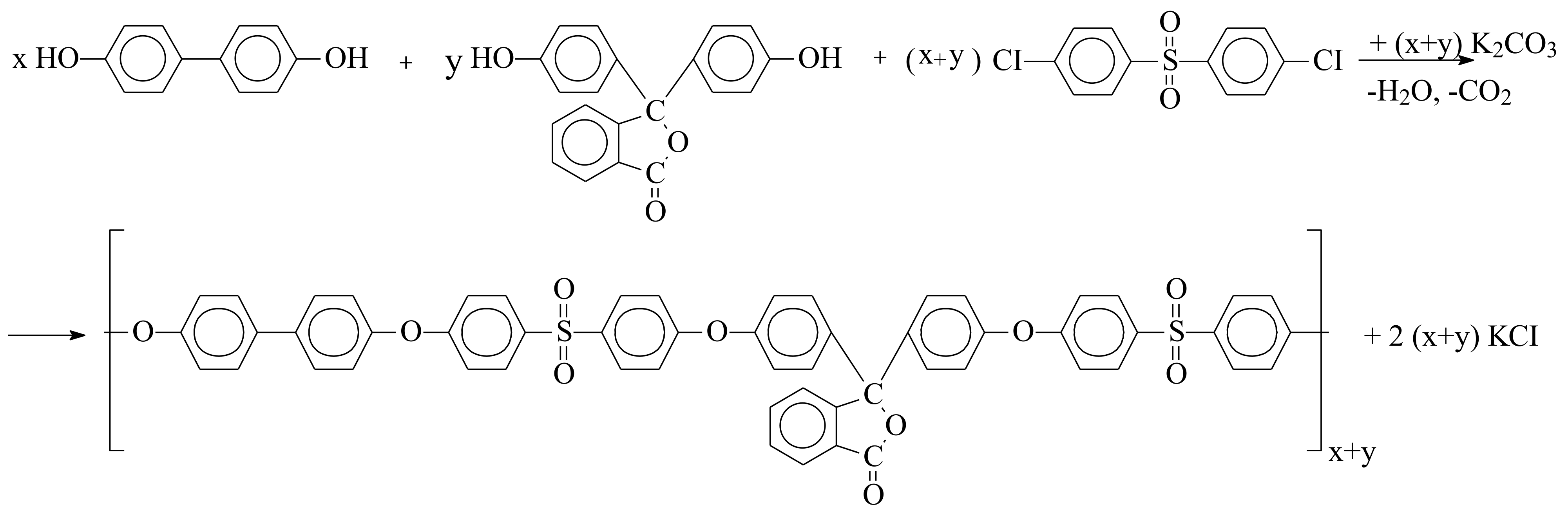
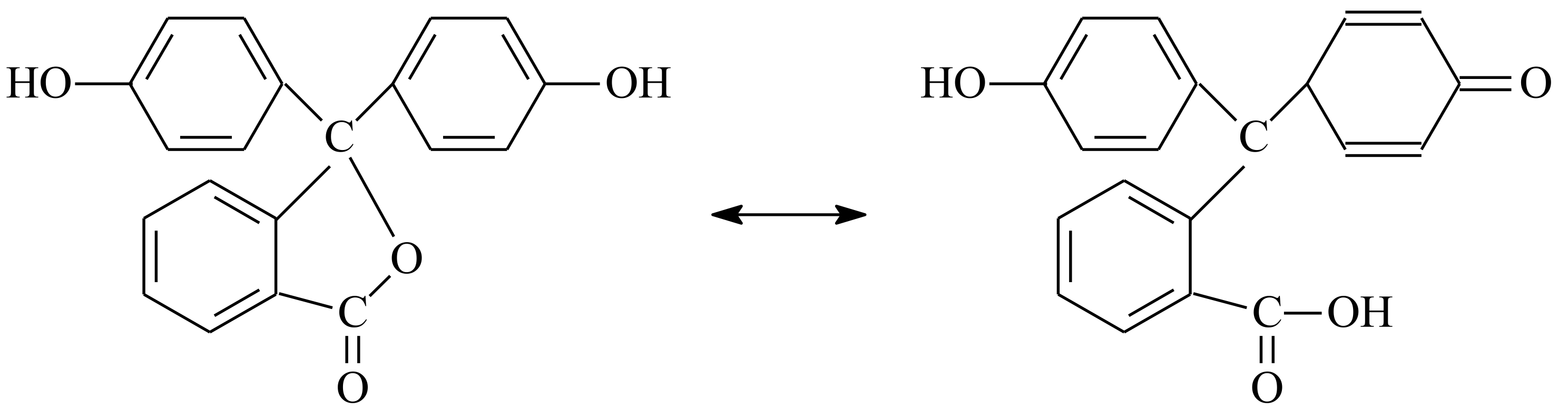
2.3.2. Synthesis of PPSU-C
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhansitov, A.A.; Khashirova, S.Y.; Slonov, A.L.; Kurdanova, Z.I.; Shabaev, A.S.; Khashirov, A.A.; Mikitaev, A.K. Development of technology of polysulfone production for 3D printing. High Perform. Polym. 2017, 29, 724–729. [Google Scholar] [CrossRef]
- Wagner, F.C. Synthetic Resin. U.S. Patent 2035578, 31 March 1936. [Google Scholar]
- Akutin, M.S. A Method of Producing a Polysulfone. SU Author’s Certificate 301073. 4 June 1969. [Google Scholar]
- Li, L.; Liu, J.; Chen, G.; Xu, L.; Mushtaq, N.; Sidra, L.R.; Wang, R.; Fang, X. Synthesis of organosoluble and transparent phenolphthalein-based cardo poly(ether sulfone imide)s via aromatic nucleophilic substitution polymerization. High Perform. Polym. 2016, 28, 1263–1271. [Google Scholar] [CrossRef]
- Yang, Y.; He, T.; Yu, F. The fracture behavior of phenolphthalein polyether–ether ketone at elevated temperature for long terms. J. Appl. Polym. Sci. 1995, 55, 627–632. [Google Scholar] [CrossRef]
- Chamkure, Y.K.; Sharma, R.K. New organosoluble polyarylates containing pendent fluorene units: Synthesis, characterization and thermal behaviour. J. Polym. Res. 2019, 26, 17. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, B.; Wang, Y. An Interesting Result of Dielectric Property for Novel Polyimides with Fluorene Groups. Mater. Sci. Forum 2010, 663–665, 511–514. [Google Scholar] [CrossRef]
- Khan, M.M.; Halder, K.; Shishatskiy, S.; Filiz, V. Synthesis and Crosslinking of Polyether-Based Main Chain Benzoxazine Polymers and Their Gas Separation Performance. Polymers 2018, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Hossain, I.; Kim, Y.; Choi, O.; Kim, T.-H. PEG/PPG-PDMS-Adamantane-based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane. Polymers 2020, 12, 1674. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, X.; Zhang, S.; Chen, T.; Wu, Y. Synthesis, properties, and gas permeation performance of cardo poly(arylene ether sulfone)s containing phthalimide side groups. J. Appl. Polym. Sci. 2007, 106, 2808–2816. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Jensen, B.J.; Havens, S.J. Poly(arylene ethers). Polymer 1988, 29, 358–369. [Google Scholar] [CrossRef]
- Singh, R.; Hay, A.S. Synthesis and physical properties of soluble, amorphous poly(ether ketone)s containing the o-dibenzoylbenzene moiety. Macromolecules 1992, 25, 1017–1024. [Google Scholar] [CrossRef]
- Korycki, A.; Garnier, C.; Abadie, A.; Nassiet, V.; Sultan, C.; Chabert, F. Poly(etheretherketone)/Poly(ethersulfone) Blends with Phenolphthalein: Miscibility, Thermomechanical Properties, Crystallization and Morphology. Polymers 2021, 13, 1466. [Google Scholar] [CrossRef]
- Xueren, L.; Congjie, G. Research on polyethersulfone, polyarylsulphone and polyetheretherketone with cardo membranes. Desalination 1994, 96, 155–161. [Google Scholar] [CrossRef]
- Pulyalina, A.; Rostovtseva, V.; Faykov, I.; Toikka, A. Application of Polymer Membranes for a Purification of Fuel Oxygenated Additive. Methanol/Methyl Tert-butyl Ether (MTBE) Separation via Pervaporation: A Comprehensive Review. Polymers 2020, 12, 2218. [Google Scholar] [CrossRef] [PubMed]
- Vasoya, P.J.; Patel, V.A.; Bhuva, B.D.; Parsania, P.H. Synthesis and Physico-Chemical Study of High Performance Cardo Copoly(Ether-Sulfone-Sulfonates). Polym. Technol. Eng. 2008, 47, 828–835. [Google Scholar] [CrossRef]
- Wang, L.-M.; Guan, G.-Y.; Chen, Z.-W. Electrospun Nanofiber-Based Cardo Poly(aryl ether sulfone) Containing Zwitterionic Side Groups as Novel Proton Exchange Membranes. Int. Polym. Process. 2018, 33, 480–485. [Google Scholar] [CrossRef]
- Blanco, J.; Nguyen, Q.; Schaetzel, P. Novel hydrophilic membrane materials: Sulfonated polyethersulfone Cardo. J. Membr. Sci. 2001, 186, 267–279. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Li, S. Synthesis and characterization of novel cardo poly(aryl ether sulfone) bearing zwitterionic side groups for proton exchange membranes. Int. J. Hydrogen Energy 2011, 36, 5512–5520. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, S. Phenolphthalein-based cardo poly(arylene ether sulfone): Preparation and application to separation membranes. J. Appl. Polym. Sci. 2013, 128, 1–12. [Google Scholar] [CrossRef]
- Zheng, G.; Dong, L.; Cai, Z.H.; Feng, Z.H. Synthesis of segmented copolymers whith polysulfones containing bisphenol A or phe-nolphthalein units as hard segment. Polym. Commun. 1985, 2, 176–179. [Google Scholar]
- Harris, J.; Maresca, L.; Metzner, M. Polyaryl Ether Sulphones. U.S. Patent No 4785072, 15 November 1988. [Google Scholar]
- Brunelle, D.J.; Steiger, D. Polyethersulfone Compositions with High Heat and Good Impact Resistance. Patent WO 2008051651, 24 October 2006. [Google Scholar]
- Kurdanova, Z.H.I.; Shakhmurzova, K.T.; Zhansitov, A.A.; Baykaziev, A.E.; Slonov, A.L.; Khashirova, S.Y.; Ligidov, M.K.H.; Mik-Itaev, A.K. Study of synthesis and properties of copolysulphones with cardium fragments. Plast. Massy. 2017, 7–8, 23–26. [Google Scholar]
- Steiner, G.; Zimmerer, C. Poly(aryl ether sulfone) (PAES). In Landolt-Börnstein—Group VIII Advanced Materials and Technologies 6A1 (Polymer Solids and Polymer Melts—Definitions and Physical Properties I); Arndt, K.-F., Lechner., M.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 6A1. [Google Scholar] [CrossRef]
- Musaev, U.I.; Mikitaev, A.K. Appreciation de Tacide Relativee des Diphenols dan le Melange de l”Alcool Tertiobuty Liquo et di-methilsulpoxide; Revue Scientifique sur la Chimie Industrielle: Annaba, Algerie, 1979; pp. 78–81. [Google Scholar]
- Mikitaev, A.K.; Korshak, V.V.; Shustov, G.B. Synthesis and properties of halide-containing polyarylatesulfonic block copoly-mers/Vysokomol. Soedin. Seriya A 1982, 24, 2558–2562. [Google Scholar]

| Sample | η (dL g−1) | MFI (g min−1) |
|---|---|---|
| PPSU | 0.50 | 14.0 |
| PPSU-C-10 | 0.47 | 13.0 |
| PPSU-C-30 | 0.40 | 12.0 |
| PPSU-C-50 | 0.31 | 9.0 |
| PPSU-C-70 | 0.31 | 10.0 |
| PPSU-C-90 | 0.30 | 4.3 |
| PPSU-C-100 | 0.28 | 5.4 |
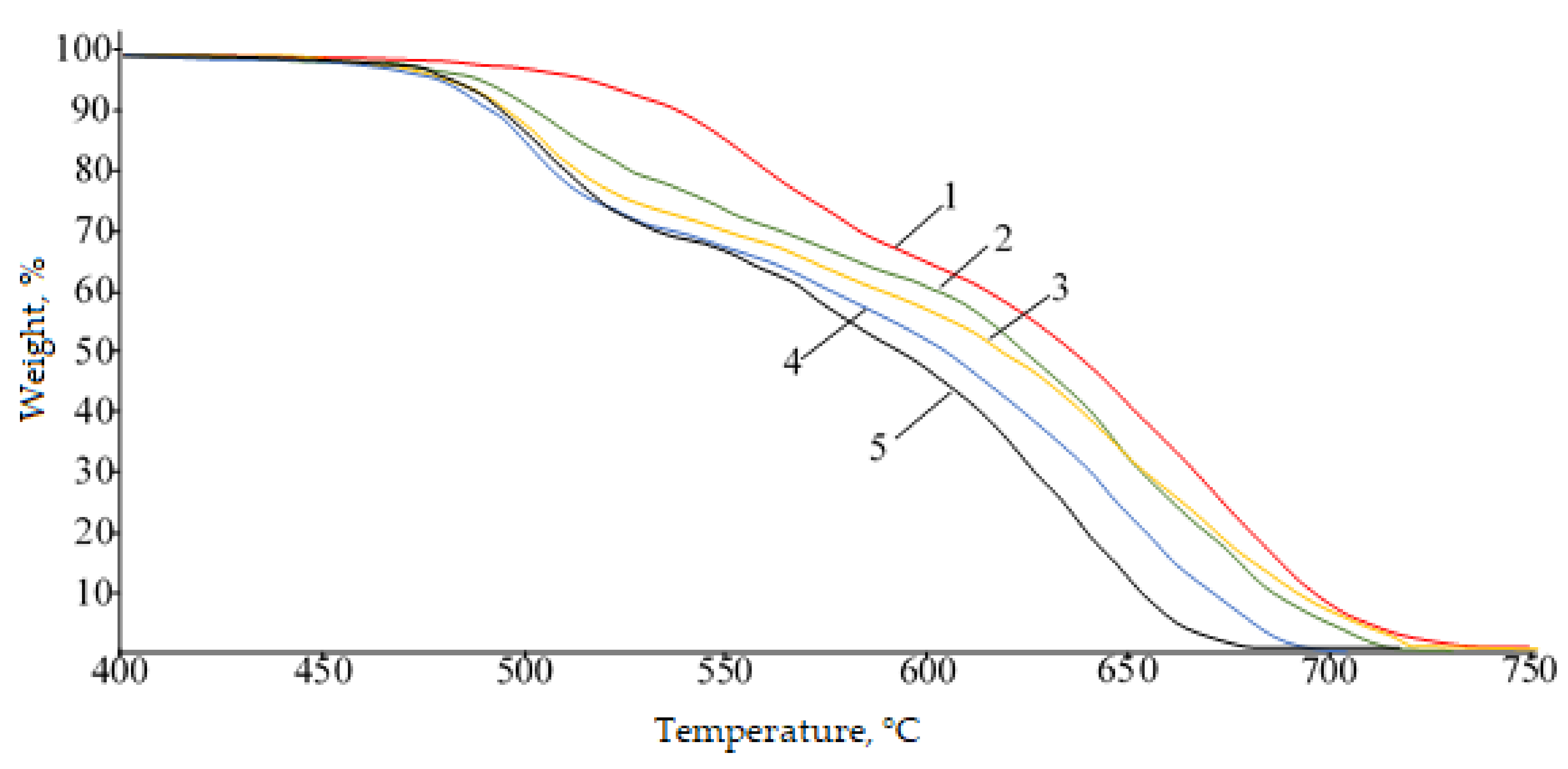
| Sample | T2%, °C | T5%, °C | T10%, °C | Tg, °C | Vicat Softening Temperature, °C |
|---|---|---|---|---|---|
| PPSU | 490 | 517 | 538 | 219 | 221 |
| PPSU-C-10 | 477 | 500 | 518 | 221 | 222 |
| PPSU-C-30 | 470 | 489 | 504 | 229 | 229 |
| PPSU-C-50 | 464 | 483 | 497 | 235 | 233 |
| PPSU-C-70 | 462 | 480 | 493 | 241 | 239 |
| PPSU-C-90 | 462 | 481 | 494 | 245 | 244 |
| PPSU-C-100 | 465 | 482 | 494 | 248 | 248 |
| Sample | Impact Strength, kJ/m2 | Efl, | σyield, | Eten, | σten, | ε, | Shore Hardness (D Scale) | |
|---|---|---|---|---|---|---|---|---|
| Unnotched | Notched | MPa | MPa | MPa | MPa | % | ||
| PPSU | n/b | 24.3 | 2390 | 87.5 | 2150 | 73 | 38.5 | 75 |
| PPSU-C-10 | 150 | 14.8 | 2500 | 83.0 | 2230 | 68 | 12.6 | 76 |
| PPSU-C-30 | 168 | 11.0 | 2650 | 87.0 | 2300 | 70 | 11.7 | 78 |
| PPSU-C-50 | 88 | 6.2 | 2780 | 90.5 | 2430 | 73 | 9.8 | 79 |
| PPSU-C-70 | 27 | 6.5 | 2970 | - | 2613 | 94 | 8.0 | 80 |
| PPSU-C-90 | 15 | 4.5 | 3110 | - | 2330 | 83 | 4.5 | 80 |
| PPSU-C-100 | 26 | 9.8 | 3270 | - | 2490 | 85 | 4.2 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurdanova, Z.; Zhansitov, A.; Shakhmurzova, K.; Slonov, A.; Baykaziev, A.; Khashirova, S. Synthesis and Properties of Copolyphenylene Sulphones with Cardo Fragments. Polymers 2021, 13, 3689. https://doi.org/10.3390/polym13213689
Kurdanova Z, Zhansitov A, Shakhmurzova K, Slonov A, Baykaziev A, Khashirova S. Synthesis and Properties of Copolyphenylene Sulphones with Cardo Fragments. Polymers. 2021; 13(21):3689. https://doi.org/10.3390/polym13213689
Chicago/Turabian StyleKurdanova, Zhanna, Azamat Zhansitov, Kamila Shakhmurzova, Azamat Slonov, Artur Baykaziev, and Svetlana Khashirova. 2021. "Synthesis and Properties of Copolyphenylene Sulphones with Cardo Fragments" Polymers 13, no. 21: 3689. https://doi.org/10.3390/polym13213689
APA StyleKurdanova, Z., Zhansitov, A., Shakhmurzova, K., Slonov, A., Baykaziev, A., & Khashirova, S. (2021). Synthesis and Properties of Copolyphenylene Sulphones with Cardo Fragments. Polymers, 13(21), 3689. https://doi.org/10.3390/polym13213689