Molecular Structure Effect of a Self-Assembled Monolayer on Thermal Resistance across an Interface
Abstract
:1. Introduction
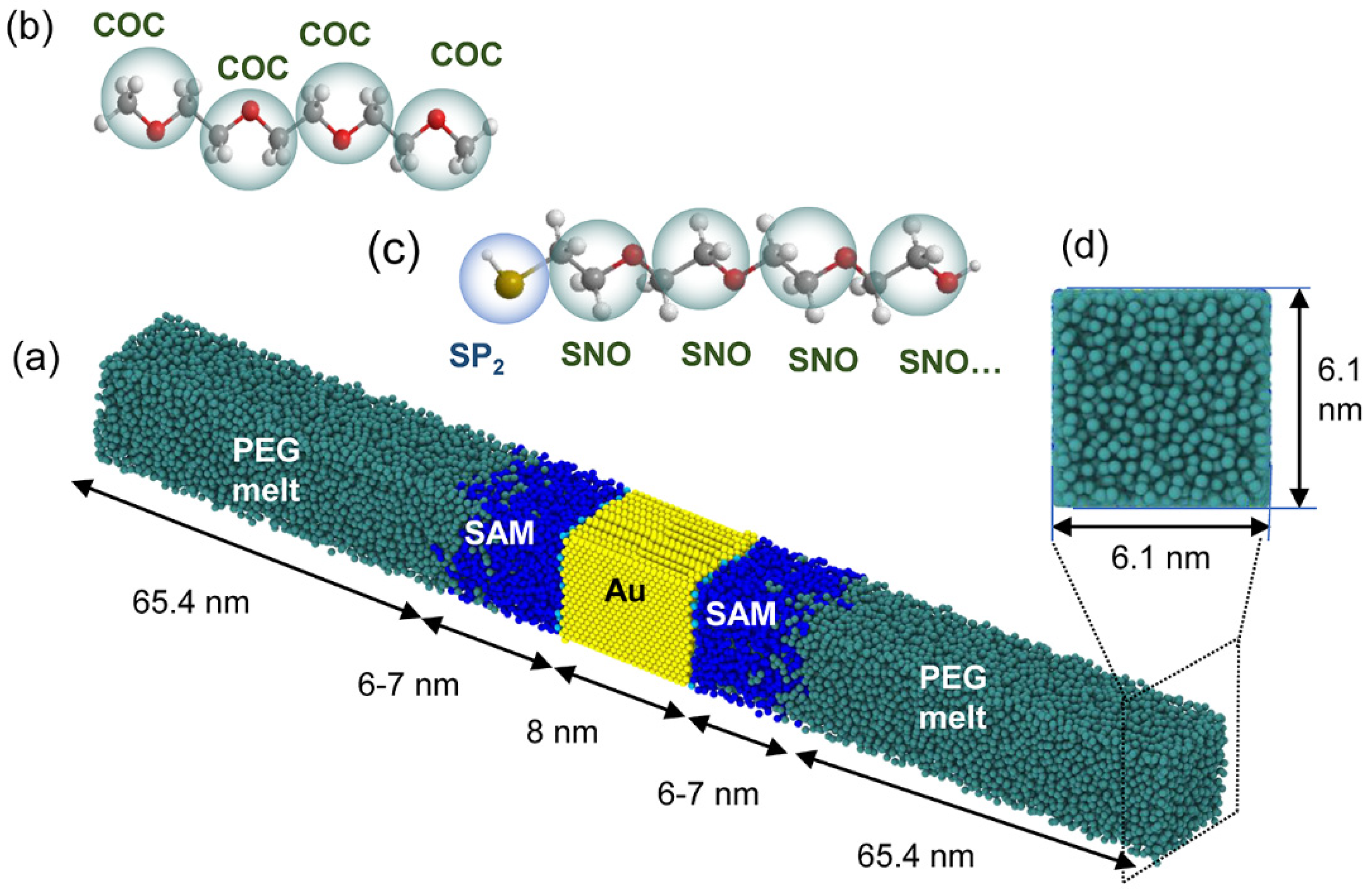
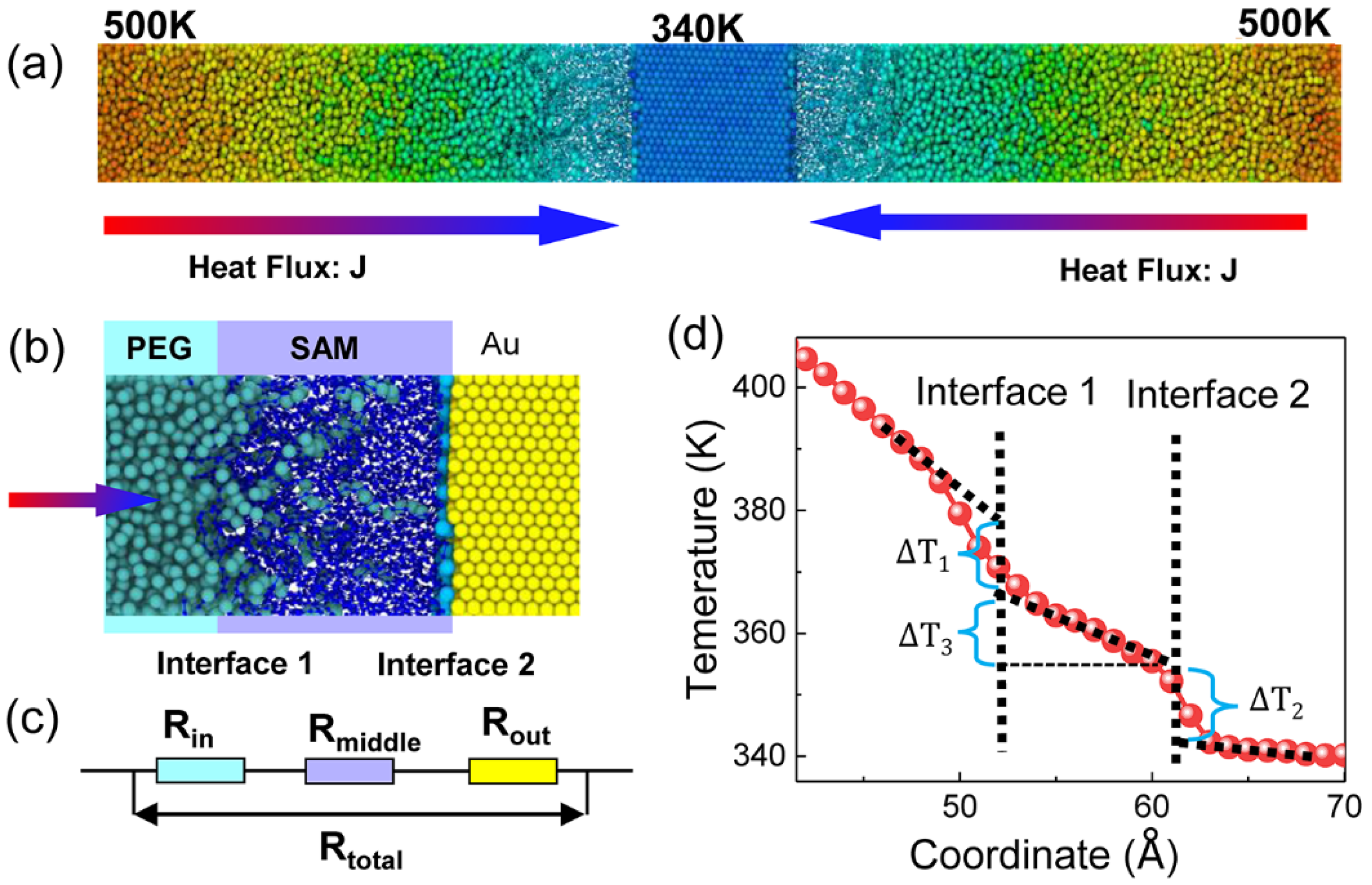
2. Material and Molecular Modelling
3. Results and Discussion
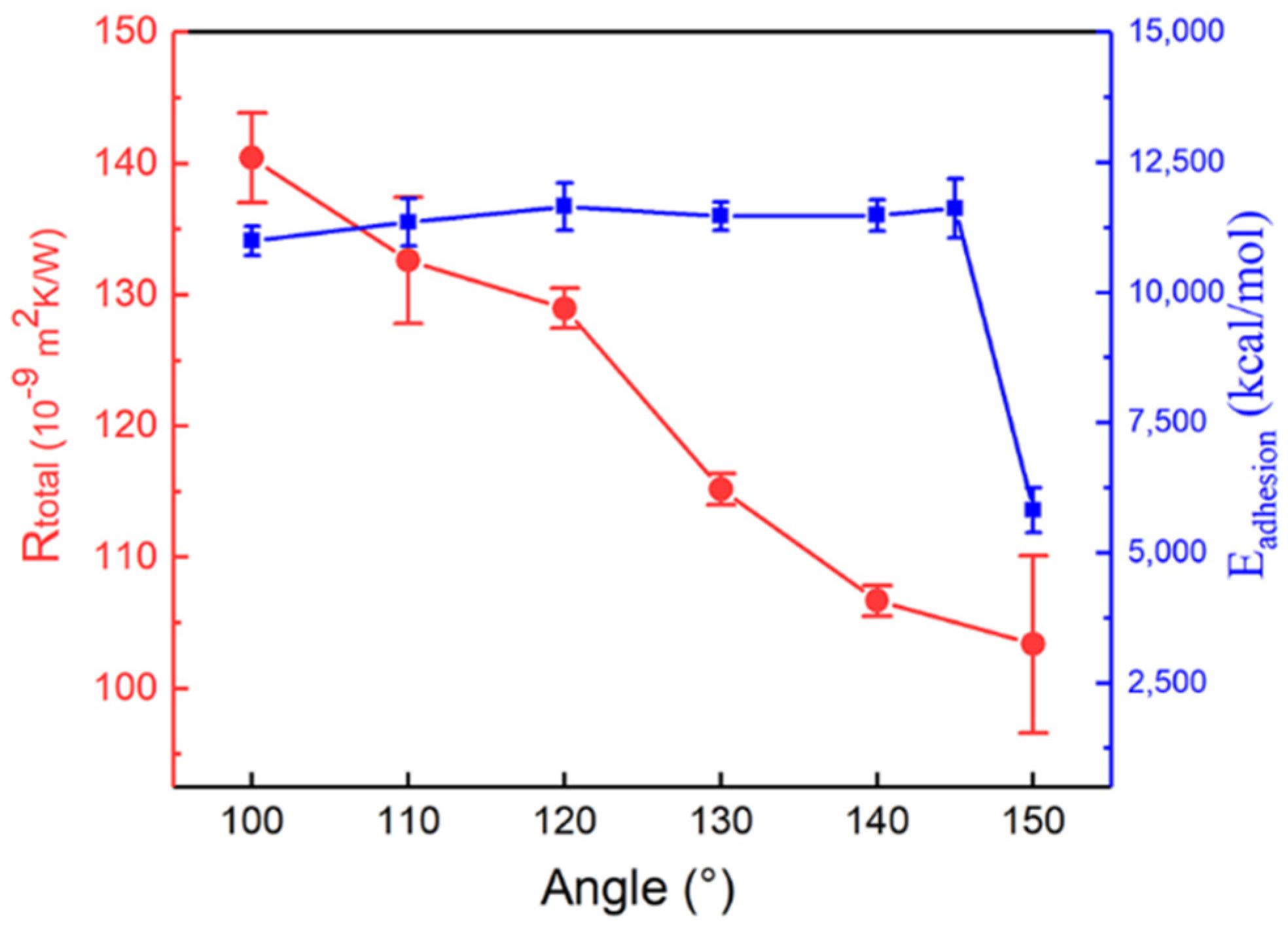
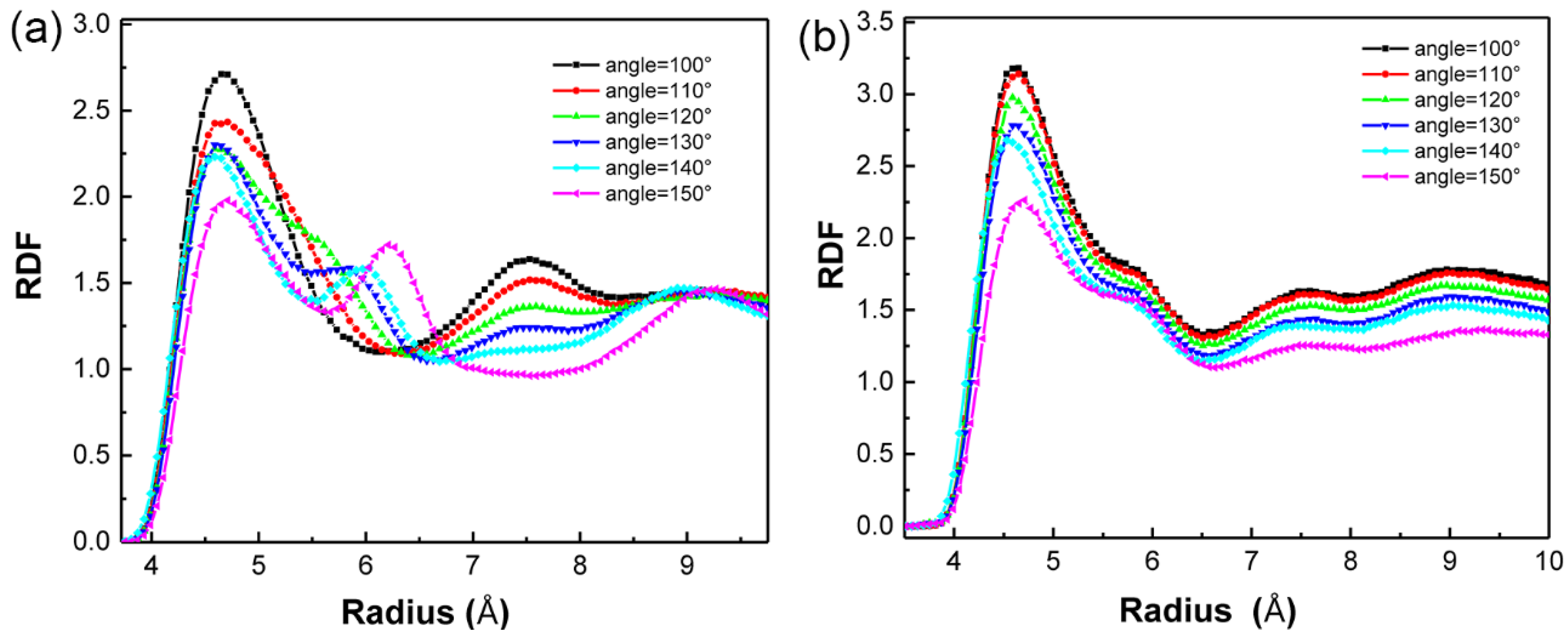
3.1. Angle Effect of SAM on
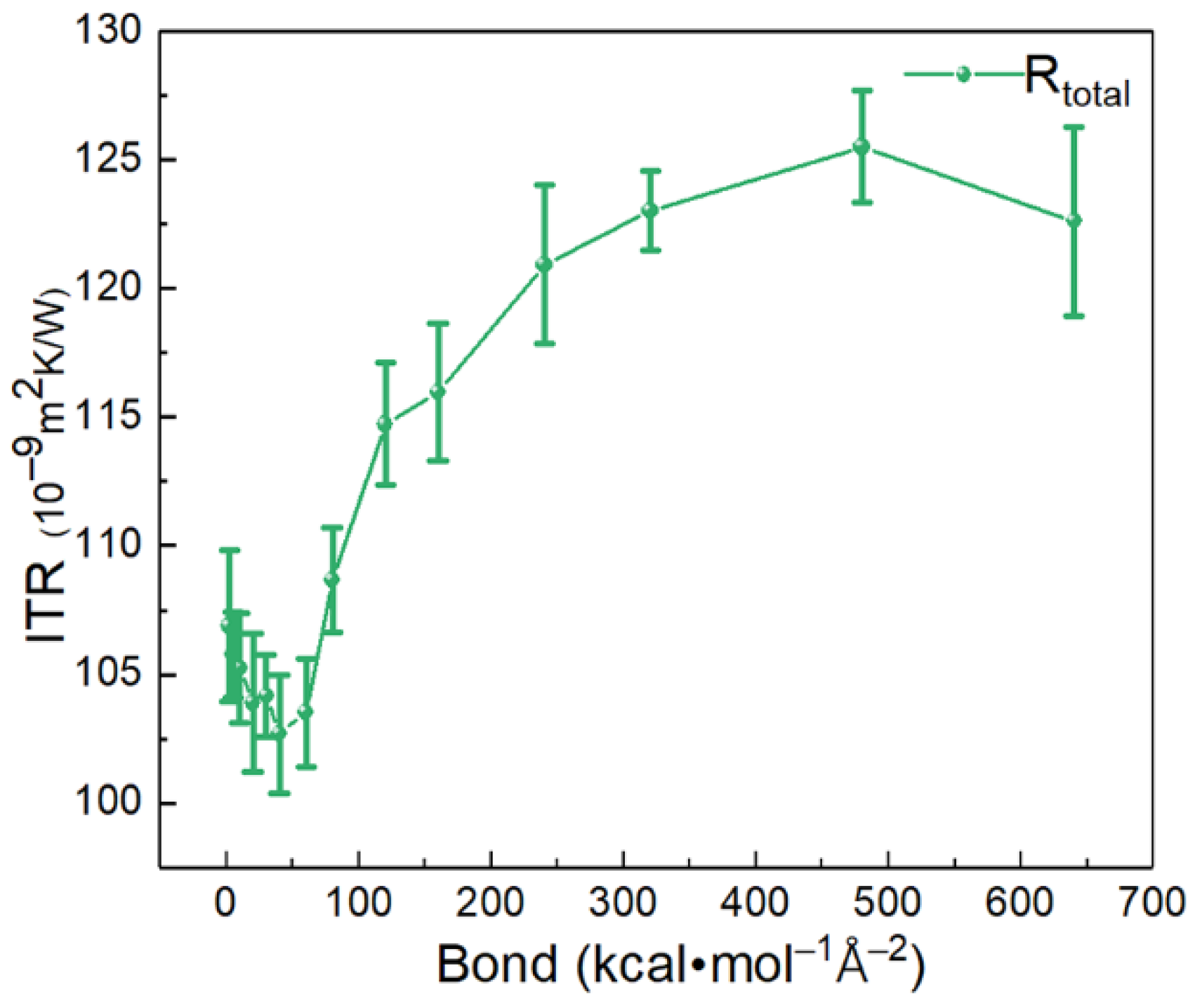
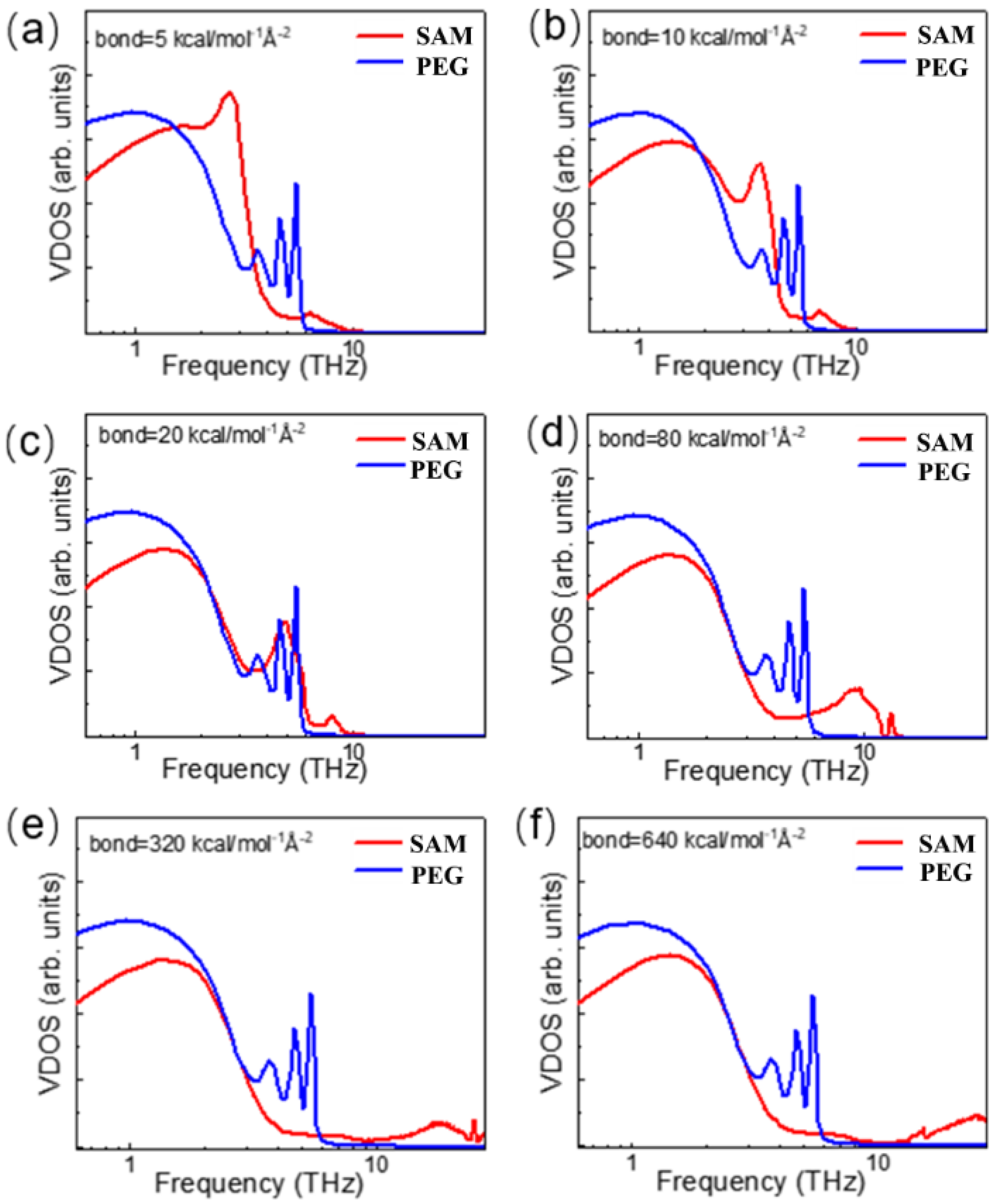
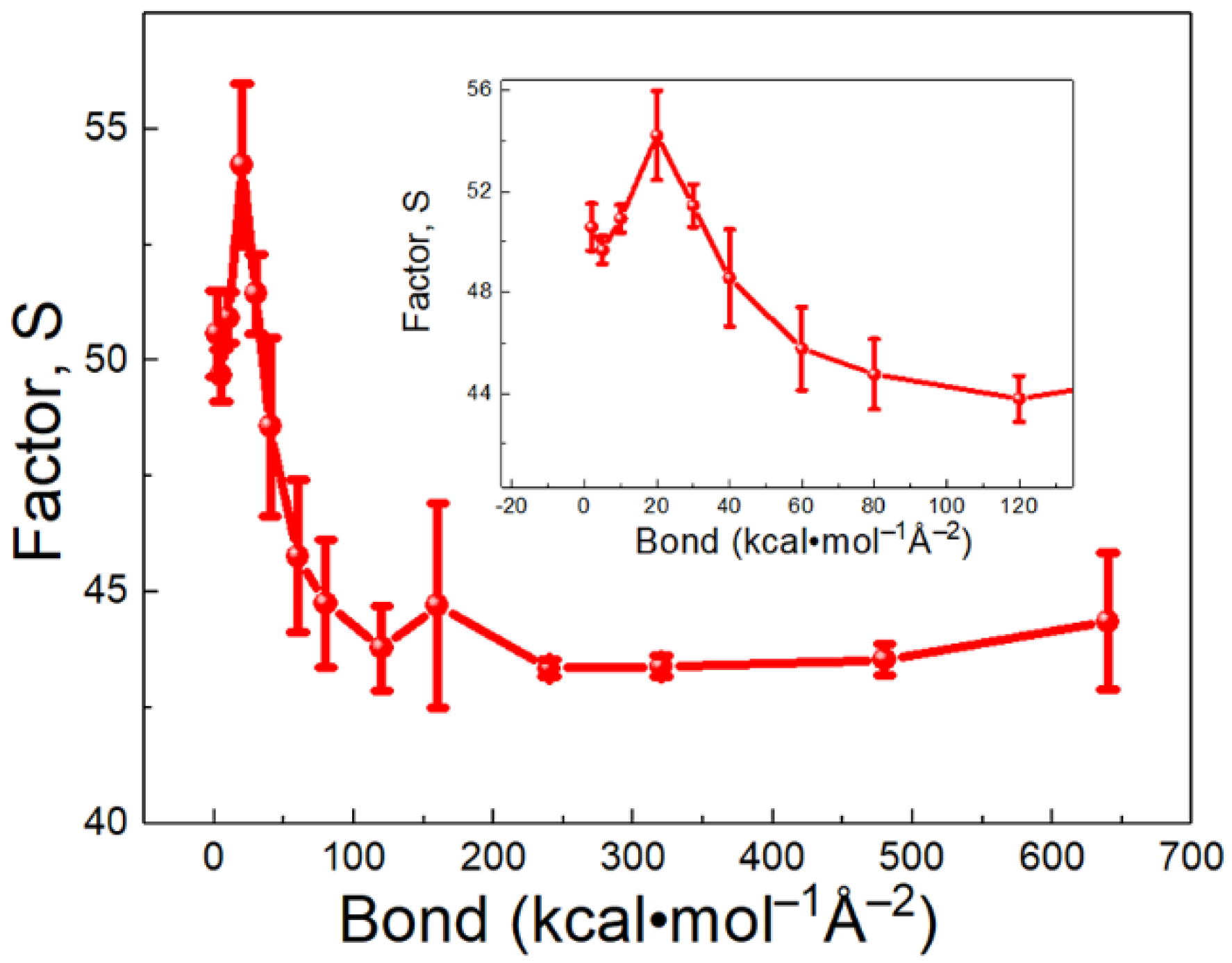
3.2. Bond Strength Effect of SAM on
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, K.J.; Shi, J.M.; Aftab, W.; Qin, M.L.; Usman, A.; Zhou, F.; Lv, Y.; Gao, S.; Zou, R.Q. Engineering the Thermal Conductivity of Functional Phase-Change Materials for Heat Energy Conversion, Storage, and Utilization. Adv. Funct. Mater. 2020, 30, 1904228. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhuo, S.W.; Zhang, Y.P. Pre-feasibility of building cooling heating and power system with thermal energy storage considering energy supply-demand mismatch. Appl. Energy 2016, 167, 125–134. [Google Scholar] [CrossRef]
- Dahash, A.; Ochs, F.; Janetti, M.B.; Streicher, W. Advances in seasonal thermal energy storage for solar district heating applications: A critical review on large-scale hot-water tank and pit thermal energy storage systems. Appl. Energy 2019, 239, 296–315. [Google Scholar] [CrossRef]
- Wang, Y.L.; Cao, Y.; Zhou, K.; Xu, Z.P. Assessment of Self-Assembled Monolayers as High-Performance Thermal Interface Materials. Adv. Mater. Interfaces 2017, 4, 1700355. [Google Scholar] [CrossRef]
- Wu, X.W.; Ni, Y.X.; Zhu, J.; Burrows, N.D.; Murphy, C.J.; Dumitrica, T.; Wang, X.J. Thermal Transport across Surfactant Layers on Gold Nanorods in Aqueous Solution. ACS Appl. Mater. Interfaces 2016, 8, 10581–10589. [Google Scholar] [CrossRef] [PubMed]
- Saha, L.C.; Kikugawa, G. Heat Conduction Performance over a Poly(ethylene glycol) Self-Assembled Monolayer/Water Interface: A Molecular Dynamics Study. J. Phys. Chem. B 2021, 125, 1896–1905. [Google Scholar] [CrossRef]
- He, J.L.; Zhang, L.; Liu, L. Improving thermal conduction across cathode/electrolyte interfaces in solid-state lithium-ion batteries by hierarchical hydrogen-bond network. Mater. Des. 2020, 194, 108927. [Google Scholar] [CrossRef]
- Kawagoe, Y.; Surblys, D.; Matsubara, H.; Kikugawa, G.; Ohara, T. Cross-Plane and In-Plane Heat Conductions in Layer-by-Layer Membrane: Molecular Dynamics Study. Langmuir 2020, 36, 6482–6493. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, M.Y.; Cheng, Y.H.; Luo, X.B. Bonding-induced thermal transport enhancement across a hard/soft material interface using molecular monolayers. Phys. Chem. Chem. Phys. 2017, 19, 7352–7358. [Google Scholar] [CrossRef]
- Lu, J.X.; Yuan, K.P.; Sun, F.Y.; Zheng, K.; Zhang, Z.Y.; Zhu, J.; Wang, X.W.; Zhang, X.L.; Zhuang, Y.F.; Ma, Y.M.; et al. Self-Assembled Monolayers for the Polymer/Semiconductor Interface with Improved Interfacial Thermal Management. ACS Appl. Mater. Interfaces 2019, 11, 42708–42714. [Google Scholar] [CrossRef]
- Tian, Z.T.; Marconnet, A.; Chen, G. Enhancing solid-liquid interface thermal transport using self-assembled monolayers. Appl. Phys. Lett. 2015, 106, 211602. [Google Scholar] [CrossRef]
- Stocker, K.M.; Gezelter, J.D. Simulations of Heat Conduction at Thiolate-Capped Gold Surfaces: The Role of Chain Length and Solvent Penetration. J. Phys. Chem. C 2013, 117, 7605–7612. [Google Scholar] [CrossRef]
- Zhang, T.; Gans-Forrest, A.R.; Lee, E.; Zhang, X.Q.; Qu, C.; Pang, Y.S.; Sun, F.; Luo, T.F. Role of Hydrogen Bonds in Thermal Transport across Hard/Soft Material Interfaces. ACS Appl. Mater. Interfaces 2016, 8, 33326–33334. [Google Scholar] [CrossRef]
- Zheng, K.; Sun, F.Y.; Zhu, J.; Ma, Y.M.; Li, X.B.; Tang, D.W.; Wang, F.S.; Wang, X.J. Enhancing the Thermal Conductance of Polymer and Sapphire Interface via Self-Assembled Monolayer. ACS Nano 2016, 10, 7792–7798. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L. Hierarchically hydrogen-bonded graphene/polymer interfaces with drastically enhanced interfacial thermal conductance. Nanoscale 2019, 11, 3656–3664. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.Z.; Ma, R.M.; Zhang, T.; Luo, T.F. Origin of Hydrophilic Surface Functionalization-Induced Thermal Conductance Enhancement across Solid-Water Interfaces. ACS Appl. Mater. Interfaces 2018, 10, 28159–28165. [Google Scholar] [CrossRef]
- Fan, H.Z.; Wang, M.; Han, D.; Zhang, J.Z.; Zhang, J.C.; Wang, X.Y. Enhancement of Interfacial Thermal Transport between Metal and Organic Semiconductor Using Self-Assembled Monolayers with Different Terminal Groups. J. Phys. Chem. C 2020, 124, 16748–16757. [Google Scholar] [CrossRef]
- Wei, X.F.; Zhang, T.; Luo, T.F. Molecular Fin Effect from Heterogeneous Self-Assembled Monolayer Enhances Thermal Conductance across Hard-Soft Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 33740–33748. [Google Scholar] [CrossRef]
- Goicochea, J.V.; Hu, M.; Michel, B.; Poulikakos, D. Surface Functionalization Mechanisms of Enhancing Heat Transfer at Solid-Liquid Interfaces. J. Heat Transf. 2011, 133, 082401. [Google Scholar] [CrossRef]
- Lee, E.; Menumerov, E.; Hughes, R.A.; Neretina, S.; Luo, T.F. Low-Cost Nanostructures from Nanoparticle-Assisted Large-Scale Lithography Significantly Enhance Thermal Energy Transport across Solid Interfaces. ACS Appl. Mater. Interfaces 2018, 10, 34690–34698. [Google Scholar] [CrossRef]
- Lee, E.; Zhang, T.; Yoo, T.H.; Guo, Z.; Luo, T.F. Nanostructures Significantly Enhance Thermal Transport across Solid Interfaces. ACS Appl. Mater. Interfaces 2016, 8, 35505–35512. [Google Scholar] [CrossRef]
- Ju, S.H.; Palpant, B.; Chalopin, Y. Adverse Effects of Polymer Coating on Heat Transport at the Solid-Liquid Interface. J. Phys. Chem. C 2017, 121, 13474–13480. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Zhang, T.; Jobbins, M.M.; Guo, Z.; Zhang, X.; Zheng, Z.; Tang, D.; Ptasinska, S.; Luo, T. Molecular Bridge Enables Anomalous Enhancement in Thermal Transport across Hard-Soft Material Interfaces. Adv. Mater. 2014, 26, 6093–6099. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, T. Role of Chain Morphology and Stiffness in Thermal Conductivity of Amorphous Polymers. J. Phys. Chem. B 2016, 120, 803–812. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Oyola-Reynoso, S.; Thuo, M.M. Properties of Self-Assembled Monolayers Revealed via Inverse Tensiometry. Langmuir 2017, 33, 13451–13467. [Google Scholar] [CrossRef]
- Thompson, D.; Nijhuis, C.A. Even the Odd Numbers Help: Failure Modes of SAM-Based Tunnel Junctions Probed via Odd-Even Effects Revealed in Synchrotrons and Supercomputers. Acc. Chem. Res. 2016, 49, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, H.; Morovati, V.; Dargazany, R. PEGylation on mixed monolayer gold nanoparticles: Effect of grafting density, chain length, and surface curvature. J. Colloid Interface Sci. 2017, 504, 325–333. [Google Scholar] [CrossRef]
- Jiao, F.; Sang, J.; Liu, Z.; Liu, W.; Liang, W. Effect of concentration of PEG coated gold nanoparticle on lung surfactant studied with coarse-grained molecular dynamics simulations. Biophys. Chem. 2020, 266, 106457. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bellucci, L.; Agostini, M.; Gagliardi, M.; Corni, S.; Cecchini, M.; Brancolini, G. Atomistic simulations of gold surface functionalization for nanoscale biosensors applications. Nanotechnology 2020, 32, 095702. [Google Scholar] [CrossRef]
- Power, A.J.; Remediakis, I.N.; Harmandaris, V. Interface and Interphase in Polymer Nanocomposites with Bare and Core-Shell Gold Nanoparticles. Polymers 2021, 13, 541. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Lloyd, J.R. Non-equilibrium molecular dynamics study of thermal energy transport in Au–SAM–Au junctions. Int. J. Heat Mass Transf. 2010, 53, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Cao, D. Aggregation of polymer-grafted nanoparticles in good solvents: A hierarchical modeling method. J. Chem. Phys. 2011, 135, 124703. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Lloyd, J.R. Equilibrium Molecular Dynamics Study of Lattice Thermal Conductivity/Conductance of Au-SAM-Au Junctions. J. Heat Transf. 2009, 132, 032401. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; de Vries, A.H.; Marrink, S.-J.; Pastor, R.W. A Coarse-Grained Model for Polyethylene Oxide and Polyethylene Glycol: Conformation and Hydrodynamics. J. Phys. Chem. B 2009, 113, 13186–13194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Subramanyan, H.; Zhang, W.; He, J.; Kim, K.; Li, X.; Liu, J. Role of angular bending freedom in regulating thermal transport in polymers. J. Appl. Phys. 2019, 125, 095104. [Google Scholar] [CrossRef]
- Stukowski, A. Computational Analysis Methods in Atomistic Modeling of Crystals. JOM 2014, 66, 399–407. [Google Scholar] [CrossRef]
- Liang, T.; Zhou, M.; Zhang, P.; Yuan, P.; Yang, D. Multilayer in-plane graphene/hexagonal boron nitride heterostructures: Insights into the interfacial thermal transport properties. Int. J. Heat Mass Transf. 2020, 151, 119395. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Zhang, Y.; Yang, W.; Tan, J.; Cheng, L. Molecular Structure Effect of a Self-Assembled Monolayer on Thermal Resistance across an Interface. Polymers 2021, 13, 3732. https://doi.org/10.3390/polym13213732
Song L, Zhang Y, Yang W, Tan J, Cheng L. Molecular Structure Effect of a Self-Assembled Monolayer on Thermal Resistance across an Interface. Polymers. 2021; 13(21):3732. https://doi.org/10.3390/polym13213732
Chicago/Turabian StyleSong, Lijian, Youchen Zhang, Weimin Yang, Jing Tan, and Lisheng Cheng. 2021. "Molecular Structure Effect of a Self-Assembled Monolayer on Thermal Resistance across an Interface" Polymers 13, no. 21: 3732. https://doi.org/10.3390/polym13213732






