Ferulic Acid as Building Block for the Lipase-Catalyzed Synthesis of Biobased Aromatic Polyesters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Ferulic Diesters
2.4. Hydrogenation of the Ferulic Diesters
2.5. Synthesis of the Ferulic Diols
2.6. Enzymatic Polymerization
3. Results and Discussion
3.1. Polyesters Based on Ferulic Diesters
3.2. Polyesters Based on Hydrogenated Ferulic Diesters
3.3. Polyesters Based on Ferulic Diols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, S.; Dey, A. PET Chemistry. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. ISBN 978-0-12-811361-5. [Google Scholar]
- John, G.; Nagarajan, S.; Vemula, P.K.; Silverman, J.R.; Pillai, C.K.S. Natural Monomers: A Mine for Functional and Sustainable Materials—Occurrence, Chemical Modification and Polymerization. Prog. Polym. Sci. 2019, 92, 158–209. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef] [PubMed]
- Satti, S.M.; Shah, A.A. Polyester-Based Biodegradable Plastics: An Approach Towards Sustainable Development. Lett. Appl. Microbiol. 2020, 70, 413–430. [Google Scholar] [CrossRef]
- Li, Q.; Ma, S.; Xu, X.; Zhu, J. Bio-based Unsaturated Polyesters. In Unsaturated Polyester Resins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 515–555. ISBN 978-0-12-816129-6. [Google Scholar]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “Perfect” Lipase Immobilized Biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Pellis, A.; Nyanhongo, G.S.; Farmer, T.J. Recent Advances on Enzymatic Catalysis as a Powerful Tool for the Sustainable Synthesis of Bio-Based Polyesters. In Biorefinery; Bastidas-Oyanedel, J.-R., Schmidt, J.E., Eds.; Springer: Cham, Switzerland, 2019; pp. 555–570. ISBN 978-3-030-10960-8. [Google Scholar]
- Todea, A.; Dreavă, D.M.; Benea, I.C.; Bîtcan, I.; Peter, F.; Boeriu, C.G. Achievements and Trends in Biocatalytic Synthesis of Specialty Polymers from Biomass-Derived Monomers Using Lipases. Processes 2021, 9, 646. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Avérous, L. Lipase-Catalyzed Synthesis of Biobased and Biodegradable Aliphatic Copolyesters from Short Building Blocks. Effect of the Monomer Length. Eur. Polym. J. 2017, 97, 328–337. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Avérous, L. Enzymatic Synthesis of Biobased Poly(1,4-Butylene Succinate- Ran -2,3-Butylene Succinate) Copolyesters and Characterization. Influence of 1,4- and 2,3-Butanediol Contents. Eur. Polym. J. 2017, 93, 103–115. [Google Scholar] [CrossRef]
- Duchiron, S.W.; Pollet, E.; Givry, S.; Avérous, L. Enzymatic Synthesis of Poly(ε-Caprolactone-co-ε-Thiocaprolactone). Eur. Polym. J. 2017, 87, 147–158. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The Quest for High Glass Transition Temperature Bioplastics. J. Mater. Chem. A 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- Albanese, M.; Boyenval, J.; Marchese, P.; Sullalti, S.; Celli, A. The Aliphatic Counterpart of PET, PPT and PBT Aromatic Polyesters: Effect of the Molecular Structure on Thermo-Mechanical Properties. AIMS Mol. Sci. 2016, 3, 32–51. [Google Scholar] [CrossRef]
- Comerford, J.W.; Byrne, F.P.; Weinberger, S.; Farmer, T.J.; Guebitz, G.M.; Gardossi, L.; Pellis, A. Thermal Upgrade of Enzymatically Synthesized Aliphatic and Aromatic Oligoesters. Materials 2020, 13, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoczinski, P.; Cangahuala, M.K.E.; Maniar, D.; Albach, R.W.; Bittner, N.; Loos, K. Biocatalytic Synthesis of Furan-Based Oligomer Diols with Enhanced End-Group Fidelity. ACS Sustain. Chem. Eng. 2020, 8, 1068–1086. [Google Scholar] [CrossRef]
- Flores, I.; de Ilarduya, A.M.; Sardon, H.; Müller, A.J.; Muñoz-Guerra, S. Synthesis of Aromatic–Aliphatic Polyesters by Enzymatic Ring Opening Polymerization of Cyclic Oligoesters and Their Cyclodepolymerization for a Circular Economy. ACS Appl. Polym. Mater. 2019, 1, 321–325. [Google Scholar] [CrossRef]
- Bazin, A.; Avérous, L.; Pollet, E. Lipase-Catalyzed Synthesis of Furan-Based Aliphatic-Aromatic Biobased Copolyesters: Impact of the Solvent. Eur. Polym. J. 2021, 159, 110717. [Google Scholar] [CrossRef]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, Polyphenols and Tannins: An Overview. In Plant Secondary Metabolites; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 1–24. ISBN 978-0-470-98855-8. [Google Scholar]
- Tinikul, R.; Chenprakhon, P.; Maenpuen, S.; Chaiyen, P. Biotransformation of Plant-Derived Phenolic Acids. Biotechnol. J. 2018, 13, e1700632. [Google Scholar] [CrossRef]
- Flourat, A.L.; Combes, J.; Bailly-Maitre-Grand, C.; Magnien, K.; Haudrechy, A.; Renault, J.; Allais, F. Accessing p-Hydroxycinnamic Acids: Chemical Synthesis, Biomass Recovery, or Engineered Microbial Production? ChemSusChem 2021, 14, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Terrett, O.M.; Dupree, P. Covalent Interactions between Lignin and Hemicelluloses in Plant Secondary Cell Walls. Curr. Opin. Biotechnol. 2019, 56, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wilkins, M. Lignin Bioconversion into Valuable Products: Fractionation, Depolymerization, Aromatic Compound Conversion, and Bioproduct Formation. Syst. Microbiol. Biomanuf. 2021, 1, 166–185. [Google Scholar] [CrossRef]
- Sandoval, G.; Quintana, P.G.; Baldessari, A.; Ballesteros, A.O.; Plou, F.J. Lipase-Catalyzed Preparation of Mono- and Diesters of Ferulic Acid. Biocatal. Biotransform. 2015, 33, 89–97. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Laguerre, M.; Villeneuve, P.; Lecomte, J. From Phenolics to Phenolipids: Optimizing Antioxidants in Lipid Dispersions. Lipid Technol. 2013, 25, 131–134. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic Acid: Pharmacological and Toxicological Aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Elias, H.-G.; Palacios, J.A. Poly(Ferulic Acid) by Thionyl Chloride Activated Polycondensation. Makromol. Chem. 1985, 186, 1027–1045. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-Derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.H.; Reis, M.H.; Qi, P.; Miller, S.A. Polyethylene Ferulate (PEF) and Congeners: Polystyrene Mimics Derived from Biorenewable Aromatics. Green Chem. 2015, 17, 4512–4517. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Short, G.N.; Qi, P.; Miller, S.A. Copolymerization of Lactones and Bioaromatics via Concurrent Ring-Opening Polymerization/Polycondensation. Green Chem. 2017, 19, 1877–1888. [Google Scholar] [CrossRef]
- Kurt, G.; Gokturk, E. Synthesis of Polyesters Mimicking Polyethylene Terephthalate and Their Thermal and Mechanical Properties. J. Polym. Res. 2020, 27, 314. [Google Scholar] [CrossRef]
- Ouimet, M.A.; Griffin, J.; Carbone-Howell, A.L.; Wu, W.-H.; Stebbins, N.D.; Di, R.; Uhrich, K.E. Biodegradable Ferulic Acid-Containing Poly(Anhydride-Ester): Degradation Products with Controlled Release and Sustained Antioxidant Activity. Biomacromolecules 2013, 14, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Pospiech, D.; Korwitz, A.; Komber, H.; Jehnichen, D.; Arnhold, K.; Brünig, H.; Scheibner, H.; Müller, M.T.; Voit, B. Polyesters with Bio-Based Ferulic Acid Units: Crosslinking Paves the Way to Property Consolidation. Polym. Chem. 2021, 12, 5139–5148. [Google Scholar] [CrossRef]
- Pion, F.; Reano, A.F.; Ducrot, P.-H.; Allais, F. Chemo-Enzymatic Preparation of New Bio-Based Bis- and Trisphenols: New Versatile Building Blocks for Polymer Chemistry. RSC Adv. 2013, 3, 8988. [Google Scholar] [CrossRef]
- Ménard, R.; Caillol, S.; Allais, F. Chemo-Enzymatic Synthesis and Characterization of Renewable Thermoplastic and Thermoset Isocyanate-Free Poly(Hydroxy)Urethanes from Ferulic Acid Derivatives. ACS Sustain. Chem. Eng. 2017, 5, 1446–1456. [Google Scholar] [CrossRef]
- Ménard, R.; Caillol, S.; Allais, F. Ferulic Acid-Based Renewable Esters and Amides-Containing Epoxy Thermosets from Wheat Bran and Beetroot Pulp: Chemo-Enzymatic Synthesis and Thermo-Mechanical Properties Characterization. Ind. Crop. Prod. 2017, 95, 83–95. [Google Scholar] [CrossRef]
- Pion, F.; Ducrot, P.-H.; Allais, F. Renewable Alternating Aliphatic-Aromatic Copolyesters Derived from Biobased Ferulic Acid, Diols, and Diacids: Sustainable Polymers with Tunable Thermal Properties. Macromol. Chem. Phys. 2014, 215, 431–439. [Google Scholar] [CrossRef]
- Barbara, I.; Flourat, A.L.; Allais, F. Renewable Polymers Derived from Ferulic Acid and Biobased Diols via ADMET. Eur. Polym. J. 2015, 62, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Rovira, J.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Human Exposure to Trace Elements through the Skin by Direct Contact with Clothing: Risk Assessment. Environ. Res. 2015, 140, 308–316. [Google Scholar] [CrossRef]
- Pellis, A.; Acero, E.H.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable Building Blocks for Sustainable Polyesters: New Biotechnological Routes for Greener Plastics: Renewable Building Blocks for Sustainable Polyesters. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H. Synthesis of Polyesters I: Hydrolase as Catalyst for Polycondensation (Condensation Polymerization). In Enzymatic Polymerization towards Green Polymer Chemistry, Green Chemistry and Sustainable Technology; Kobayashi, S., Uyama, H., Kadokawa, J., Eds.; Springer: Singapore, 2019; pp. 105–163. ISBN 9789811338120. [Google Scholar]
- Vosmann, K.; Wiege, B.; Weitkamp, P.; Weber, N. Preparation of Lipophilic Alkyl (Hydroxy)Benzoates by Solvent-Free Lipase-Catalyzed Esterification and Transesterification. Appl. Microbiol. Biotechnol. 2008, 80, 29–36. [Google Scholar] [CrossRef]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.-M.; Papaspyrides, C.D. A Review on Enzymatic Polymerization to Produce Polycondensation Polymers: The Case of Aliphatic Polyesters, Polyamides and Polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Parthiban, A.; Vasantha, V.A. Biorenewable Functional Oligomers and Polymers—Direct Copolymerization of Ferulic Acid to Obtain Polymeric UV Absorbers and Multifunctional Materials. Polymer 2020, 188, 122122. [Google Scholar] [CrossRef]
- Christelle, B.; Eduardo, B.D.O.; Latifa, C.; Elaine-Rose, M.; Bernard, M.; Evelyne, R.-H.; Mohamed, G.; Jean-Marc, E.; Catherine, H. Combined Docking and Molecular Dynamics Simulations to Enlighten the Capacity of Pseudomonas Cepacia and Candida Antarctica Lipases to Catalyze Quercetin Acetylation. J. Biotechnol. 2011, 156, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, H.; Sereti, V.; Kolisis, F.N. Studies on the Enzymatic Synthesis of Lipophilic Derivatives of Natural Antioxidants. J. Am. Oil Chem. Soc. 1999, 76, 1505. [Google Scholar] [CrossRef]
- Cassani, J.; Luna, H.; Navarro, A.; Castillo, E. Comparative Esterification of Phenylpropanoids versus Hydrophenylpropanoids Acids Catalyzed by Lipase in Organic Solvent Media. Electron. J. Biotechnol. 2007, 10, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Zoumpanioti, M.; Merianou, E.; Karandreas, T.; Stamatis, H.; Xenakis, A. Esterification of Phenolic Acids Catalyzed by Lipases Immobilized in Organogels. Biotechnol. Lett. 2010, 32, 1457–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, R.T.; Scheib, H.; Bornscheuer, U.T.; Pleiss, J.; Syldatk, C.; Schmid, R.D. Substrate Specificity of Lipase B from Candida Antarctica in the Synthesis of Arylaliphatic Glycolipids. J. Mol. Catal. B Enzym. 2000, 8, 201–211. [Google Scholar] [CrossRef]
- Mahapatro, A.; Kalra, B.; Kumar, A.; Gross, R.A. Lipase-Catalyzed Polycondensations: Effect of Substrates and Solvent on Chain Formation, Dispersity, and End-Group Structure. Biomacromolecules 2003, 4, 544–551. [Google Scholar] [CrossRef]
- Pellis, A.; Comerford, J.W.; Maneffa, A.J.; Sipponen, M.H.; Clark, J.H.; Farmer, T.J. Elucidating Enzymatic Polymerisations: Chain-Length Selectivity of Candida Antarctica Lipase B towards Various Aliphatic Diols and Dicarboxylic Acid Diesters. Eur. Polym. J. 2018, 106, 79–84. [Google Scholar] [CrossRef]
- Nasr, K.; Meimoun, J.; Favrelle-Huret, A.; Winter, J.D.; Raquez, J.-M.; Zinck, P. Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate: A Statistical Approach Predicting the Key-Parameters in Solution and in Bulk. Polymers 2020, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Kreye, O.; Tóth, T.; Meier, M.A.R. Copolymers Derived from Rapeseed Derivatives via ADMET and Thiol-Ene Addition. Eur. Polym. J. 2011, 47, 1804–1816. [Google Scholar] [CrossRef]
- Oh, S.-C.; Lee, D.-G.; Kwak, H.; Bae, S.-Y. Combustion Kinetics Of Polyethylene Terephthalate. Environ. Eng. Res. 2006, 11, 250–256. [Google Scholar] [CrossRef]
- Jawerth, M.; Lawoko, M.; Lundmark, S.; Perez-Berumen, C.; Johansson, M. Allylation of a Lignin Model Phenol: A Highly Selective Reaction under Benign Conditions towards a New Thermoset Resin Platform. RSC Adv. 2016, 6, 96281–96288. [Google Scholar] [CrossRef] [Green Version]
- Debuissy, T.; Sangwan, P.; Pollet, E.; Avérous, L. Study on the Structure-Properties Relationship of Biodegradable and Biobased Aliphatic Copolyesters Based on 1,3-Propanediol, 1,4-Butanediol, Succinic and Adipic Acids. Polymer 2017, 122, 105–116. [Google Scholar] [CrossRef]
- Pellis, A.; Weinberger, S.; Gigli, M.; Guebitz, G.M.; Farmer, T.J. Enzymatic Synthesis of Biobased Polyesters Utilizing Aromatic Diols as the Rigid Component. Eur. Polym. J. 2020, 130, 109680. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Guo, B. Use of MALDI-TOF To Measure Molecular Weight Distributions of Polydisperse Poly(Methyl Methacrylate). Anal. Chem. 1998, 70, 131–135. [Google Scholar] [CrossRef]
- Pezzana, L.; Mousa, M.; Malmström, E.; Johansson, M.; Sangermano, M. Bio-Based Monomers for UV-Curable Coatings: Allylation of Ferulic Acid and Investigation of Photocured Thiol-Ene Network. Prog. Org. Coat. 2021, 150, 105986. [Google Scholar] [CrossRef]
- Masuku, C.P. Thermolytic Decomposition of Coniferyl Alcohol. J. Anal. Appl. Pyrolysis 1992, 23, 195–208. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An Engineered PET Depolymerase to Break down and Recycle Plastic Bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Salam, M.D.; Varma, A.; Prashar, R.; Choudhary, D. Review on Efficacy of Microbial Degradation of Polyethylene Terephthalate and Bio-Upcycling as a Part of Plastic Waste Management. Appl. Ecol. Environ. Sci. 2021, 9, 695–703. [Google Scholar] [CrossRef]
- Magnin, A.; Entzmann, L.; Bazin, A.; Pollet, E.; Avérous, L. Green Recycling Process for Polyurethane Foams by a Chem-Biotech Approach. ChemSusChem 2021, 14, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Carniel, A.; Valoni, É.; Nicomedes, J.; Gomes, A.D.C.; de Castro, A.M. Lipase from Candida Antarctica (CALB) and Cutinase from Humicola Insolens Act Synergistically for PET Hydrolysis to Terephthalic Acid. Process. Biochem. 2017, 59, 84–90. [Google Scholar] [CrossRef]


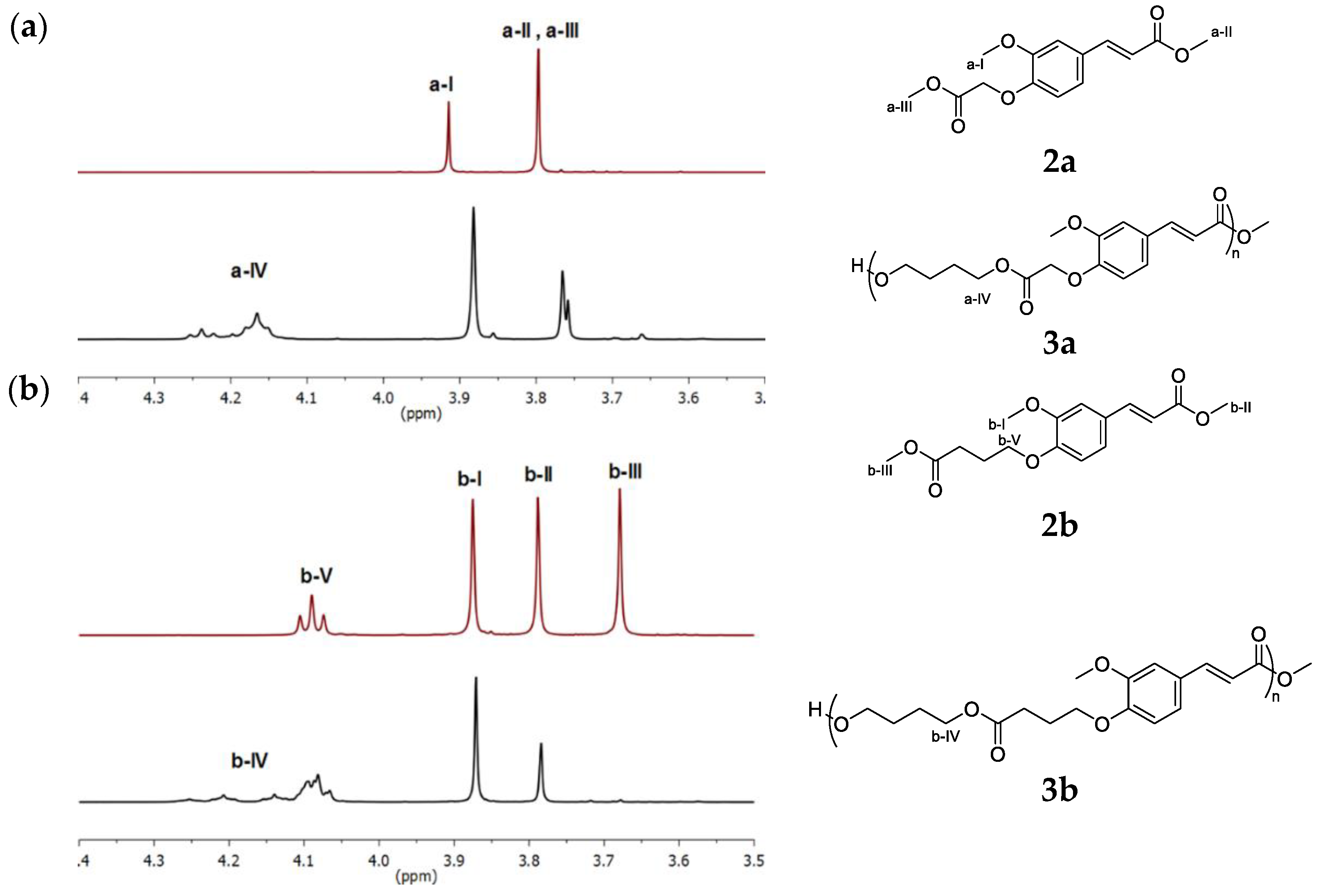

 5a,
5a,  5b.
5b.


 7a,
7a,  7b.
7b.


| Ferulic-Based Monomer | Co-Monomer | Yield a | Mn (g·mol−1) | Mw (g·mol−1) | DPn | Tg (°C) | Tdmax (°C) | Td5% (°C) |
|---|---|---|---|---|---|---|---|---|
(2a) | 1,4-BDO | n.d. b | 1300 | 1600 | 4 | n.d. b | n.d. b | n.d. b |
(2b) | 1,4-BDO | n.d. b | 2000 | 2500 | 5 | n.d. b | n.d. b | n.d. b |
(4a) | 1,4-BDO | 58% | 17,900 | 33,100 | 58 | 7 | 373 | 346 |
(4b) | 1,4-BDO | 96% | 12,600 | 29,800 | 37 | −9 | 394 | 350 |
(6a) | DEA | 82% | 6500 | 15,000 | 19 | 21 | 403 | 316 |
(6b)  | DEA | 79% | 6300 | 15,500 | 17 | 2 | 418 | 308 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazin, A.; Avérous, L.; Pollet, E. Ferulic Acid as Building Block for the Lipase-Catalyzed Synthesis of Biobased Aromatic Polyesters. Polymers 2021, 13, 3693. https://doi.org/10.3390/polym13213693
Bazin A, Avérous L, Pollet E. Ferulic Acid as Building Block for the Lipase-Catalyzed Synthesis of Biobased Aromatic Polyesters. Polymers. 2021; 13(21):3693. https://doi.org/10.3390/polym13213693
Chicago/Turabian StyleBazin, Alfred, Luc Avérous, and Eric Pollet. 2021. "Ferulic Acid as Building Block for the Lipase-Catalyzed Synthesis of Biobased Aromatic Polyesters" Polymers 13, no. 21: 3693. https://doi.org/10.3390/polym13213693
APA StyleBazin, A., Avérous, L., & Pollet, E. (2021). Ferulic Acid as Building Block for the Lipase-Catalyzed Synthesis of Biobased Aromatic Polyesters. Polymers, 13(21), 3693. https://doi.org/10.3390/polym13213693









