Ternary Nanocomposite System Composing of Graphene Nanoplatelet, Cellulose Nanofiber and Jatropha Oil Based Waterborne Polyurethane: Characterizations, Mechanical, Thermal Properties and Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Jatropha Oil Based Polyol (JOL)
2.3. Preparation of Jatropha Oil Based Polyurethane Dispersion
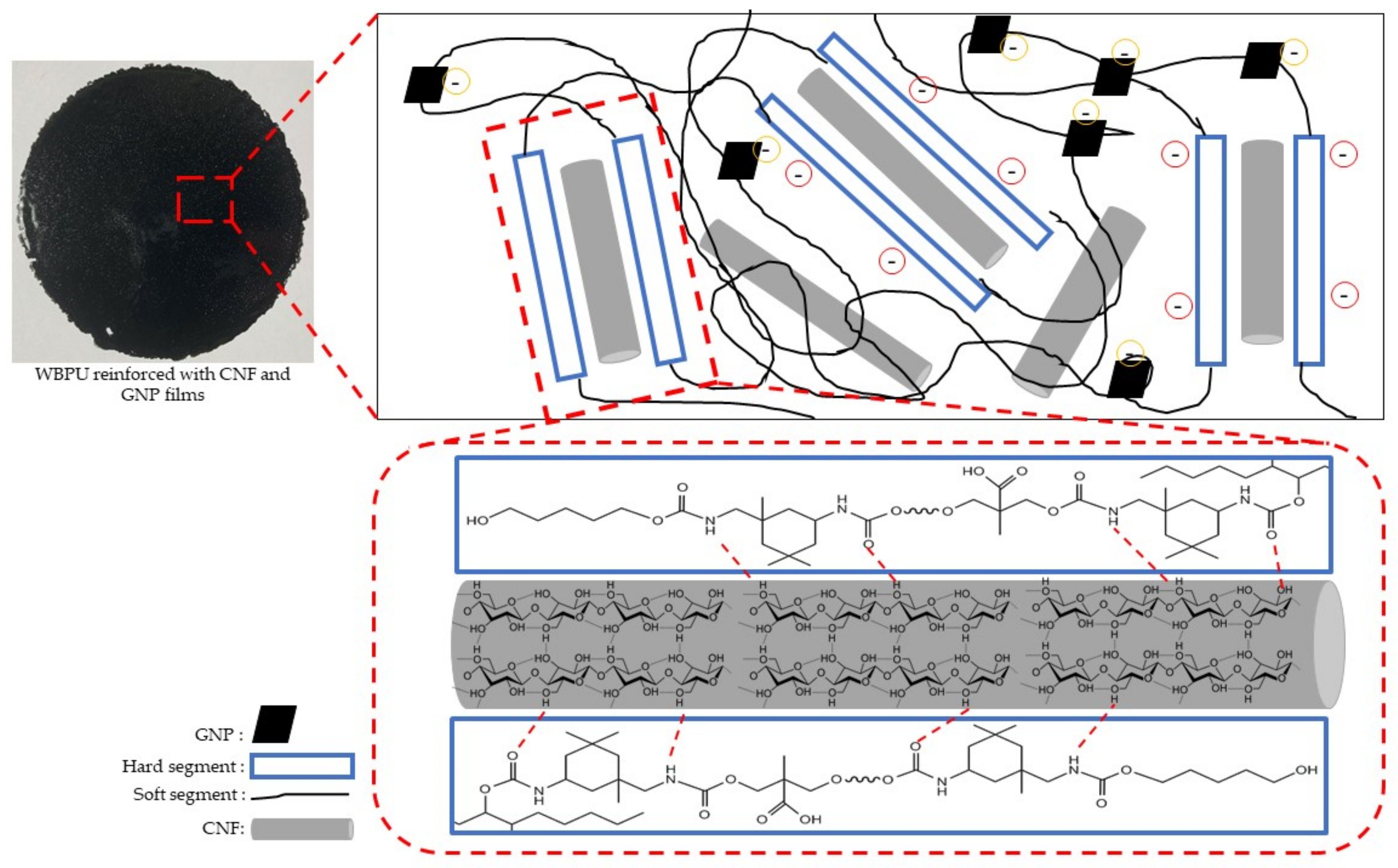
2.4. Preparation of WBPU-CNF-GNP Nanocomposite Film
2.5. Characterisation
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR) Characterisation
2.5.2. Mechanical Properties Characterisation
2.5.3. Thermal Degradation Analysis
2.5.4. Morphology Evaluation
2.5.5. Water Uptake Analysis
2.5.6. Water Contact Angle
2.5.7. Conductivity Analysis
3. Result and Discussion
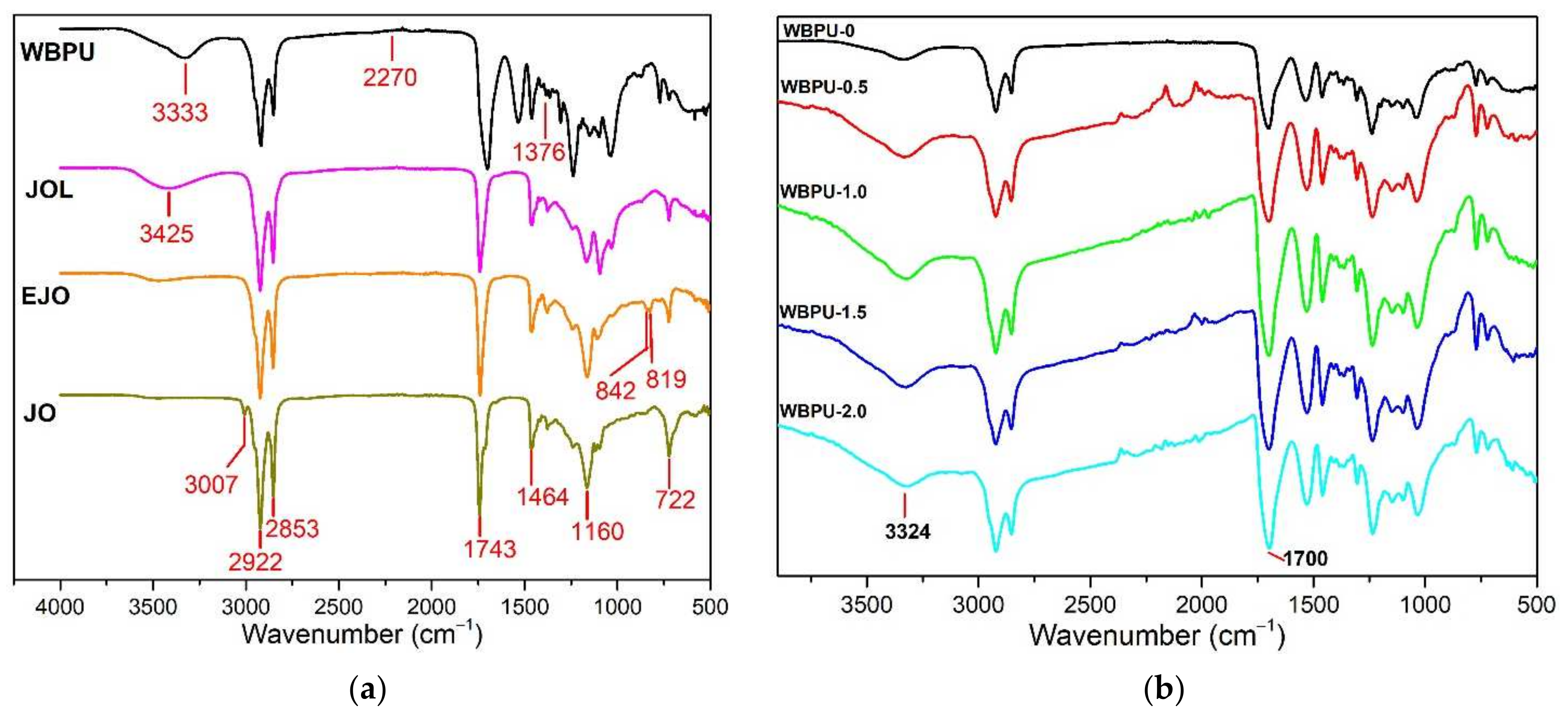
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Surface Morphology
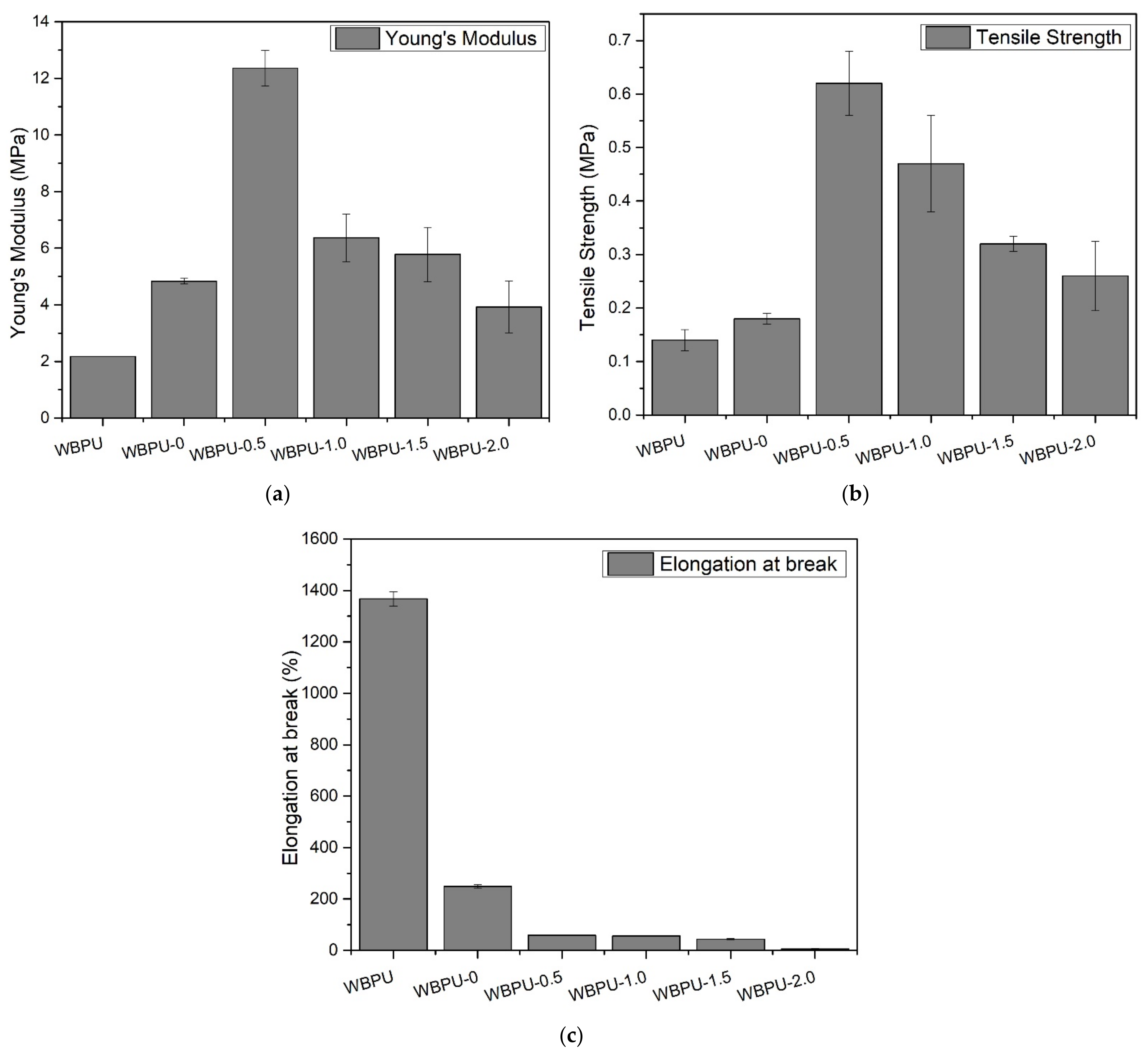
3.3. Mechanical Properties
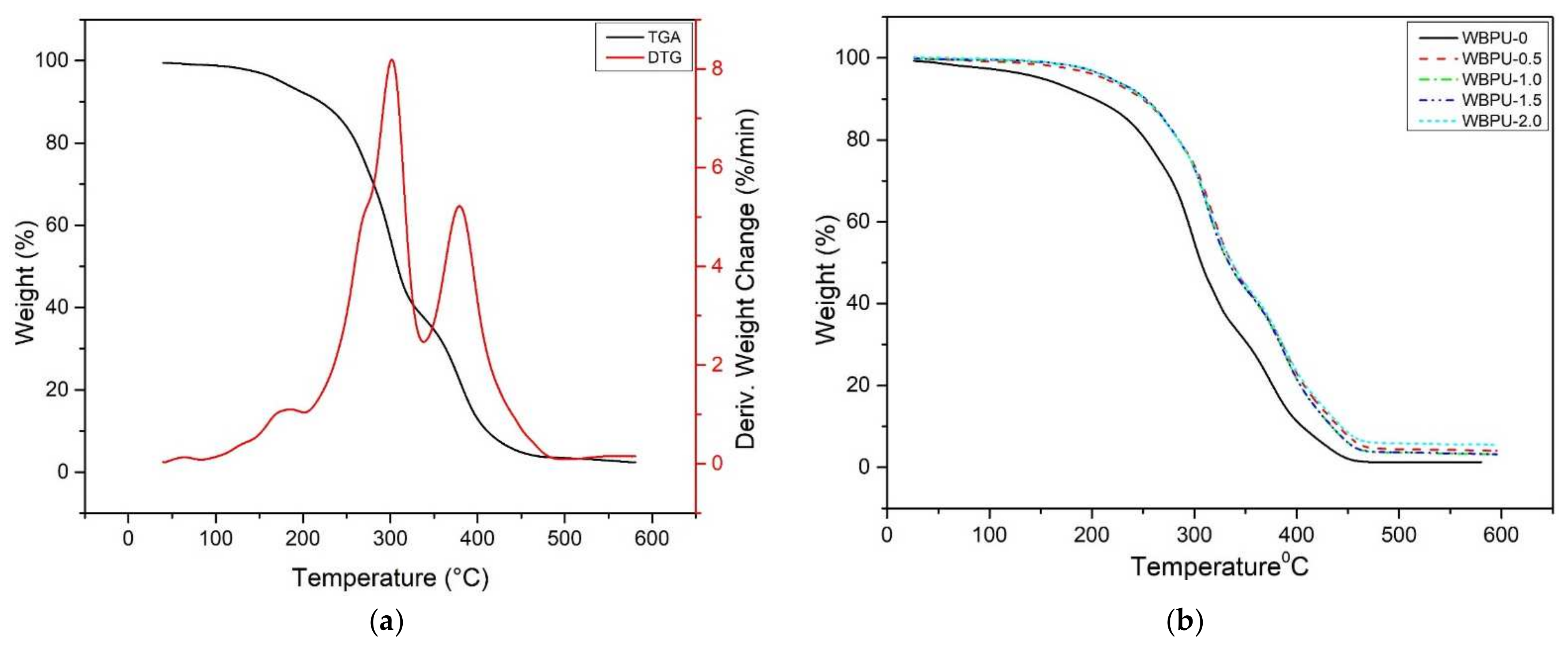
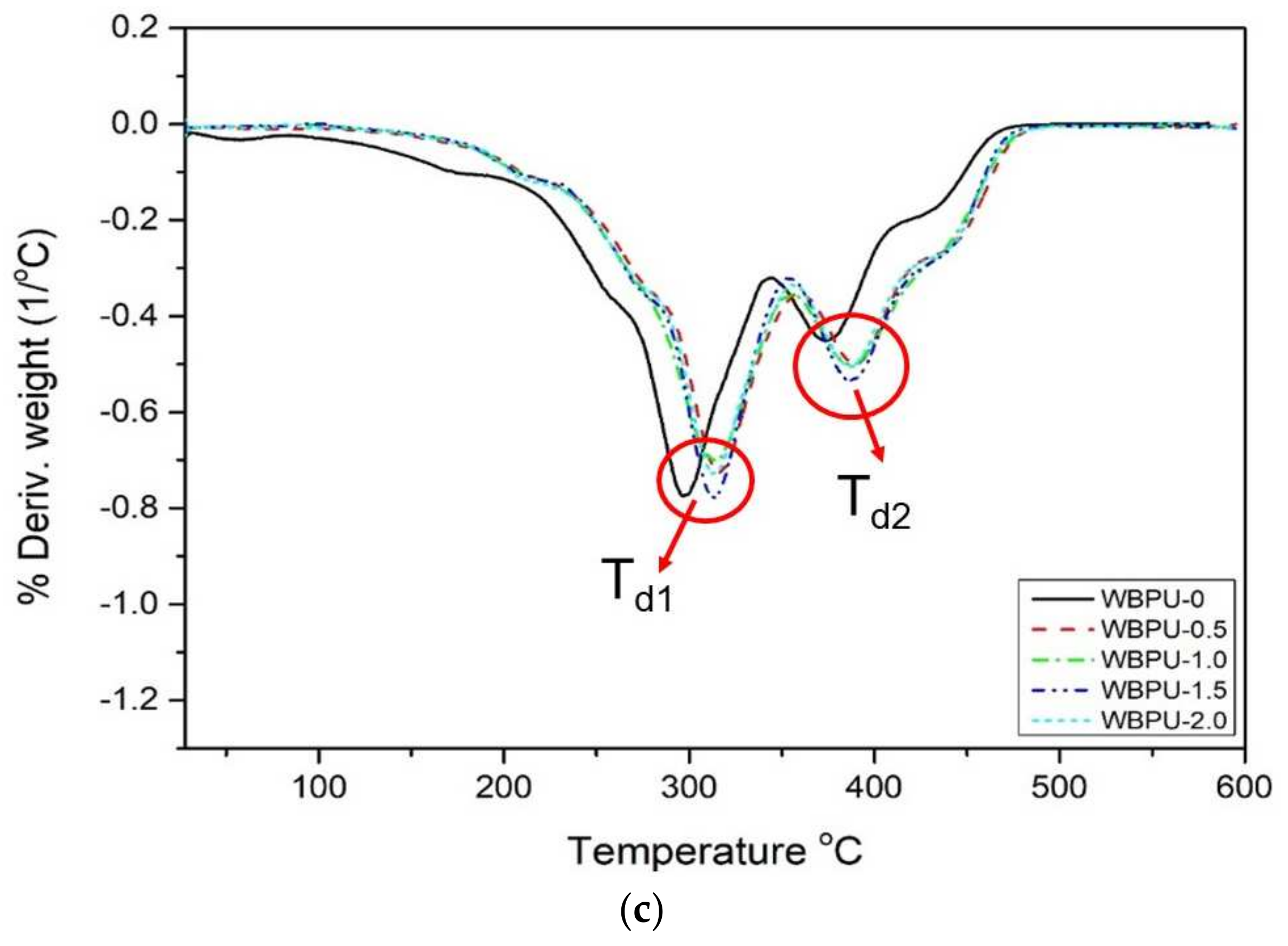
3.4. Thermal Properties
3.5. Water Contact Angle Measurement
3.6. Water Uptake Measurement
3.7. Conductivity Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gübitz, G.; Mittelbach, M.; Trabi, M. Exploitation of the tropical oil seed plant Jatropha curcas L. Bioresour. Technol. 1999, 67, 73–82. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Pan, Y.; Schubert, D.W.; Liu, C. Shear-induced rheological and electrical properties of molten poly(methyl methacrylate)/carbon black nanocomposites. Compos. Part B Eng. 2019, 164, 37–44. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, B.; Zheng, G.; Liu, X.; Li, T.; Yan, C.; Cheng, C.; Dai, K.; Liu, C.; Shen, C.; et al. Continuously prepared highly conductive and stretchable SWNT/MWNT synergistically composited electrospun thermoplastic polyurethane yarns for wearable sensing. J. Mater. Chem. C 2018, 6, 2258–2269. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Pang, H.; Xu, L.; Yan, D.-X.; Li, Z.-M. Conductive polymer composites with segregated structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Zheng, G.; Schubert, D.W. Rheological and electrical behavior of poly(methyl methacrylate)/carbon black composites as investigated by creep recovery in shear. Compos. Sci. Technol. 2016, 128, 1–7. [Google Scholar] [CrossRef]
- Rueda, L.; D’Arlas, B.F.; Corcuera, M.A.; Eceiza, A. Biostability of polyurethanes. Study from the viewpoint of microphase separated structure. Polym. Degrad. Stab. 2014, 108, 195–200. [Google Scholar] [CrossRef]
- Leitsch, E.K.; Heath, W.H.; Torkelson, J.M. Polyurethane/polyhydroxyurethane hybrid polymers and their applications as adhesive bonding agents. Int. J. Adhes. Adhes. 2016, 64, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Qiang, S.; Yang, F.; Zhao, C.; Wang, C.; Yin, Y. Synthesis of blocked and branched waterborne polyurethanes for pigment printing applications. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, F.; Chen, S.; Yang, H.; Ge, Z.; Chen, S. Development of shape memory polyurethane based on polyethylene glycol and liquefied 4,4′-diphenylmethane diisocyanate using a bulk method for biomedical applications. Polym. Int. 2015, 64, 477–485. [Google Scholar] [CrossRef]
- Si, H.; Liu, H.; Shang, S.; Song, J.; Liao, S.; Wang, D.; Song, Z. Maleopimaric acid-modified two-component waterborne polyurethane for coating applications. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Kong, L.; Xu, D.; He, Z.; Wang, F.; Gui, S.; Fan, J.; Pan, X.; Dai, X.; Dong, X.; Liu, B.; et al. Nanocellulose-Reinforced Polyurethane for Waterborne Wood Coating. Molecules 2019, 24, 3151. [Google Scholar] [CrossRef] [Green Version]
- Amri, M.; Guan, C.; Al-Edrus, S.O.; Yasin, F.M.; Mohamad, S. Effect of Cellulose Nanofibrils on the Properties of Jatropha Oil-Based Waterborne Polyurethane Nanocomposite Film. Polymers 2021, 13, 1460. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent advances in the synthesis and applications of graphene–polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Chen, Z. Graphene-related nanomaterials: Tuning properties by functionalization. Nanoscale 2013, 5, 4541–4583. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, P.; Chen, Y.; Su, Z.; Wei, G. Recent advances in the fabrication and structure-specific applications of graphene-based inorganic hybrid membranes. Nanoscale 2015, 7, 5080–5093. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An Overview of the Applications of Graphene-Based Materials in Supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, P.; Liu, R.; Li, W.; Ge, B.; Cao, M. Preparation and physical properties of functionalized graphene/waterborne polyurethane UV-curing composites by click chemistry. Polym. Int. 2016, 65, 415–422. [Google Scholar] [CrossRef]
- Lee, Y.R.; Raghu, A.V.; Jeong, H.M.; Kim, B.K. Properties of Waterborne Polyurethane/Functionalized Graphene Sheet Nanocomposites Prepared by an in situ Method. Macromol. Chem. Phys. 2009, 210, 1247–1254. [Google Scholar] [CrossRef]
- SaifulAzry, S.O.A.; Chuah, T.G.; Paridah, M.T.; Aung, M.M.; A Ridzuan, M.; Lee, C.H.; Sariah, S.; Lee, S.H.; Juliana, A.H. Influence of cellulose II polymorph nanowhiskers on bio-based nanocomposite film from Jatropha oil polyurethane. Mater. Res. Express 2021, 8, 015003. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Basri, M.; Jusoh, E.R. Waterborne polyurethane dispersions synthesized from jatropha oil. Ind. Crop. Prod. 2015, 64, 194–200. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, X.; Yu, Q.; Liu, S.; Guo, D.; Yu, R.; Hu, J. Synthesis and characterization of low crystalline waterborne polyurethane for potential application in water-based ink binder. Prog. Org. Coatings 2014, 77, 61–71. [Google Scholar] [CrossRef]
- Gao, K.; Shao, Z.; Wu, X.; Wang, X.; Li, J.; Zhang, Y.; Wang, W.; Wang, F. Cellulose nanofibers/reduced graphene oxide flexible transparent conductive paper. Carbohydr. Polym. 2013, 97, 243–251. [Google Scholar] [CrossRef]
- Das, T.K.; Prusty, S. Graphene-Based Polymer Composites and Their Applications. Polym. Plast. Technol. Eng. 2013, 52, 319–331. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Wang, Y.; Liu, Y.; Liu, Y.; Chen, Y. New nanocomposite materials reinforced with cellulose nanocrystals in nitrile rubber. Polym. Test. 2013, 32, 819–826. [Google Scholar] [CrossRef]
- Benhamou, K.; Kaddami, H.; Magnin, A.; Dufresne, A.; Ahmad, A. Bio-based polyurethane reinforced with cellulose nanofibers: A comprehensive investigation on the effect of interface. Carbohydr. Polym. 2015, 122, 202–211. [Google Scholar] [CrossRef]
- SaifulAzry, S.O.A.; Chuah, T.G.; Paridah, M.T.; Aung, M.M.; Edi, S.Z. Effects of polymorph transformation via mercerisation on microcrystalline cellulose fibres and isolation of nanocrystalline cellulose fibres. Pertanika J. Sci. Technol. 2017, 25, 1275–1290. [Google Scholar]
- Jiménez-Pardo, I.; Sun, P.; Van Benthem, R.; Esteves, A. Design of self-dispersible charged-polymer building blocks for waterborne polyurethane dispersions. Eur. Polym. J. 2018, 101, 324–331. [Google Scholar] [CrossRef]
- Hsiao, S.-T.; Ma, C.-C.M.; Tien, H.-W.; Liao, W.-H.; Wang, Y.-S.; Li, S.-M.; Huang, Y.-C. Using a non-covalent modification to prepare a high electromagnetic interference shielding performance graphene nanosheet/water-borne polyurethane composite. Carbon 2013, 60, 57–66. [Google Scholar] [CrossRef]
- Cunha, E.; Paiva, M.C. Composite Films of Waterborne Polyurethane and Few-Layer Graphene—Enhancing Barrier, Mechanical, and Electrical Properties. J. Compos. Sci. 2019, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Król, P.; Król, B.; Zenker, M.; Subocz, J. Composites prepared from the waterborne polyurethane cationomers-modified graphene. Part II. Electrical properties of the polyurethane films. Colloid Polym. Sci. 2015, 293, 2941–2947. [Google Scholar] [CrossRef] [Green Version]
- Raghu, A.V.; Lee, Y.R.; Jeong, H.M.; Shin, C.M. Preparation and Physical Properties of Waterborne Polyurethane/Functionalized Graphene Sheet Nanocomposites. Macromol. Chem. Phys. 2008, 209, 2487–2493. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, D.H.; Raghu, A.V.; Reddy, K.R.; Lee, H.-I.; Yoon, K.S.; Jeong, H.M.; Kim, B.K. Properties of Graphene/Waterborne Polyurethane Nanocomposites Cast from Colloidal Dispersion Mixtures. J. Macromol. Sci. Part B 2011, 51, 197–207. [Google Scholar] [CrossRef]
- SaifulAzry, S.O.A.; Chuah, T.G.; Paridah, T.; Aung, M.M.; Zainudin, E.S. 17. Green nanocomposites from cellulose nanowhiskers and Jatropha oil–based polyurethane. In Cellulose-Reinforced Nanofibre Composites Production, Properties and Applications; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Park, S.H.; Oh, K.W.; Kim, S.H. Reinforcement effect of cellulose nanowhisker on bio-based polyurethane. Compos. Sci. Technol. 2013, 86, 82–88. [Google Scholar] [CrossRef]
- Larraza, I.; Vadillo, J.; Santamaria-Echart, A.; Tejado, A.; Azpeitia, M.; Vesga, E.; Orue, A.; Saralegi, A.; Arbelaiz, A.; Eceiza, A. The effect of the carboxylation degree on cellulose nanofibers and waterborne polyurethane/cellulose nanofiber nanocomposites properties. Polym. Degrad. Stab. 2020, 173, 109084. [Google Scholar] [CrossRef]
- Gao, Z.; Peng, J.; Zhong, T.; Sun, J.; Wang, X.; Yue, C. Biocompatible elastomer of waterborne polyurethane based on castor oil and polyethylene glycol with cellulose nanocrystals. Carbohydr. Polym. 2012, 87, 2068–2075. [Google Scholar] [CrossRef]
- Pei, A.; Malho, J.-M.; Ruokolainen, J.; Zhou, Q.; Berglund, L.A. Strong Nanocomposite Reinforcement Effects in Polyurethane Elastomer with Low Volume Fraction of Cellulose Nanocrystals. Macromolecules 2011, 44, 4422–4427. [Google Scholar] [CrossRef]
- Shao, W.; Wang, S.; Liu, H.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Preparation of bacterial cellulose/graphene nanosheets composite films with enhanced mechanical performances. Carbohydr. Polym. 2016, 138, 166–171. [Google Scholar] [CrossRef]
- Mucci, V.L.; Ivdre, A.; Buffa, J.M.; Cabulis, U.; Stefani, P.M.; Aranguren, M.I. Composites made from a soybean oil biopolyurethane and cellulose nanocrystals. Polym. Eng. Sci. 2017, 58, 125–132. [Google Scholar] [CrossRef]
- Kim, M.S.; Ryu, K.M.; Lee, S.H.; Choi, Y.C.; Jeong, Y.G. Influences of cellulose nanofibril on microstructures and physical properties of waterborne polyurethane-based nanocomposite films. Carbohydr. Polym. 2019, 225, 115233. [Google Scholar] [CrossRef]
- Thiyagu, C.; Manjubala, I.; Narendrakumar, U. Thermal and morphological study of graphene based polyurethane composites. Mater. Today Proc. 2019, 45, 3982–3985. [Google Scholar] [CrossRef]
- Cao, X.; Habibi, Y.; Lucia, L.A. One-pot polymerization, surface grafting, and processing of waterborne polyurethane-cellulose nanocrystal nanocomposites. J. Mater. Chem. 2009, 19, 7137–7145. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Amri, M.R.; Al-Edrus, S.S.O.; Guan, C.T.; Yasin, F.M.; Hua, L.S. Jatropha Oil as a Substituent for Palm Oil in Biobased Polyurethane. Int. J. Polym. Sci. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Aung, M.M.; Li, W.J.; Lim, H.N. Improvement of Anticorrosion Coating Properties in Bio-Based Polymer Epoxy Acrylate Incorporated with Nano Zinc Oxide Particles. Ind. Eng. Chem. Res. 2020, 59, 1753–1763. [Google Scholar] [CrossRef]
- Ccorahua, R.; Troncoso, O.P.; Rodriguez, S.; Lopez, D.; Torres, F.G. Hydrazine treatment improves conductivity of bacterial cellulose/graphene nanocomposites obtained by a novel processing method. Carbohydr. Polym. 2017, 171, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Drzal, L.T. The use of cellulose nanofibrils to enhance the mechanical properties of graphene nanoplatelets papers with high electrical conductivity. Ind. Crop. Prod. 2018, 124, 519–529. [Google Scholar] [CrossRef]
- Howard, G.T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Chaharmahali, M.; Hamzeh, Y.; Ebrahimi, G.; Ashori, A.; Ghasemi, I. Effects of nano-graphene on the physico-mechanical properties of bagasse/polypropylene composites. Polym. Bull. 2014, 71, 337–349. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Water-Based Polyurethane Filled with Multi-Walled Carbon Nanotubes Prepared by a Colloidal-Physics Method. Macromol. Chem. Phys. 2007, 208, 1183–1189. [Google Scholar] [CrossRef]






| GNP a | CNF a | JOL a | DMPA a | IPDI a | HDO a | TEA a | Water a | |
|---|---|---|---|---|---|---|---|---|
| WBPU | 0 | 0 | 52.54 | 11.2 | 24.52 | 5.17 | 6.57 | 16 |
| WBPU-0 | 0 | 0.5 | 52.54 | 11.2 | 24.52 | 5.17 | 6.57 | 16 |
| WBPU-0.5 | 0.5 | 0.5 | 52.54 | 11.2 | 24.52 | 5.17 | 6.57 | 16 |
| WBPU-1.0 | 1 | 0.5 | 52.54 | 11.2 | 24.52 | 5.17 | 6.57 | 16 |
| WBPU-1.5 | 1.5 | 0.5 | 52.54 | 11.2 | 24.52 | 5.17 | 6.57 | 16 |
| WBPU-2.0 | 2 | 0.5 | 52.54 | 11.2 | 24.52 | 5.17 | 6.57 | 16 |
| Band Assignment | Wavenumber (cm−1) |
|---|---|
| Neat WBPU | |
| ~3600–3000 | |
| ~1700 | |
| ~1550 | |
| ~1376 | |
| CNF | |
| ~3372 | |
| ~2894 | |
| ~1592 | |
| ~1059 | |
| GNP | |
| ~3324 | |
| ~1700 |
| Tdec (°C) | Weight Loss (%) | Residue at 600 °C (%) | |||
|---|---|---|---|---|---|
| Td1 | Td2 | Wd1 | Wd2 | ||
| WBPU | 299.76 | 378.79 | 53.82 | 40.72 | 0.9962 |
| WBPU-0 | 313.42 | 380.25 | 60.70 | 35.25 | 0.0001 |
| WBPU-0.5 | 316.28 | 389.25 | 53.43 | 25.98 | 0.0001 |
| WBPU-1.0 | 315.18 | 388.19 | 53.55 | 24.66 | 0.0001 |
| WBPU-1.5 | 316.55 | 388.30 | 51.59 | 28.87 | 0.0001 |
| WBPU-2.0 | 316.05 | 387.88 | 53.84 | 24.37 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amri, M.R.; Md Yasin, F.; Abdullah, L.C.; Al-Edrus, S.S.O.; Mohamad, S.F. Ternary Nanocomposite System Composing of Graphene Nanoplatelet, Cellulose Nanofiber and Jatropha Oil Based Waterborne Polyurethane: Characterizations, Mechanical, Thermal Properties and Conductivity. Polymers 2021, 13, 3740. https://doi.org/10.3390/polym13213740
Amri MR, Md Yasin F, Abdullah LC, Al-Edrus SSO, Mohamad SF. Ternary Nanocomposite System Composing of Graphene Nanoplatelet, Cellulose Nanofiber and Jatropha Oil Based Waterborne Polyurethane: Characterizations, Mechanical, Thermal Properties and Conductivity. Polymers. 2021; 13(21):3740. https://doi.org/10.3390/polym13213740
Chicago/Turabian StyleAmri, Mohamad Ridzuan, Faizah Md Yasin, Luqman Chuah Abdullah, Syeed Saifulazry Osman Al-Edrus, and Siti Fatahiyah Mohamad. 2021. "Ternary Nanocomposite System Composing of Graphene Nanoplatelet, Cellulose Nanofiber and Jatropha Oil Based Waterborne Polyurethane: Characterizations, Mechanical, Thermal Properties and Conductivity" Polymers 13, no. 21: 3740. https://doi.org/10.3390/polym13213740








