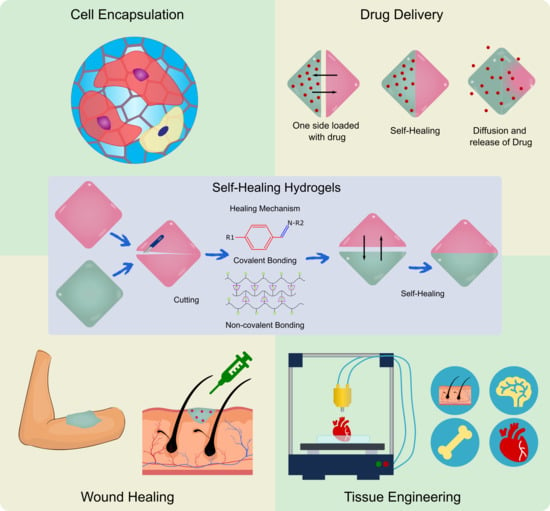
Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications
Abstract
:1. Introduction
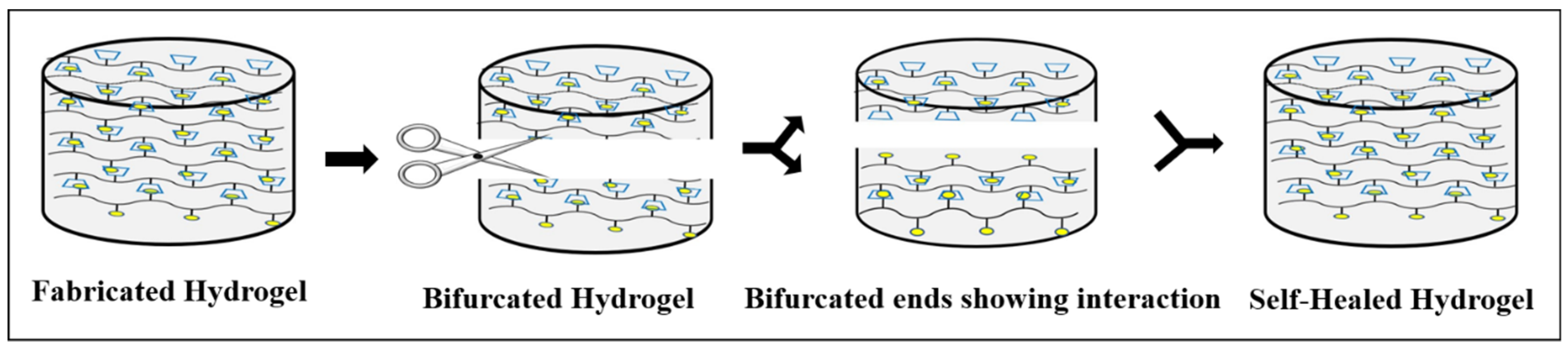
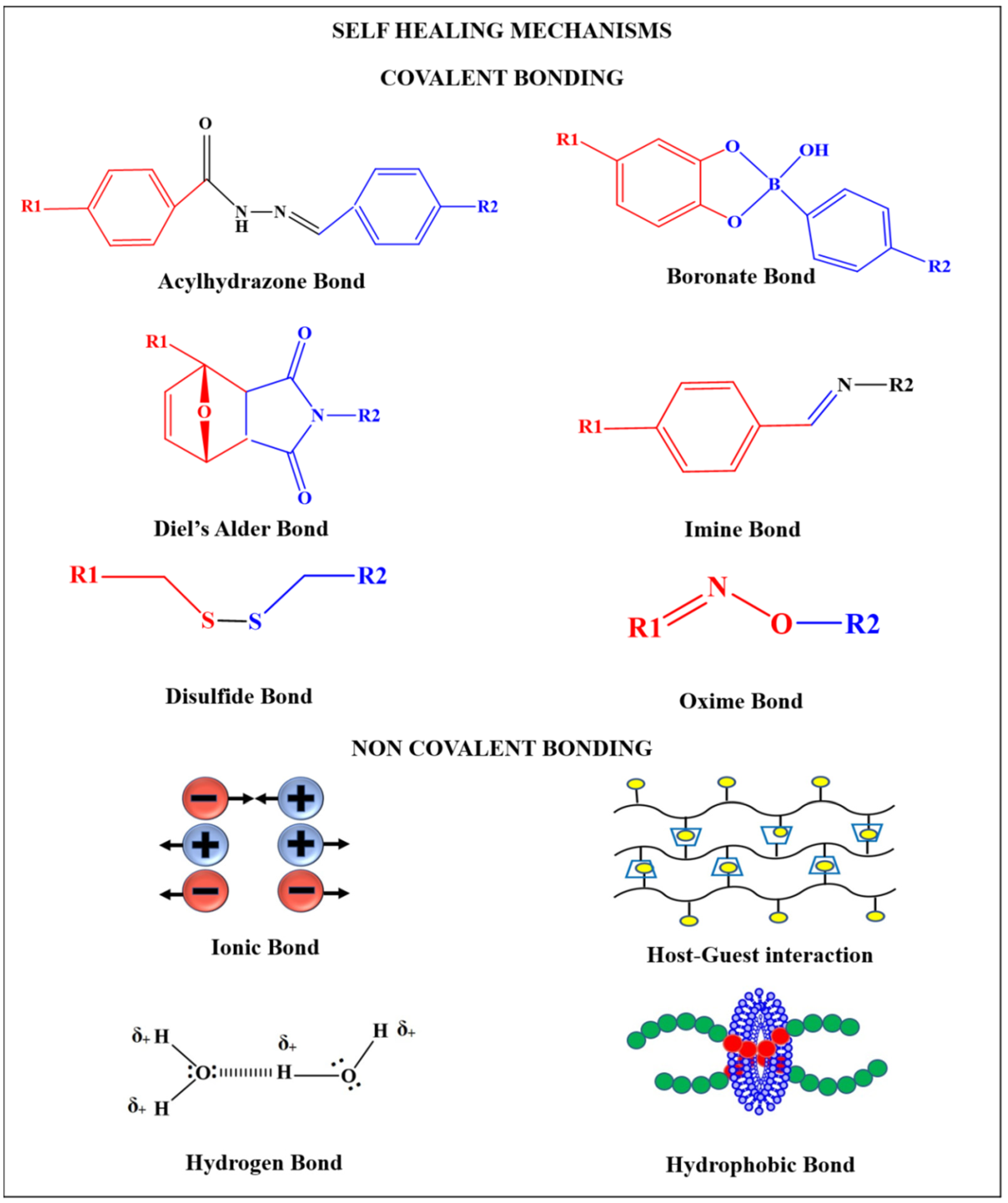
2. Self-Healing Mechanism
2.1. Hydrogen Bonding
2.2. Ionic Bonds
2.3. Host-Guest Interactions
2.4. Hydrophobic Bonds
2.5. Imine Bonds
2.6. Disulfide Bond
2.7. Acylhydrazone Bonds
2.8. Diels-Alder Reaction
2.9. Boronate Bonds
2.10. Oxime Bonds
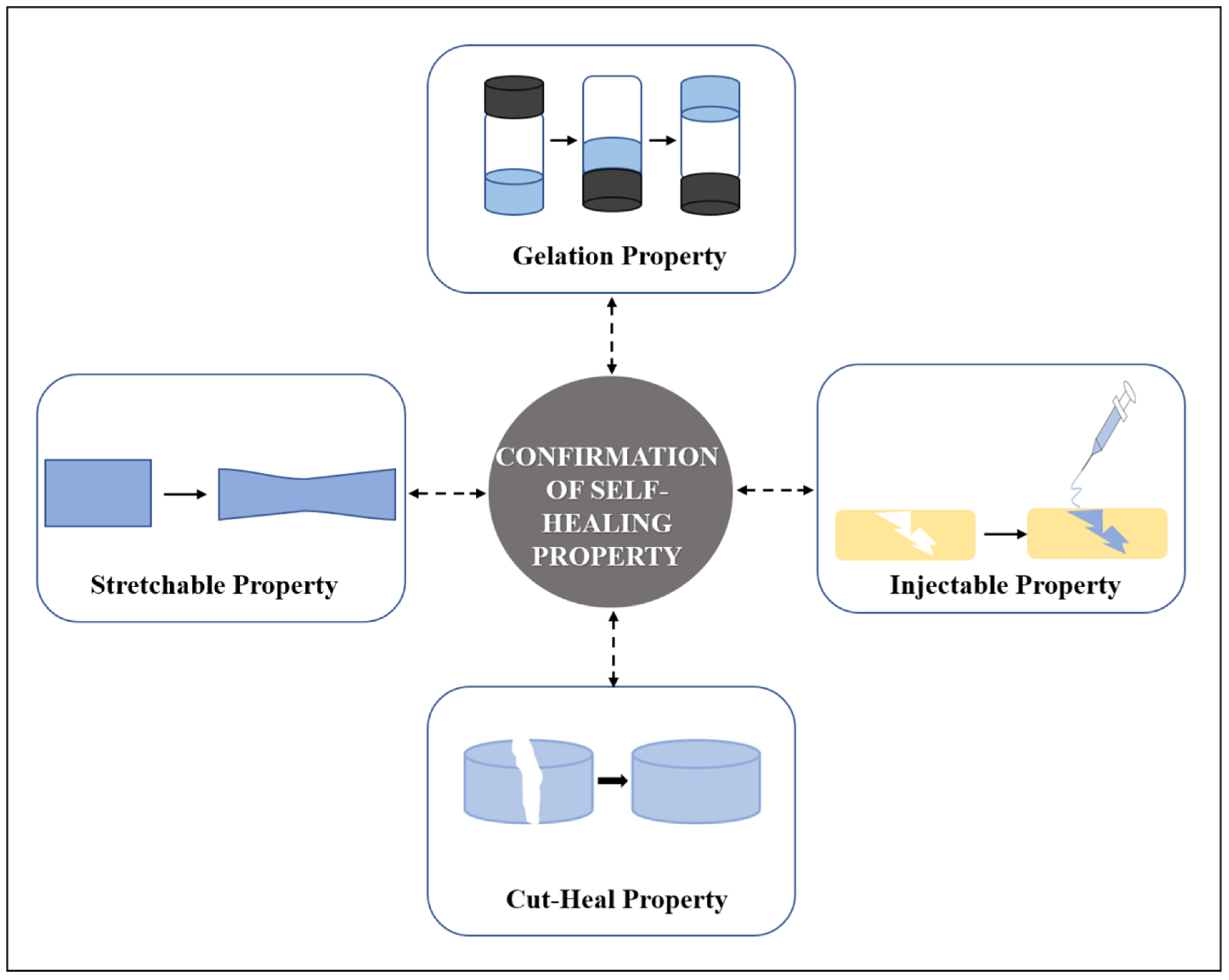
3. Testing of Self-Healing Property
3.1. Common Testings of Self Healing Hydrogels
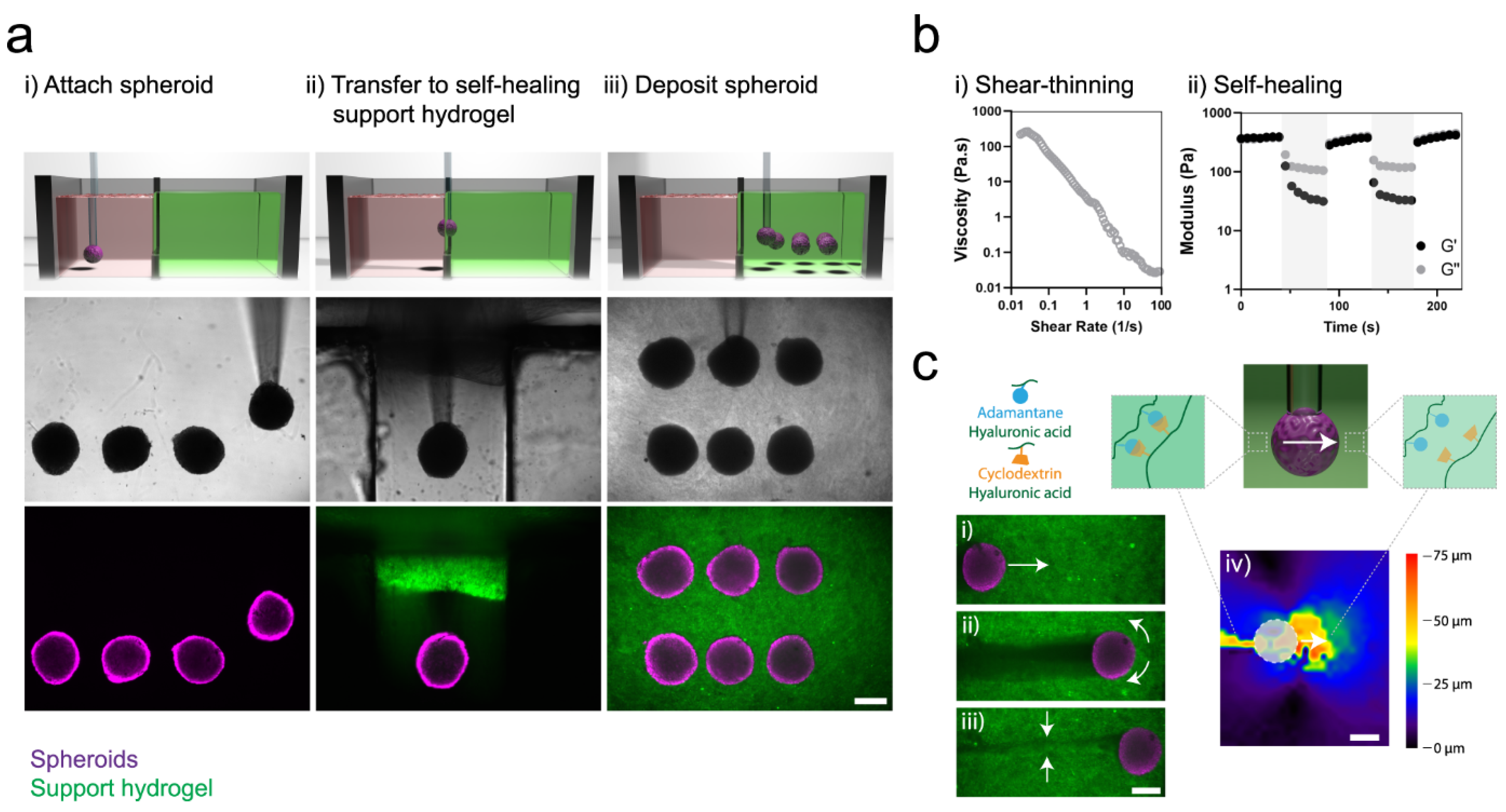
3.2. Rheological Recovery Test
4. Applications of Self-Healing Hydrogels
4.1. Self-Healing Hydrogels in Wound Healing
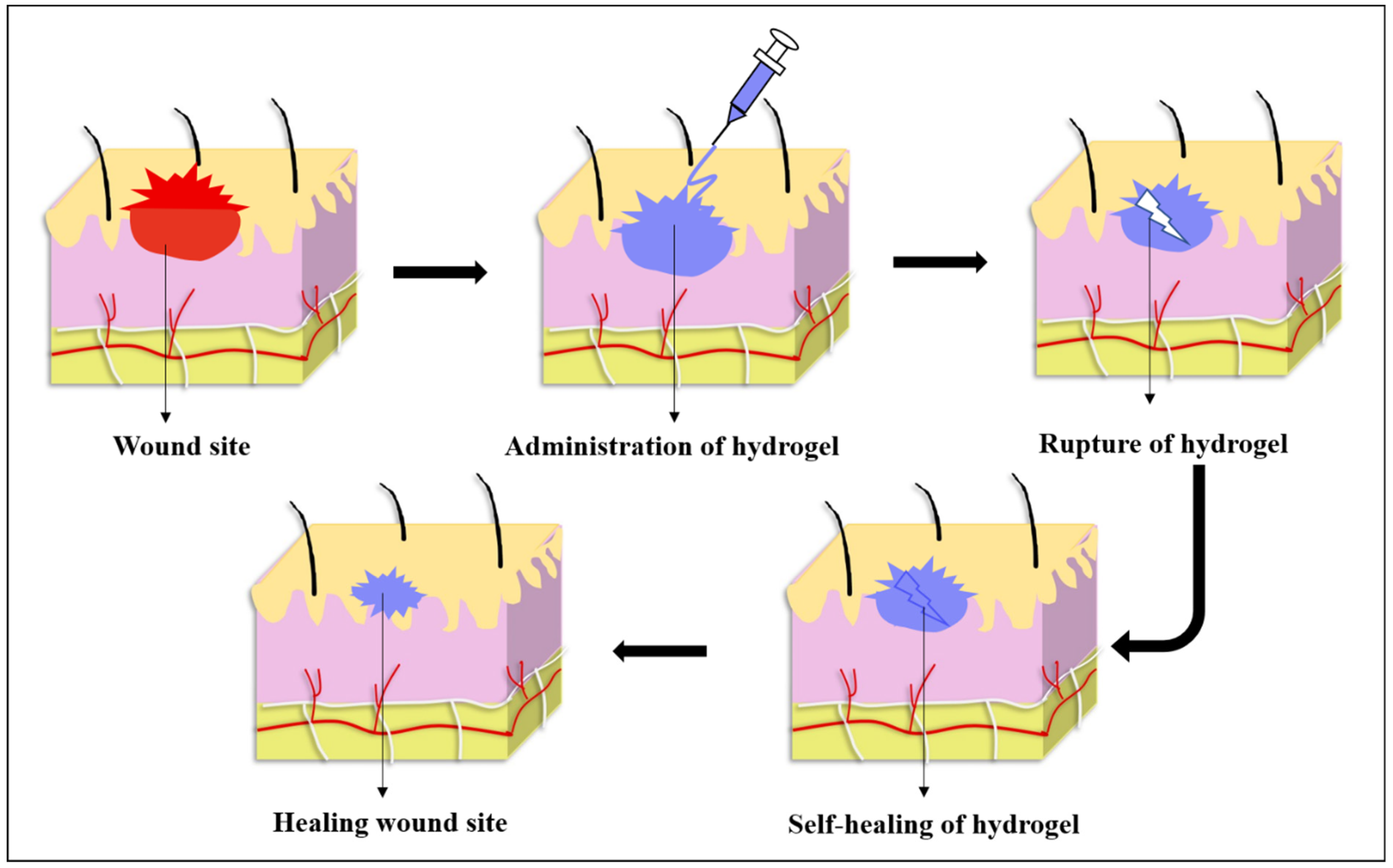
4.1.1. Importance of Self-Healing Hydrogels in Wound Healing
4.1.2. Advances of Self-Healing Hydrogels in Wound Healing
4.1.3. Mussel Inspired Self-Healing Hydrogels for Wound Healing
4.1.4. Nanoparticles in Self-Healing Hydrogels for Wound Healing
4.1.5. Bacterial Cellulose Based Self-Healing Hydrogel for Wound Healing
4.1.6. Leap towards 3D/4D Bioprinted Self-Healing Hydrogels
4.2. Self-Healing Hydrogels in Drug Delivery
4.2.1. Self-Healing Hydrogels as Drug Delivery Platforms for Cancer Therapy
4.2.2. Natural Polymer Based Self-Healing Hydrogels as Drug Delivery Platforms for Cancer Therapy
4.2.3. Synthetic Polymer Based Self-Healing Hydrogels as Drug Delivery Platforms for Cancer Therapy
4.2.4. Hybrid Polymeric Self-Healing Hydrogels as Drug Delivery Platforms for Anti-Cancer Therapy
4.2.5. Nanocomposite Self-Healing Hydrogels as Drug Delivery Platforms for Anti-Cancer Therapy
4.2.6. Self-Healing Hydrogels as Drug Delivery Platforms for Antimicrobial Therapy
4.2.7. Self-Healing Hydrogels as Drug Delivery Platforms for other Applications
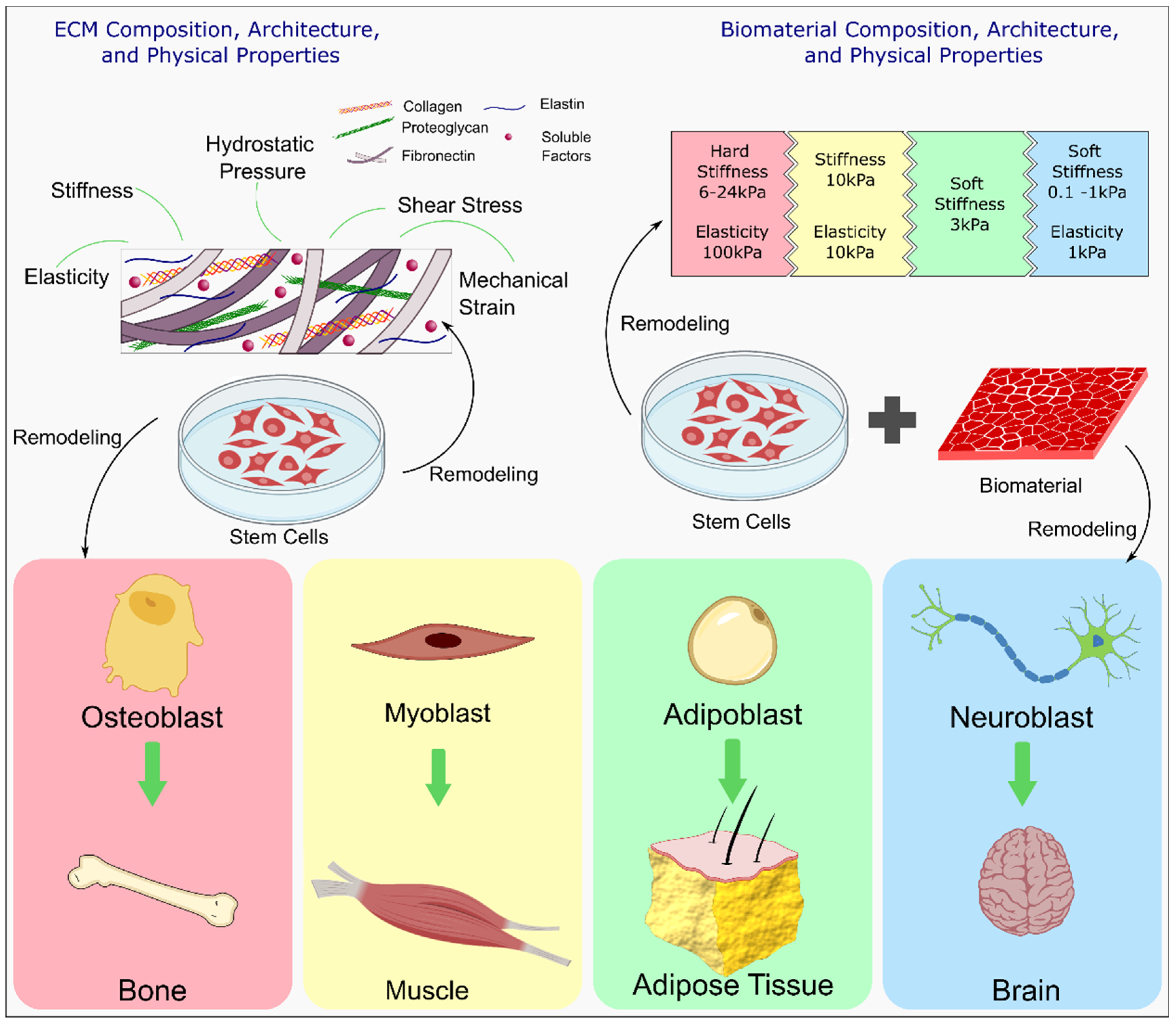
4.3. Effect of Self-Healing Properties on Cells
4.3.1. Maintaining Cell Stemness
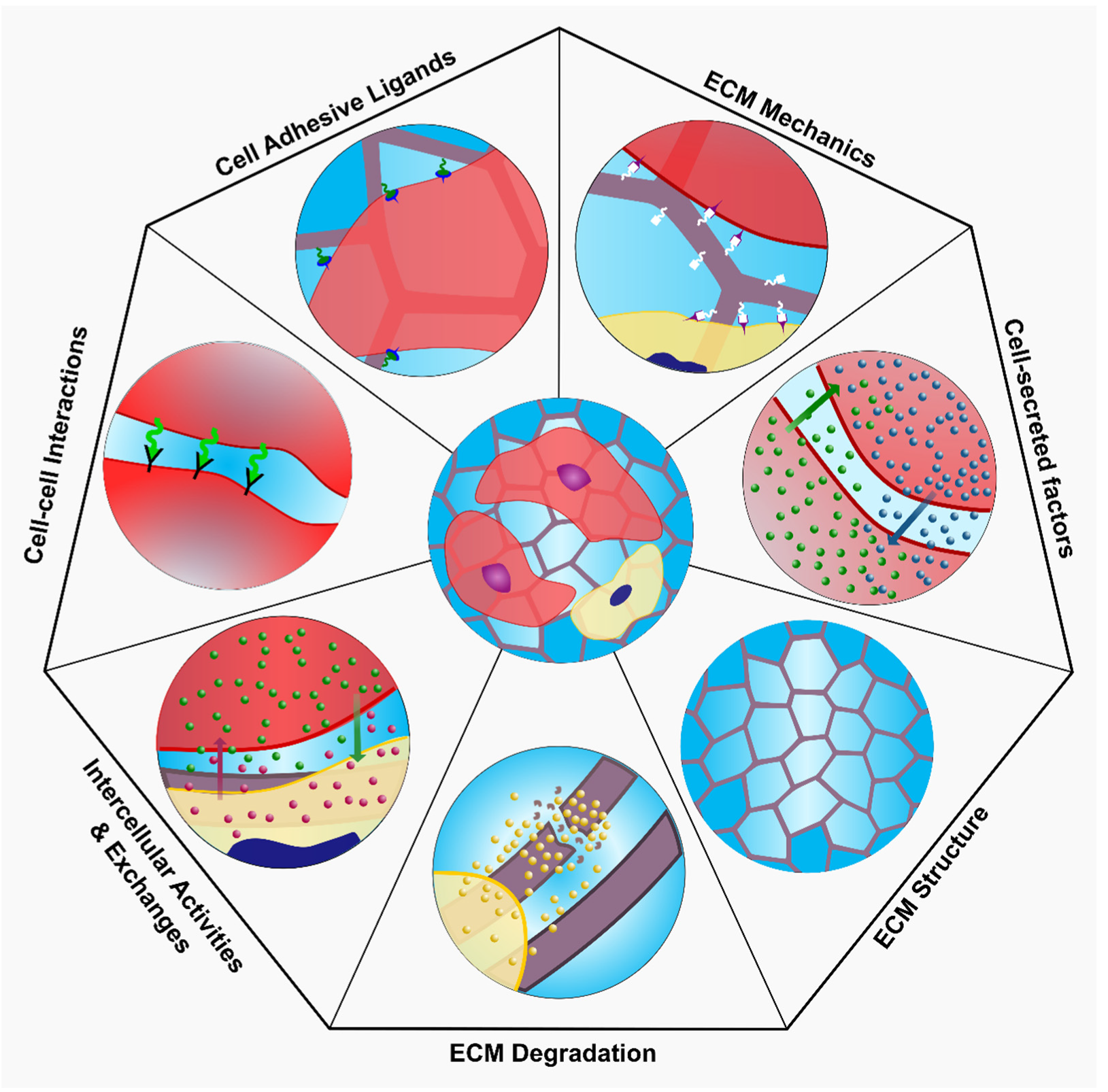
4.3.2. ECM Mechanics
4.3.3. Cell-Adhesive Ligands
4.3.4. Cell-Cell Interactions
4.3.5. Cell-Secreted Factors
4.3.6. Cell Encapsulation and Stem Cell Differentiation
4.4. Self-Healing Hydrogels in Cartilage and Bone Tissue Engineering
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| S No | Hydrogel Components Involved in Bond Formation | Self-Healing Mechanism | Potential Application | Ref. |
|---|---|---|---|---|
| 1 | N-carboxyethyl chitosan and PF127 | Imine bond | Wound healing | [5] |
| 2 | Guar gum, a water-soluble galactomannan with acidic poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) | Hydrogen bond | Wound healing | [6] |
| 3 | Cytosine, guanosine, modified hyaluronic acid | Hydrogen bond | Injectable drug delivery systems, tissue engineering, cell scaffold materials, and regenerative medicine | [14] |
| 4 | Polyvinyl alcohol, 3,4-dihydroxyphenyl-L-alanine (DOPA) and Fe complex | Hydrogen bond | Biomedical applications | [15] |
| 5 | Poly (ethylene oxide), poly(N-isopropylacrylamide) and ureido pyrimidinone | Hydrogen bond | 3D cellencapsulation and protein delivery | [18] |
| 6 | Poly acrylic acid grafted vinyl hybrid silica nanoparticles | Hydrogen bonding and ferric ion-mediated ionic interactions | Tissue engineering | [18] |
| 7 | Cholic acid with β-cyclodextrin | Host Guest Interaction | Suitable for bio applications | [29] |
| 8 | Cationic β-cyclodextrin oligomer crosslinked by epichlorohydrin and modified by allyl glycidyl ether and glycidyl trimethylammonium chloride | Electrostatic interaction, host-guest complexation, and C-C bonds as the macrocrosslinker | Suitable for biomedical application in future | [32] |
| 9 | Cyclodextrin modified alginate, and a methacrylated gelatin | Host Guest Interaction | Suitable for biomedical application in future | [33] |
| 10 | Acryloyl-6-aminocaproic acid | Hydrophobic bond | Tissue Engineering, Biomedical application | [40] |
| 11 | Dibenzaldehyde-modified PFG2000 | Imine bond | Biology, medicine, and engineering | [42] |
| 12 | Chitosan and dialdehyde bacterial cellulose | Imine bond | Wound healing | [45] |
| 13 | Chondroitin sulfate multiple aldehyde and N-succinyl-chitosan | Imine bond | Cell encapsulation | [46] |
| 14 | Agarose-ethylenediamine conjugate with dialdehyde-functionalized PEG | Imine bond | Tissue adhesive, Wound dressing | [48] |
| 15 | N,O-carboxymethyl chitosan/oxidized chondroitin sulfate | Imine bond | Wound healing | [50] |
| 16 | Carboxymethyl chitosan was incorporated with zinc | Imine bond | Biomedical field | [51] |
| 17 | Konjac glucomannan-chitosan | Imine bond | Wound healing | [52] |
| 18 | Quaternized chitosan and benzaldehyde-terminated poly(ethylene oxide)-b-poly(propyleneoxide)-b-poly(ethyleneoxide) | Imine bond | Drug delivery | [54] |
| 19 | Glycol chitosan and telechelic difunctional poly (ethylene glycol) | Imine bond | Wound healing | [55] |
| 20 | Aldehyde modified methyl cellulose and chitosan grafted polyethylene glycol loaded with exosome | dynamic covalent Schiff base linkage | Wound healing | [58] |
| 21 | Carboxymethyl cellulose dialdehyde and carboxymethyl chitosan | Schiff base linkage | Study the effects of paracrine signals due to cell secretion factors | [59] |
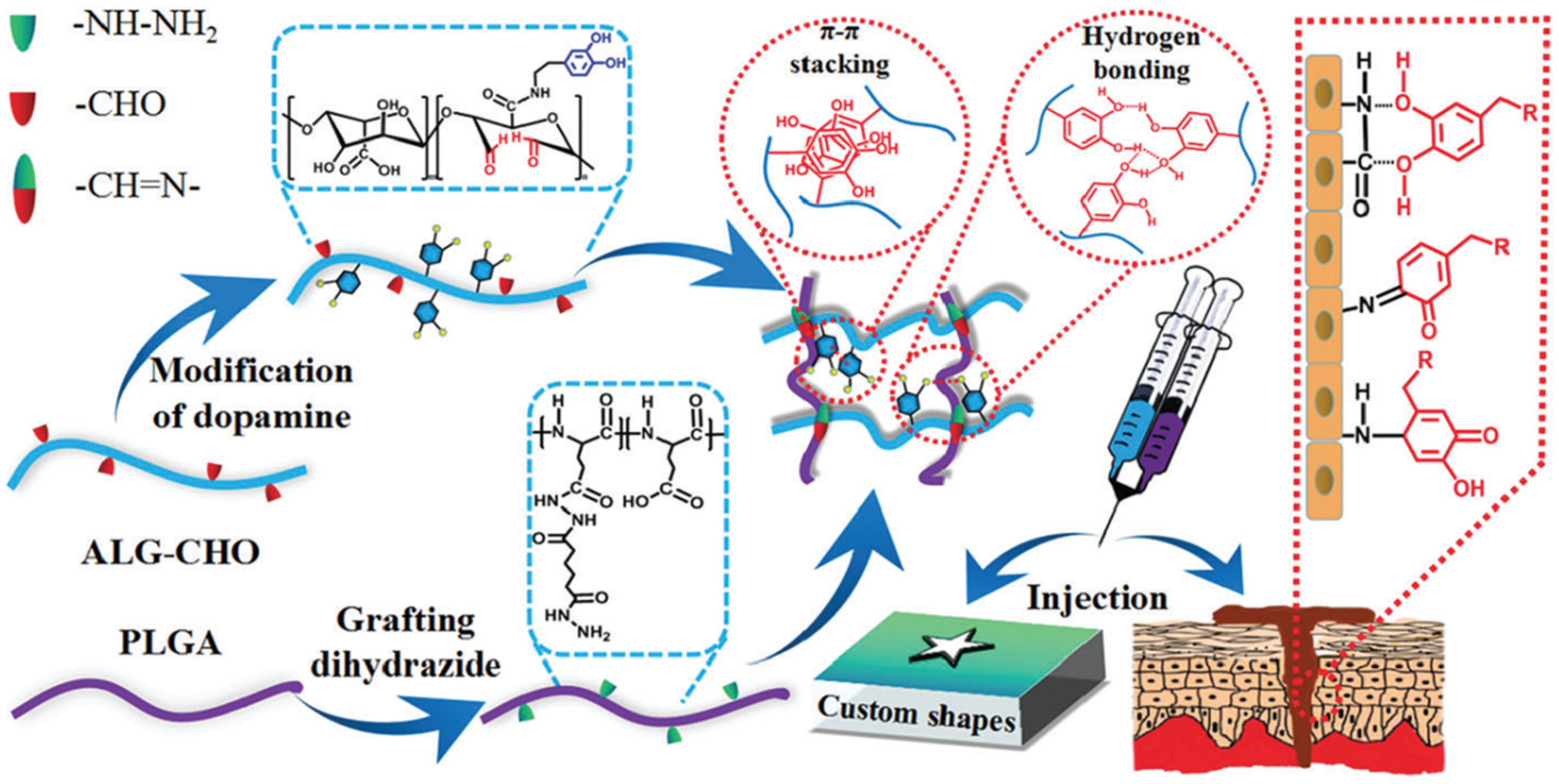
| 22 | Ablated porous PLGA grafted with hydrophobic PCL | Schiff base reaction | Bone tissue engineering application, efficient permeability and diffusion of oxygens and removal of waste from cell metabolism | [62] |
| 23 | Gelatin and oxidized dextran | Imine cross-linkage | Protection of cells from shear forces during extrusion, ECM degradation, cellular migration | [64] |
| 24 | Oxidized dextran and acid dihydrazide | Acylhydrazone bond | Wound healing | [67] |
| 25 | Polyethylene oxide and tris[(4-formylphenoxy) methyl ethane | Acylhydrazone bond | Wound Healing | [67] |
| 26 | Sodium alginate dialdehyde reacts with hydrazide | Schiff base reaction and acylhydrazone bonds | Tissue Engineering, drug delivery | [73] |
| 27 | Protein-polymer conjugates | Oxime bond | Biomedical Applications | [74] |
| 28 | Poly(vinyl alcohol) -borax gel as a matrix with dopamine-grafted oxidized carboxymethylcellulose and cellulose nanofibers | dynamic reversible borate ester linkages and hydrogen bonds along with dynamic cross-linking imine linkages | Wound healing | [114] |
| 29 | Carboxymethyl chitosan and aldehyde functionalized sodium alginate | Imine bond | Wound healing | [115] |
| 30 | Cationically charged chitosan with anionically modified bacterial cellulose | Hydrogen bond | Wound healing | [111] |
| 31 | Chitosan hydrogel cross-linked with telechelic difunctional poly (ethylene glycol) (DF-PEG-DF). | Schiff base linkage | co-delivery system for synergistic chemo-hyperthermic therapy for triple-negative breast cancer | [128] |
| 32 | Carboxyethyl-modified chitosan (CEC) and aldehyde modified hyaluronic acid (A-HA) | Dynamic Schiff base bonds between amine groups on CEC and aldehyde groups on A-HA | pH based Doxorubicin (Dox) release for cancer therapy | [129] |
| 33 | Chitosan-catechol groups | Catechol-Fe (III) coordinative interactions | Dox and Docetaxel (DTX) release for cancer therapy | [130] |
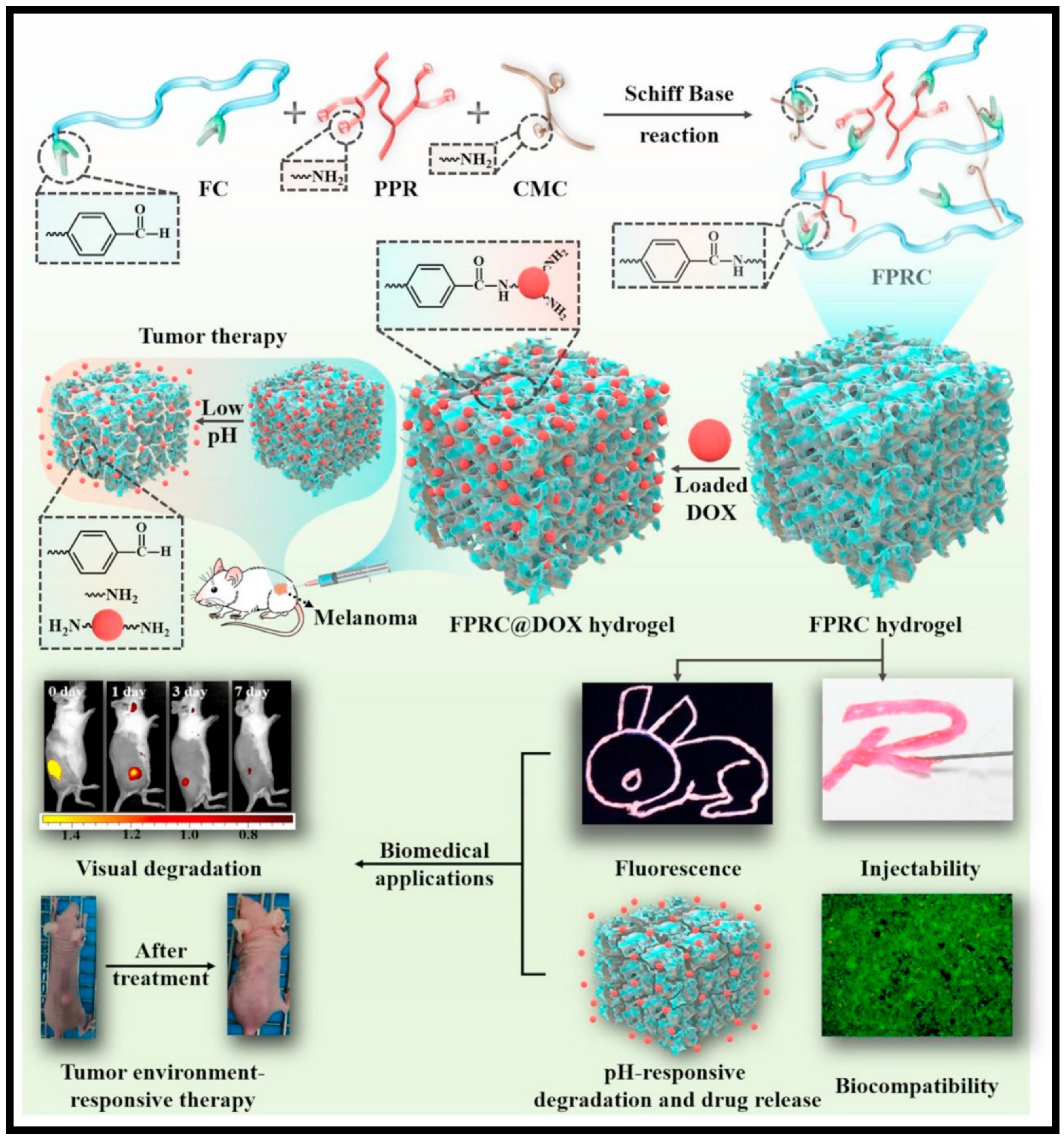
| 34 | F127–CHO (FC) [Pluoronic F127 with aldehyde group], PPR [red fluorescence-emissive polycitrate-polymine-rhodamine B polymer] and CMC [Carboxymethyl chitosan] | Schiff’s base reaction | pH-responsive Dox release for cancer therapy | [131] |
| 35 | Boric acids and diols derivatives of guanosine and isoguanosine | Boronate ester bonds | Guanosine delivery for cancer therapy | [133] |
| 36 | Hyaluronic acid was modified to have phenylboronic acid and dopamine moieties | Boronate ester bonds | Erlotinib (ERT) delivery for cancer therapy | [134] |
| 37 | Ureidopyrimidinone (UPy) side chains present in gelatin and Fe3+ ions | Co-ordination bond | 5-fluorouracil (5-FU) delivery for cancer therapy | [135] |
| 38 | Gum Arabic (GA) was modified with multi-aldehyde group (GAMA) to be reacted with the succinic anhydride-modified CS (SCS) | Schiff base linkage | controlled release of nano curcumin as an anti-cancer treatment | [136] |
| 39 | Cellulose | Ketoester-type acyl-hydrazone bond | pH-responsive Dox release for cancer therapy | [137] |
| 40 | Amino groups in polyetherimide (PEI) and the acetoacetate groups of the four-armed star-shaped poly(2-(dimethylamino) ethyl methacrylate-co-2-hydroxyethyl methacry-late) modified with tert-butyl acetoacetate (t-BAA), SP(DMAEMA-co-HEMA-AA) and PDA NPs. | Dynamic covalent enamine bonds | Dox release for cancer therapy | [138] |
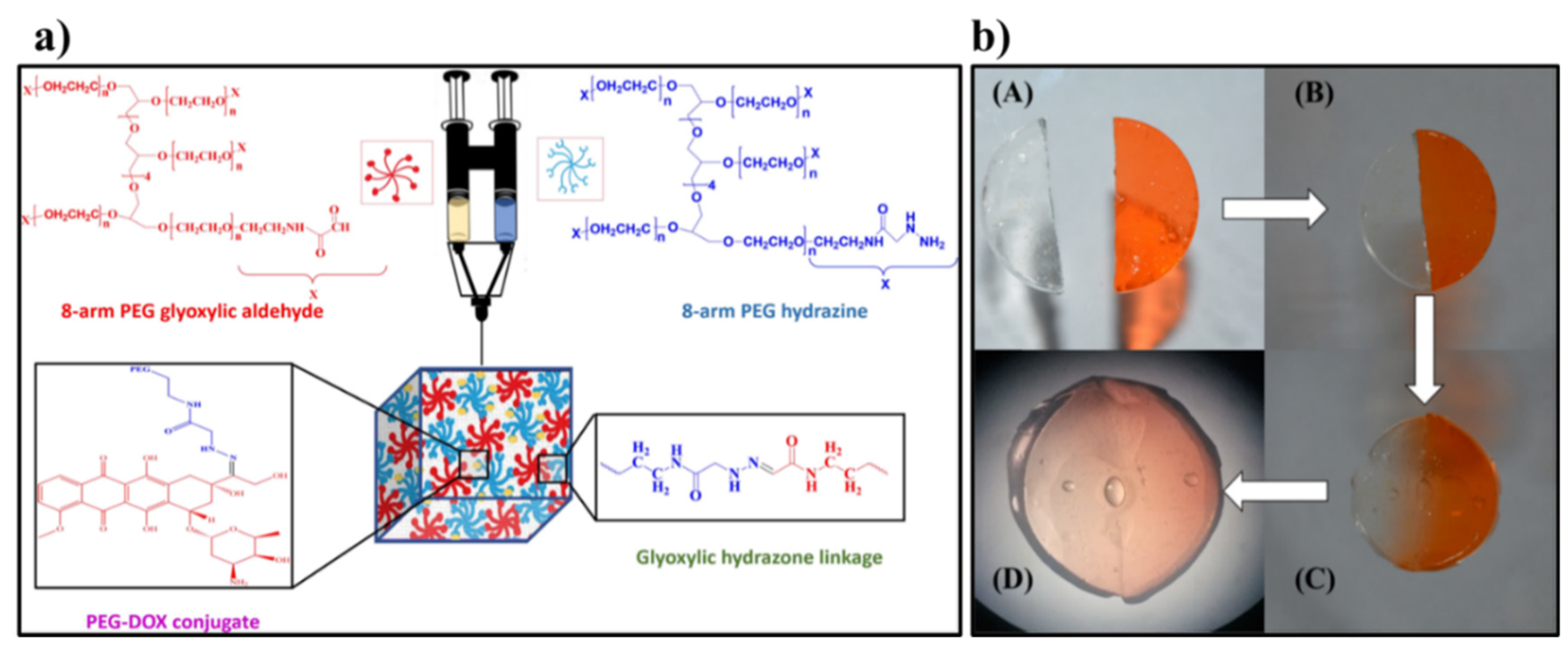
| 41 | 8-arm PEG glyoxylic aldehyde and 8-arm PEG hydrazine | Glyoxylic hydrazone linkages | pH-responsive Dox release for cancer therapy | [139] |
| 42 | TPE-poly(N,N-dimethylacrylamide-stat-Diacetone acrylamide) [TPE-P(DMA-stat-DAA)] | Diacylhydrazide bonds | pH-responsive Dox release for cancer therapy | [140] |
| 43 | Pentablock terpolypeptide [PLys-b-(PHIS-co-PBLG)-PLys-b-(PHIS-co-PBLG)-b-PLys] | Pentablock peptides’ macromolecular architecture | pH- and enzyme-responsive Gemcitabine release for pancreatic cancer therapy | [141] |
| 44 | Four-arms star polymer, poly (ethylene gly-col)-b-poly(g-o-nitrobenzyl-L-glutamate) | Hydrophobic interaction | Light -responsive Dox release for cancer therapy | [142] |
| 45 | Amine groups of N-carboxyethyl chitosan (CEC) and benzaldehyde groups from poly (ethylene glycol) (PEGDA) | Covalent Schiff-base linkage | pH-dependent Dox release for hepatocellular carcinoma | [143] |
| 46 | 4-arm PEG-benzaldehyde (4armPEGDA) and N-carboxyethyl chitosan (CEC) | Covalent Schiff-base linkage | pH-dependent Dox release for hepatocellular carcinoma | [144] |
| 47 | Oxidized xanthan and 8-arm PEG hydrazine | Biodegradable hydrazone linkages | Controlled release of Dox | [145] |
| 48 | α-cyclodextrin (α- CD) and poly (ethylene glycol) (PEG) chains of the poly (ethylene glycol)-block-poly (lactic acid) (PEG-b-PLA) micelles | Host-guest interaction | Controlled release of Dox against HeLa cells | [146] |
| 49 | Dialdehyde-functionalized polyethylene glycol (DF-PEG) and β-glycerophosphate (GP) cross-linked chitosan (CS) hydrogels | Schiff’s base chemistry | Controlled release of Dox in Heps tumor-bearing mice | [147] |
| 50 | Pectin aldehyde (pectin-CHO) and acylhydrazide func-tionalized polymer poly(N-isopropylacrylamide-stat-acylhydrazide) P(NIPAM-stat-AH) | Acylhydrazone bonds | Controlled release of two drugs: Dox and combretastatin A4 disodium phosphate (CA4) | [148] |
| 51 | Chitosan and the oxidized pectin | Imine bond formed through Schiff’s base reaction | Sustained release of 5-FU. Hydrogel with magnetic, pH- and thermo-responsive properties | [149] |
| 52 | Chondroitin sulfate multialdehyde (CSMA), branched polyethylenimine (BPEI) and BPEI conjugated graphene oxide (BPEI-GO) | Schiff-base linkage | Controlled Dox release with photothermal therapy against breast cancer | [150] |
| 53 | Benzaldehyde-functionalized pullulan (PULL-CHO) and chitosan-g-PEG (CS-g-PEG) | Schiff base linkage | synergistic magnetothermal-chemo-chemodynamic therapy for cancer | [151] |
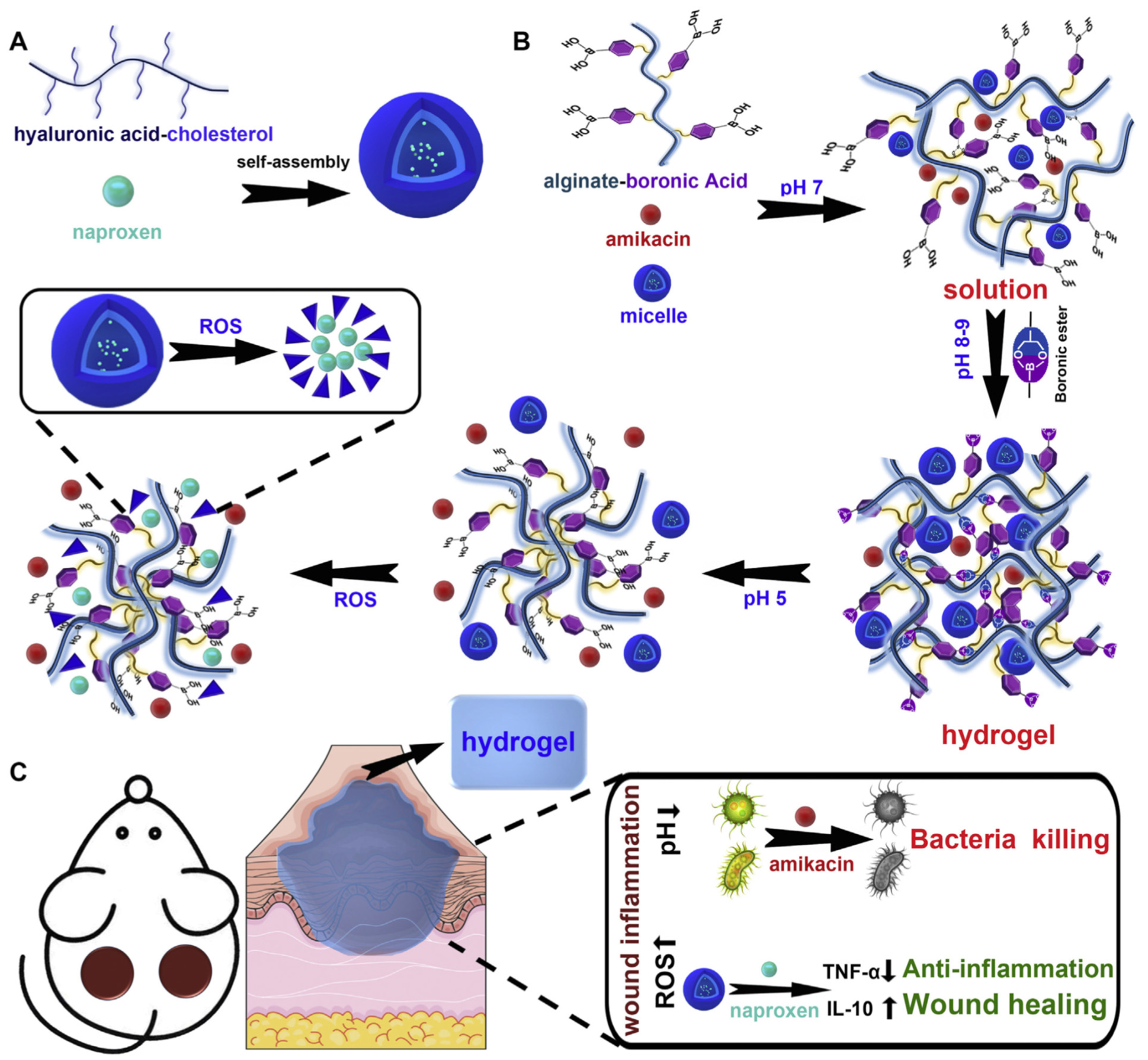
| 54 | Grafting phenylboronic acid to the side chain of the alginate and hyaluronic acid grafted with cholesterol | Boronic ester bonds | Smart pH- and reactive oxygen species (ROS)-responsive injectable hydrogel—dual drug (amikacin and naproxen) delivery | [152] |
| 55 | Chitosan and amino acid (acryloyl-phenylalanine) | Hydrogen bonds | Controlled release of levofloxacin | [153] |
| 56 | Chitosan-cuminaldehyde | Dynamic imine bond | Controlled release of levofloxacin | [154] |
| 57 | In situ free radical polymerization of guar gum-graft-acrylic acid (GG-PAA) in presence of L-Alanine as crosslinker | Hydrogen bond | Controlled release of levofloxacin | [155] |
| 58 | Phenyl boronic acid-modified hyaluronic acid (HA–PBA) and plant-derived polyphenol-tannic acid (TA) | Boronic ester dynamic covalent bond | Dual (pH- and reactive oxygen species (ROS)) responsive silver nanoparticles based antimicrobial hydrogel | [156] |
| 59 | PLGA-PEG-PLGA co-polymeric system, comprising of a derivative of isoniazid (DINH) loaded liposomes | Hydrophobic interactions | A local drug delivery system for bone tuberculosis therapy | [157] |
| 60 | Thiol group of 4-arm-PEG-SH and silver ions | Dynamic reversible coordination bond | Acute bacterial rhinosinusitis (ABRS) therapy | [158] |
| 61 | Amine functionalized PDMS based polyzwitterionic polymersomes and polyethylene glycol dialdehyde (PEG-DA) | Schiff’s base reaction | Curcumin delivery for antimicrobial applications | [159] |
| 62 | Hyaluronic acid-g-dopamine and reduced Graphene oxide (rGO) | Physical bonding between rGO@PDA and HA-DA including hydrogen bonding, and π–π stacking | Hemostatic antioxidant conductive photothermal antibacterial hydrogels | [160] |
| 63 | Guanosine-quartet Na+-borate | Dynamic guanosine–borate diester bond | Controlled release of Acyclovir, an antiviral agent | [161] |
| 64 | β-cyclodextrin modified hyaluronic acid (HA-CD) and adamantane modified 4-arm-PEG (4-arm-PEG-Ad) | Host-guest interaction | Controlled release of Dexamethasone | [162] |
| 65 | Octa-cyclodextrin polyhedral oligomeric silsesquioxane (OCDPOSS) and acryla-mide-modified adamantane (Ad-AAm) | Host-guest interactions | Controlled release of Dexamethasone | [163] |
| 66 | DNA-based hydrogel crosslinked with oxidized alginate (OA) + Silica nanoparticles | Dynamic imine linkages | Sustained release of simvastatin | [164] |
| 67 | Carboxyethyl chitosan and oxidized pullulan | Dynamic imine bond | Controlled release of Dexamethasone | [165] |
| 68 | Hydroxy-propyl-methyl cellulose derivatives (HPMC-x) and PEG-b-PLA nanoparticles | Hydrophobic interactions | Dual drug delivery applications | [166] |
| 69 | Ureido-pyrimidinone (UPy) and dextran (DEX) | Hydrogen bond | Regeneration of cartilage–bone tissue complex | [199] |
| 70 | hydrazide-modified poly(L-glutamic acid) (PLGA–ADH) & alginate(catechol- and aldehyde-modified alginate, ALG–CHO–Catechol) | Hydrogen bond | Tissue engineering, bone bleeding treatment. | [205] |
| 71 | Catechol-conjugated chitosan (CHI-C) | Imine bond | Hemostasis and bone regeneration, bio-adhesive | [206] |
| 72 | chondroitin sulfate (ChS) | Acylhydrazone bond | Bone tissue engineering | [207] |
| 73 | Gelatin hydrogels with cell-infiltration and injectable (Ci-I) | Host Guest Interaction | Continuous delivery of small drugs | [209] |
| 74 | Coralline hydroxyapatite (CHA)/silk fibroin (SF)/glycol chitosan (GCS)/difunctionalized polyethylene glycol (DF-PEG) | Imine bond | Bone defect treatment | [210] |
| 75 | PEG hydrogel incorporated with adhesive liposomes (A-LIP) loaded with BMP-2 | Disulfide bond | Osteoporotic fractures and bone marrow cavity treatment | [211] |
| 76 | polysaccharide matrices & LAPONITE® (LAP) nanoplatelets | Hydrogen bond | Orthopedic treatment | [212] |
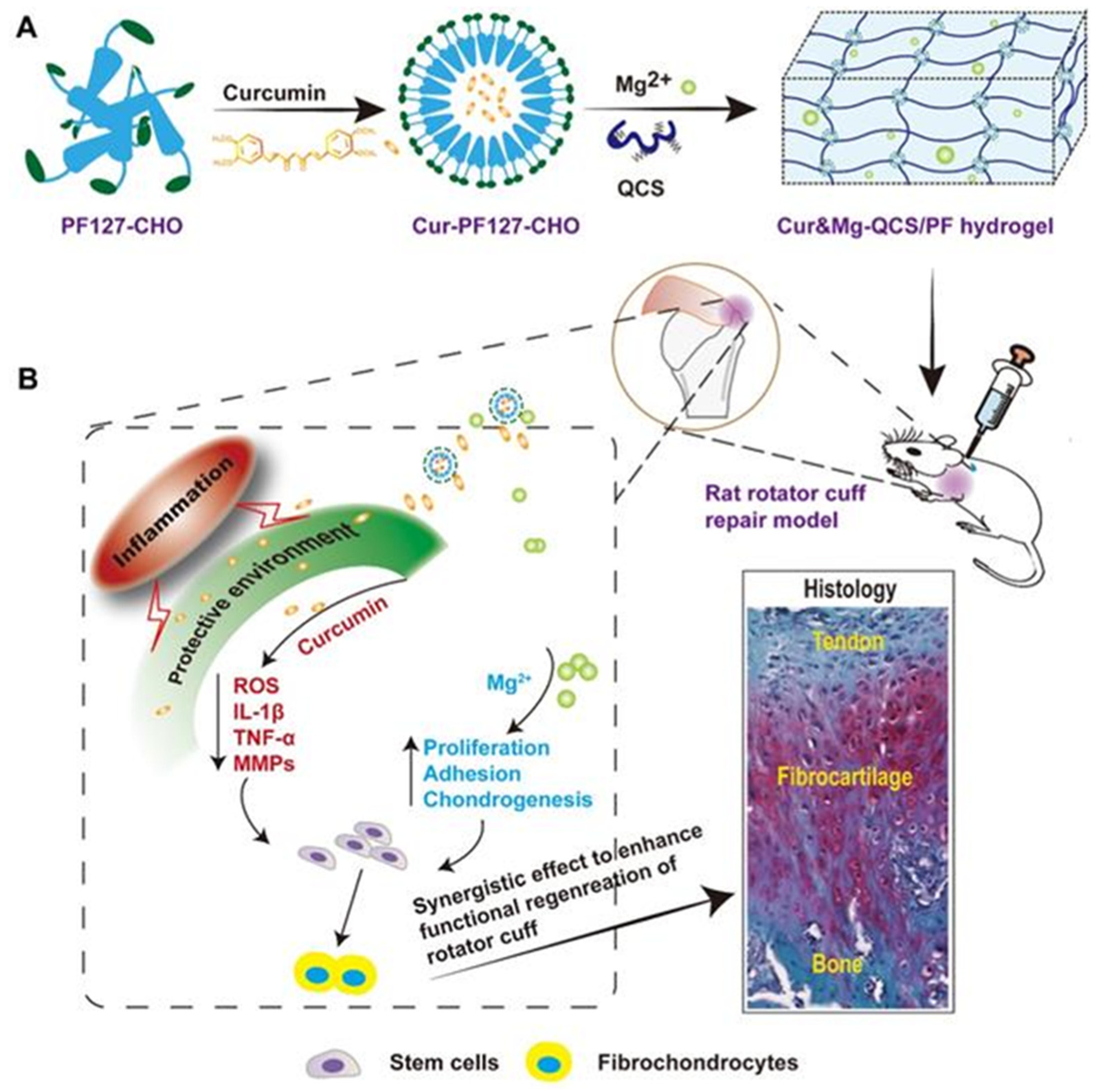
| 77 | Mg2+/curcumin, QCS and Pluronic®F127 (PF127-CHO) | Imine bond | Tendon-to-bone healing | [213] |
| 78 | PVA based hydrogel | Hydrogen bond | Multiple applications | [215] |
| 79 | poly(N-hydroxyethyl acrylamide-co-methyl vinyl ketone) (PHEAA-co-PMVK) with a bifunctional hydroxylamine. | Hydrogen bond | Tunable mechanical property for various applications | [216] |
| 80 | Chitosan methacrylate (CHMA) and polyvinyl alcohol (PVA) | Hydrogen bond | Bone tissue engineering | [217] |
| 81 | Fluorenylmethoxycarbonyl-diphenylalanine (Fmoc-FF), Fmoc-RGD (arginine-glycine-aspartate) | Hydrogen bonds | Promotion of cell adhesion | [220] |
References
- Hussain, I.; Fu, G. Self-healing hydrogels. In Self-Healing Polymer-Based Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 369–423. [Google Scholar]
- Jeon, I.; Cui, J.; Illeperuma, W.R.K.; Aizenberg, J.; Vlassak, J.J. Extremely Stretchable and Fast Self-Healing Hydrogels. Adv. Mater. 2016, 28, 4678–4683. [Google Scholar] [CrossRef]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Wang, H.; Ma, P.X.; Guo, B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12, 1224–1246. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zheng, W.; Yang, G.; Jiang, X. Rapid Fabrication of Self-Healing, Conductive, and Injectable Gel as Dressings for Healing Wounds in Stretchable Parts of the Body. Adv. Funct. Mater. 2020, 30, 2002370. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Poly(vinyl alcohol) Hydrogel Can Autonomously Self-Heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Lang, C.; Qiao, S.; An, G.; Fan, X.; Zhao, L.; Hou, C.; Liu, J. Enzyme-Regulated Fast Self-Healing of a Pillararene-Based Hydrogel. Biomacromolecules 2017, 18, 1885–1892. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Liu, S.; Guo, J.; Jia, Y.G. Polypeptide-based self-healing hydrogels: Design and biomedical applications. Acta Biomater. 2020, 113, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.; Wen, J.; Wu, Y.; Shan, Y.; Liao, J. The design, mechanism and biomedical application of self-healing hydrogels. Chin. Chem. Lett. 2017, 28, 1857–1874. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S. Synthesis and Biomedical Applications of Self-healing Hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Li, X.; Shen, Y.; Chang, G.; Yang, J.; Gu, Z. Self-healing pH-sensitive cytosine- and guanosine-modified hyaluronic acid hydrogels via hydrogen bonding. Polymer 2017, 108, 348–360. [Google Scholar] [CrossRef]
- Shi, D.; Liu, R.; Dong, W.; Li, X.; Zhang, H.; Chen, M.; Akashi, M. pH-dependent and self-healing properties of mussel modified poly(vinyl alcohol) hydrogels in a metal-free environment. RSC Adv. 2015, 5, 82252–82258. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Deng, Y.; Ngai, T.; Wang, C. Dynamic Supramolecular Hydrogels: Regulating Hydrogel Properties through Self-Complementary Quadruple Hydrogen Bonds and Thermo-Switch. ACS Macro Lett. 2017, 6, 641–646. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Y.; Gao, L.; Bai, T.; Wang, W.; Cui, Y.; Liu, W. A Mechanically Strong, Highly Stable, Thermoplastic, and Self-Healable Supramolecular Polymer Hydrogel. Adv. Mater. 2015, 27, 3566–3571. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, X.-Y.; Shi, F.-K.; Zhang, L.-Q.; Wang, X.-P.; Cheetham, A.G.; Cui, H.; Xie, X.-M. Self-healable, tough and highly stretchable ionic nanocomposite physical hydrogels. Soft Matter 2015, 11, 4235–4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Zhou, X.; Tang, Q.; Bao, H.; Wang, G.; Saha, P. A self-healable and easily recyclable supramolecular hydrogel electrolyte for flexible supercapacitors. J. Mater. Chem. A 2016, 4, 8769–8776. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Srivastava, A. Robust, self-healing hydrogels synthesised from catechol rich polymers. J. Mater. Chem. B 2015, 3, 899–910. [Google Scholar] [CrossRef]
- Huang, S.; Yang, L.; Liu, M.; Phua, S.L.; Yee, W.A.; Liu, W.; Zhou, R.; Lu, X. Complexes of Polydopamine-Modified Clay and Ferric Ions as the Framework for Pollutant-Absorbing Supramolecular Hydrogels. Langmuir 2013, 29, 1238–1244. [Google Scholar] [CrossRef]
- Bai, T.; Liu, S.; Sun, F.; Sinclair, A.; Zhang, L.; Shao, Q.; Jiang, S. Zwitterionic fusion in hydrogels and spontaneous and time-independent self-healing under physiological conditions. Biomaterials 2014, 35, 3926–3933. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cai, L.; Jia, Y.-G.; Liu, S.; Chen, Y.; Ren, L. Progress in self-healing hydrogels assembled by host–guest interactions: Preparation and biomedical applications. J. Mater. Chem. B 2019, 7, 1637–1651. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [Green Version]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef]
- Rodell, C.B.; Dusaj, N.N.; Highley, C.B.; Burdick, J.A. Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv. Mater. 2016, 28, 8419–8424. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, K.; Nakahata, M.; Takashima, Y.; Harada, A. Self-Healing, Expansion-Contraction, and Shape-Memory Properties of a Preorganized Supramolecular Hydrogel through Host-Guest Interactions. Angew. Chem. Int. Ed. 2015, 54, 8984–8987. [Google Scholar] [CrossRef]
- Jia, Y.-G.; Zhu, X.X. Self-Healing Supramolecular Hydrogel Made of Polymers Bearing Cholic Acid and β-Cyclodextrin Pendants. Chem. Mater. 2015, 27, 387–393. [Google Scholar] [CrossRef]
- Miao, T.; Fenn, S.L.; Charron, P.N.; Oldinski, R.A. Self-Healing and Thermoresponsive Dual-Cross-Linked Alginate Hydrogels Based on Supramolecular Inclusion Complexes. Biomacromolecules 2015, 16, 3740–3750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Ma, X.; Wu, S.; Tian, H. A Rapidly Self-Healing Supramolecular Polymer Hydrogel with Photostimulated Room-Temperature Phosphorescence Responsiveness. Angew. Chem. Int. Ed. 2014, 53, 14149–14152. [Google Scholar] [CrossRef]
- Jeong, D.; Joo, S.-W.; Shinde, V.V.; Jung, S. Triple-crosslinkedβ-cyclodextrin oligomer self-healing hydrogel showing high mechanical strength, enhanced stability and pH responsiveness. Carbohydr. Polym. 2018, 198, 563–574. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Shavandi, A. Shear thinning/self-healing hydrogel based on natural polymers with secondary photocrosslinking for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 90, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Can, V.; Kochovski, Z.; Reiter, V.; Severin, N.; Siebenbürger, M.; Kent, B.; Just, J.; Rabe, J.P.; Ballauff, M.; Okay, O. Nanostructural Evolution and Self-Healing Mechanism of Micellar Hydrogels. Macromolecules 2016, 49, 2281–2287. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and Self-Healing Hydrogels Formed via Hydrophobic Interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Argun, A.; Sahin, M.; Sari, M.; Okay, O. Structure optimization of self-healing hydrogels formed via hydrophobic interactions. Polymer 2012, 53, 5513–5522. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sahin, M.; Argun, A.; Oppermann, W.; Okay, O. Dynamics and Large Strain Behavior of Self-Healing Hydrogels with and without Surfactants. Macromolecules 2012, 45, 1991–2000. [Google Scholar] [CrossRef]
- Gulyuz, U.; Okay, O. Self-Healing Poly(acrylic acid) Hydrogels with Shape Memory Behavior of High Mechanical Strength. Macromolecules 2014, 47, 6889–6899. [Google Scholar] [CrossRef]
- Ziółkowski, B.; Florea, L.; Theobald, J.; Benito-Lopez, F.; Diamond, D. Self-protonating spiropyran-co-NIPAM-co-acrylic acid hydrogel photoactuators. Soft Matter 2013, 9, 8754. [Google Scholar] [CrossRef]
- Algi, M.P.; Okay, O. Highly stretchable self-healing poly(N,N-dimethylacrylamide) hydrogels. Eur. Polym. J. 2014, 59, 113–121. [Google Scholar] [CrossRef]
- Gao, T.T.; Niu, N.; Liu, Y.D.; Liu, X.L.; Gao, G.; Liu, F.Q. Synthesis and characterization of hydrophobic association hydrogels with tunable mechanical strength. RSC Adv. 2016, 6, 43463–43469. [Google Scholar] [CrossRef]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.-C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid self-healing hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [Green Version]
- McKay, C.S.; Finn, M.G. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trask, R.S.; Williams, H.R.; Bond, I.P. Self-healing polymer composites: Mimicking nature to enhance performance. Bioinspir. Biomim. 2007, 2, P1–P9. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Zhang, M.; Wu, Z.; Wei, J.; Jiang, Y.; Sheng, N.; Liang, Q.; Zhang, D.; Chen, S. All-natural injectable hydrogel with self-healing and antibacterial properties for wound dressing. Cellulose 2020, 27, 2637–2650. [Google Scholar] [CrossRef]
- Lü, S.; Gao, C.; Xu, X.; Bai, X.; Duan, H.; Gao, N.; Feng, C.; Xiong, Y.; Liu, M. Injectable and Self-Healing Carbohydrate-Based Hydrogel for Cell Encapsulation. ACS Appl. Mater. Interfaces 2015, 7, 13029–13037. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Tian, M.; Feng, R.; Dang, Y.; Zhang, M. Novel Self-Healing Hydrogel with Injectable, pH-Responsive, Strain-Sensitive, Promoting Wound-Healing, and Hemostatic Properties Based on Collagen and Chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-Forming and Self-Healing Agarose-Based Hydrogels for Tissue Adhesives and Potential Wound Dressings. Biomacromolecules 2018, 19, 980–988. [Google Scholar] [CrossRef]
- Ma, Y.-H.; Yang, J.; Li, B.; Jiang, Y.-W.; Lu, X.; Chen, Z. Biodegradable and injectable polymer–liposome hydrogel: A promising cell carrier. Polym. Chem. 2016, 7, 2037–2044. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C 2021, 118, 111324. [Google Scholar] [CrossRef]
- Wahid, F.; Zhou, Y.-N.; Wang, H.-S.; Wan, T.; Zhong, C.; Chu, L.-Q. Injectable self-healing carboxymethyl chitosan-zinc supramolecular hydrogels and their antibacterial activity. Int. J. Biol. Macromol. 2018, 114, 1233–1239. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, B.; Zhang, X.; Xu, L.; Tao, L.; Li, S.; Wei, Y. A magnetic self-healing hydrogel. Chem. Commun. 2012, 48, 9305. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Fu, Y.; Wei, Y.; Zhao, L.; Tao, L. Self-Adapting Hydrogel to Improve the Therapeutic Effect in Wound-Healing. ACS Appl. Mater. Interfaces 2018, 10, 26046–26055. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wei, Y.; Tao, L. Chitosan-based self-healing hydrogel for bioapplications. Chin. Chem. Lett. 2017, 28, 2053–2057. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2020, 8, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Sharma, A.; Panwar, V.; Chopra, V.; Ghosh, D. Polysaccharide-Based Hybrid Self-Healing Hydrogel Supports the Paracrine Response of Mesenchymal Stem Cells. ACS Appl. Bio Mater. 2019, 2, 2013–2027. [Google Scholar] [CrossRef]
- Bai, H.; Kyu-Cheol, N.; Wang, Z.; Cui, Y.; Liu, H.; Liu, H.; Feng, Y.; Zhao, Y.; Lin, Q.; Li, Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J. Tissue Eng. 2020, 11, 204173142094724. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, K.; Yan, S.; Wu, J.; Yin, J. A tough and self-healing poly(L-glutamic acid)-based composite hydrogel for tissue engineering. J. Mater. Chem. B 2018, 6, 6865–6876. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wu, S.; Wang, S.; Xiong, Y.; Li, Y.; Li, B.; Deng, H.; Du, Y.; Xiao, L.; Shi, X. A dynamic and self-crosslinked polysaccharide hydrogel with autonomous self-healing ability. Soft Matter 2015, 11, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gerecht, S. A self-healing hydrogel as an injectable instructive carrier for cellular morphogenesis. Biomaterials 2018, 185, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, X.; Wang, S.; Yuan, L.; Ge, L.; Li, D.; Mu, C. Facile Fabrication of Biocompatible Gelatin-Based Self-Healing Hydrogels. ACS Appl. Polym. Mater. 2019, 1, 1350–1358. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, L.; Gong, L.; Hu, W.; Huang, W.; Chen, Y.; Asha, A.B.; Srinivas, S.; Chen, L.; Narain, R.; et al. Injectable, Self-Healing Hydrogel with Tunable Optical, Mechanical, and Antimicrobial Properties. Chem. Mater. 2019, 31, 2366–2376. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Wang, Q.; Xu, C.; Tang, Q.; Yang, H. An Injectable, Dual Responsive, and Self-Healing Hydrogel Based on Oxidized Sodium Alginate and Hydrazide-Modified Poly(ethyleneglycol). Molecules 2018, 23, 546. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Wang, L.; Deng, F.; Wang, W.; Li, A.; Lu, C.; Chen, H.; Wu, G.; Nan, K.; Li, L. Construction of Injectable Self-Healing Macroporous Hydrogels via a Template-Free Method for Tissue Engineering and Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 36721–36732. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef] [Green Version]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chem.-Eur. J. 2016, 22, 844–857. [Google Scholar] [CrossRef]
- Ding, C.; Yang, Q.; Tian, M.; Guo, C.; Deng, F.; Dang, Y.; Zhang, M. Novel collagen-based hydrogels with injectable, self-healing, wound-healing properties via a dynamic crosslinking interaction. Polym. Int. 2020, 69, 858–866. [Google Scholar] [CrossRef]
- Wang, Y.; Adokoh, C.K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Expert Opin. Drug Deliv. 2018, 15, 77–91. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Ma, X.; Lei, Y. Functional self-healing materials and their potential applications in biomedical engineering. Adv. Compos. Hybrid Mater. 2018, 1, 94–113. [Google Scholar] [CrossRef]
- Balitaan, J.N.I.; Hsiao, C.-D.; Yeh, J.-M.; Santiago, K.S. Innovation inspired by nature: Biocompatible self-healing injectable hydrogels based on modified-β-chitin for wound healing. Int. J. Biol. Macromol. 2020, 162, 723–736. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Kleinschek, K.S.; Raščan, I.M.; Smrke, D.M. Advanced therapies of skin injuries. Wien. Klin. Wochenschr. 2015, 127, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liang, Y.; He, J.; Zhang, H.; Guo, B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing to Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Ponsubha, S.; Jaiswal, A.K. Effect of interpolymer complex formation between chondroitin sulfate and chitosan-gelatin hydrogel on physico-chemical and rheological properties. Carbohydr. Polym. 2020, 238, 116179. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Curr. Med. Chem. 2019, 25, 5782–5797. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Wang, W.; Narain, R.; Zeng, H. Rational Design of Self-Healing Tough Hydrogels: A Mini Review. Front. Chem. 2018, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wang, H.; Bai, Y.; Wang, Y.; Tao, L.; Wei, Y.; Fan, Y.; Guo, X.; Liu, H. Improving Chronic Diabetic Wound Healing through an Injectable and Self-Healing Hydrogel with Platelet-Rich Plasma Release. ACS Appl. Mater. Interfaces 2020, 12, 55659–55674. [Google Scholar] [CrossRef]
- Sun, F.; Bu, Y.; Chen, Y.; Yang, F.; Yu, J.; Wu, D. An Injectable and Instant Self-Healing Medical Adhesive for Wound Sealing. ACS Appl. Mater. Interfaces 2020, 12, 9132–9140. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Z.; Yang, Y.; Ren, F.; Li, J.; Zhu, S.; Ma, F.; Wu, R.; Lv, Y.; He, G.; et al. Injectable Self-Healing Adhesive pH-Responsive Hydrogels Accelerate Gastric Hemostasis and Wound Healing. Nano-Micro Lett. 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Xu, Y.; Chen, J.; Ning, N.; Yang, Z.; Guo, Y.; Hu, X.; Wang, Y. Highly Stretchable and Conductive Self-Healing Hydrogels for Temperature and Strain Sensing and Chronic Wound Treatment. ACS Appl. Mater. Interfaces 2020, 12, 40990–40999. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon N. Y. 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Ren, Y.; Wang, P.; Pu, Y.; Yang, R.; Wang, X.; Tan, X.; Ye, Z.; Maurizot, V.; et al. Mussel-Inspired Dual-Cross-linking Hyaluronic Acid/ε-Polylysine Hydrogel with Self-Healing and Antibacterial Properties for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 27876–27888. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-Healing Mussel-Inspired Multi-pH-Responsive Hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef]
- He, X.; Liu, L.; Han, H.; Shi, W.; Yang, W.; Lu, X. Bioinspired and Microgel-Tackified Adhesive Hydrogel with Rapid Self-Healing and High Stretchability. Macromolecules 2019, 52, 72–80. [Google Scholar] [CrossRef]
- Chen, S.; Tang, F.; Tang, L.; Li, L. Synthesis of Cu-Nanoparticle Hydrogel with Self-Healing and Photothermal Properties. ACS Appl. Mater. Interfaces 2017, 9, 20895–20903. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, T.; Tu, Z.; Dai, W.; Xue, Y.; Tang, C.; Gao, W.; Mao, C.; Lei, B.; Lin, C. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics 2020, 10, 4929–4943. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, L.; Zhang, M.; Ullah, M.W.; Liu, L.; Zhao, W.; Li, Y.; Ahmed, A.A.Q.; Cheng, H.; Shi, Z.; et al. In Situ Synthesized Selenium Nanoparticles-Decorated Bacterial Cellulose/Gelatin Hydrogel with Enhanced Antibacterial, Antioxidant, and Anti-Inflammatory Capabilities for Facilitating Skin Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100402. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, W.; He, F.; Ullah, M.W.; Ikram, M.; Shah, S.M.; Khan, R.; Khan, T.; Khalid, A.; Yang, G.; Wahid, F. Fabrication of Bacterial Cellulose-Curcumin Nanocomposite as a Novel Dressing for Partial Thickness Skin Burn. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Wang, L.; Mao, L.; Qi, F.; Li, X.; Wajid Ullah, M.; Zhao, M.; Shi, Z.; Yang, G. Synergistic effect of highly aligned bacterial cellulose/gelatin membranes and electrical stimulation on directional cell migration for accelerated wound healing. Chem. Eng. J. 2021, 424, 130563. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Ghosh, A.K.; Sarkar, P.; Kundu, P.P. AgNPs Ornamented Modified Bacterial Cellulose Based Self-Healable L-B-L Assembly via a Schiff Base Reaction: A Potential Wound Healing Patch. ACS Appl. Bio Mater. 2021, 4, 428–440. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Kundu, P.P. Modified bacterial cellulose based self-healable polyeloctrolyte film for wound dressing application. Carbohydr. Polym. 2017, 174, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Wu, K.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. Synthesis of cellulose-based double-network hydrogels demonstrating high strength, self-healing, and antibacterial properties. Carbohydr. Polym. 2017, 168, 112–120. [Google Scholar] [CrossRef]
- Chen, Y.M.; Sun, L.; Yang, S.A.; Shi, L.; Zheng, W.J.; Wei, Z.; Hu, C. Self-healing and photoluminescent carboxymethyl cellulose-based hydrogels. Eur. Polym. J. 2017, 94, 501–510. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef]
- Xuan, H.; Wu, S.; Fei, S.; Li, B.; Yang, Y.; Yuan, H. Injectable nanofiber-polysaccharide self-healing hydrogels for wound healing. Mater. Sci. Eng. C 2021, 128, 112264. [Google Scholar] [CrossRef]
- Sharma, A.K.; Priya; Kaith, B.S.; Bhagya Shree; Simran; Saiyam. Borax mediated synthesis of a biocompatible self-healing hydrogel using dialdehyde carboxymethyl cellulose-dextrin and gelatin. React. Funct. Polym. 2021, 166, 104977. [Google Scholar] [CrossRef]
- Invernizzi, M.; Turri, S.; Levi, M.; Suriano, R. 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers. Eur. Polym. J. 2018, 101, 169–176. [Google Scholar] [CrossRef]
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Lee, S.; Sarkar, K.; Vozzi, G.; et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater. Today 2017, 20, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, M.; Paleczny, J.; Junka, A.; Shavandi, A.; Dawiec-Liśniewska, A.; Podstawczyk, D. 3D Printing of Thermoresponsive Hydrogel Laden with an Antimicrobial Agent towards Wound Healing Applications. Bioengineering 2021, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Naficy, S.; Brown, H.R.; Razal, J.M.; Spinks, G.M.; Whitten, P.G. Progress toward Robust Polymer Hydrogels. Aust. J. Chem. 2011, 64, 1007. [Google Scholar] [CrossRef]
- Gupta, D.; Gangwar, A.; Jyoti, K.; Sainaga Jyothi, V.G.S.; Sodhi, R.K.; Mehra, N.K.; Singh, S.B.; Madan, J. Self healing hydrogels: A new paradigm immunoadjuvant for delivering peptide vaccine. Colloids Surf. B Biointerfaces 2020, 194, 111171. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F. Development of Hydrogels with Self-Healing Properties for Delivery of Bioactive Agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef]
- Schnipper, L.E.; Davidson, N.E.; Wollins, D.S.; Tyne, C.; Blayney, D.W.; Blum, D.; Dicker, A.P.; Ganz, P.A.; Hoverman, J.R.; Langdon, R.; et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J. Clin. Oncol. 2015, 33, 2563–2577. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.K.; Noone, A.; Mariotto, A.B.; Simard, E.P.; Boscoe, F.P.; Henley, S.J.; Jemal, A.; Cho, H.; Anderson, R.N.; Kohler, B.A.; et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014, 120, 1290–1314. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Q.; Guo, Z.; Wang, D.; Gao, F.; Wang, X.; Wei, Y.; Zhao, L. Injectable and Self-Healing Thermosensitive Magnetic Hydrogel for Asynchronous Control Release of Doxorubicin and Docetaxel to Treat Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 33660–33673. [Google Scholar] [CrossRef]
- Qian, C.; Zhang, T.; Gravesande, J.; Baysah, C.; Song, X.; Xing, J. Injectable and self-healing polysaccharide-based hydrogel for pH-responsive drug release. Int. J. Biol. Macromol. 2019, 123, 140–148. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Pal, S.; Kumar, S.; Kar, A.; Awasthi, A.K.; Naaz, A.; Srivastava, A.; Bajaj, A. Injectable, Self-Healing Chimeric Catechol-Fe(III) Hydrogel for Localized Combination Cancer Therapy. ACS Biomater. Sci. Eng. 2017, 3, 3404–3413. [Google Scholar] [CrossRef]
- Wang, M.; Chen, M.; Niu, W.; Winston, D.D.; Cheng, W.; Lei, B. Injectable biodegradation-visual self-healing citrate hydrogel with high tissue penetration for microenvironment-responsive degradation and local tumor therapy. Biomaterials 2020, 261, 120301. [Google Scholar] [CrossRef]
- Tang, F.; Feng, H.; Du, Y.; Xiao, Y.; Dan, H.; Zhao, H.; Chen, Q. Developing a Self-Healing Supramolecular Nucleoside Hydrogel Based on Guanosine and Isoguanosine. Chem.-Asian J. 2018, 13, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, H.; Liu, J.; Tang, F.; Du, Y.; Ji, N.; Xie, L.; Zhao, X.; Wang, Z.; Chen, Q. Dual-functional guanosine-based hydrogel integrating localized delivery and anticancer activities for cancer therapy. Biomaterials 2020, 230, 119598. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yang, M.; Seo, J.-H.; Jeong, D.I.; Hwang, C.; Kim, H.-J.; Lee, J.; Lee, K.; Park, J.; Cho, H.-J. Serially pH-Modulated Hydrogels Based on Boronate Ester and Polydopamine Linkages for Local Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 2189–2203. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, H.; Zhu, H.; Jiang, L.; Yang, H. Self-healing gelatin-based shape memory hydrogels via quadruple hydrogen bonding and coordination crosslinking for controlled delivery of 5-fluorouracil. J. Biomater. Sci. Polym. Ed. 2020, 31, 712–728. [Google Scholar] [CrossRef]
- Pandit, A.H.; Mazumdar, N.; Imtiyaz, K.; Alam Rizvi, M.M.; Ahmad, S. Self-Healing and Injectable Hydrogels for Anticancer Drug Delivery: A Study with Multialdehyde Gum Arabic and Succinic Anhydride Chitosan. ACS Appl. Bio Mater. 2020, 3, 8460–8470. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, X.; Yang, B.; Zhang, L.; Lu, A. Highly self-healable and injectable cellulose hydrogels via rapid hydrazone linkage for drug delivery and 3D cell culture. Carbohydr. Polym. 2021, 273, 118547. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, N.; Yuan, W. NIR/Thermoresponsive Injectable Self-Healing Hydrogels Containing Polydopamine Nanoparticles for Efficient Synergistic Cancer Thermochemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 9118–9131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Singh, Y. Glyoxylic Hydrazone Linkage-Based PEG Hydrogels for Covalent Entrapment and Controlled Delivery of Doxorubicin. Biomacromolecules 2019, 20, 2174–2184. [Google Scholar] [CrossRef]
- Hou, F.; Xi, B.; Wang, X.; Yang, Y.; Zhao, H.; Li, W.; Qin, J.; He, Y. Self-healing hydrogel with cross-linking induced thermo-response regulated light emission property. Colloids Surf. B Biointerfaces 2019, 183, 110441. [Google Scholar] [CrossRef]
- Bilalis, P.; Skoulas, D.; Karatzas, A.; Marakis, J.; Stamogiannos, A.; Tsimblouli, C.; Sereti, E.; Stratikos, E.; Dimas, K.; Vlassopoulos, D.; et al. Self-Healing pH- and Enzyme Stimuli-Responsive Hydrogels for Targeted Delivery of Gemcitabine to Treat Pancreatic Cancer. Biomacromolecules 2018, 19, 3840–3852. [Google Scholar] [CrossRef]
- Zhao, D.; Tang, Q.; Zhou, Q.; Peng, K.; Yang, H.; Zhang, X. A photo-degradable injectable self-healing hydrogel based on star poly(ethylene glycol)- b-polypeptide as a potential pharmaceuticals delivery carrier. Soft Matter 2018, 14, 7420–7428. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Wu, Y.; Wang, H.; Liu, J.; Ma, Q.; Xiao, K.; Li, Z.; Li, J.; Luo, F.; Tan, H. An injectable hydrogel with pH-sensitive and self-healing properties based on 4armPEGDA and N-carboxyethyl chitosan for local treatment of hepatocellular carcinoma. Int. J. Biol. Macromol. 2020, 163, 1208–1222. [Google Scholar] [CrossRef]
- Sharma, P.K.; Taneja, S.; Singh, Y. Hydrazone-Linkage-Based Self-Healing and Injectable Xanthan–Poly(ethylene glycol) Hydrogels for Controlled Drug Release and 3D Cell Culture. ACS Appl. Mater. Interfaces 2018, 10, 30936–30945. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.J.; He, F.; Huang, L.; Xiao, L.; Yang, G. Supramolecular hydrogels based on poly (ethylene glycol)-poly (lactic acid) block copolymer micelles and α-cyclodextrin for potential injectable drug delivery system. Carbohydr. Polym. 2018, 194, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Meng, X.; Wu, Z.; Wu, Z.; Qi, X. Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection. Mater. Sci. Eng. C 2018, 93, 1064–1072. [Google Scholar] [CrossRef]
- An, H.; Yang, Y.; Zhou, Z.; Bo, Y.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Pectin-based Injectable and Biodegradable Self-Healing Hydrogels for Enhanced Synergistic Anticancer Therapy. Acta Biomater. 2021, 131, 149–161. [Google Scholar] [CrossRef]
- Li, D.; Wang, S.; Meng, Y.; Li, J.; Li, J. An injectable, self-healing hydrogel system from oxidized pectin/chitosan/γ-Fe2O3. Int. J. Biol. Macromol. 2020, 164, 4566–4574. [Google Scholar] [CrossRef]
- Li, Q.; Wen, J.; Liu, C.; Jia, Y.; Wu, Y.; Shan, Y.; Qian, Z.; Liao, J. Graphene-Nanoparticle-Based Self-Healing Hydrogel in Preventing Postoperative Recurrence of Breast Cancer. ACS Biomater. Sci. Eng. 2019, 5, 768–779. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, N.; Huang, Y.; He, R.; Xu, S.; Yuan, W. Coordination of injectable self-healing hydrogel with Mn-Zn ferrite@mesoporous silica nanospheres for tumor MR imaging and efficient synergistic magnetothermal-chemo-chemodynamic therapy. Chem. Eng. J. 2020, 401, 126100. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, A.; Deepak; Kumar, R.; Rana, N.K.; Koch, B. Development of a novel chitosan based biocompatible and self-healing hydrogel for controlled release of hydrophilic drug. Int. J. Biol. Macromol. 2018, 116, 37–44. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, P.; Tiwari, S. Dynamic imine bond based chitosan smart hydrogel with magnified mechanical strength for controlled drug delivery. Int. J. Biol. Macromol. 2020, 160, 489–495. [Google Scholar] [CrossRef]
- Sharma, S.; Afgan, S.; Deepak; Kumar, A.; Kumar, R. l-Alanine induced thermally stable self-healing guar gum hydrogel as potential drug vehicle for sustained release of hydrophilic drug. Mater. Sci. Eng. C 2019, 99, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Kong, Y.; Su, Y.; Kuss, M.A.; Jiang, X.; Li, X.; Xie, J.; Duan, B. Tannic acid-inspired, self-healing, and dual stimuli responsive dynamic hydrogel with potent antibacterial and anti-oxidative properties. J. Mater. Chem. B 2021. [CrossRef]
- Liu, P.; Guo, B.; Wang, S.; Ding, J.; Zhou, W. A thermo-responsive and self-healing liposome-in-hydrogel system as an antitubercular drug carrier for localized bone tuberculosis therapy. Int. J. Pharm. 2019, 558, 101–109. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, Z.; Zhu, C.; Song, X.; Qin, Y.; Zhu, M.; Zhang, T.; Cui, W.; Tang, H.; Zheng, H. Injectable and Self-Healing Hydrogel with Anti-Bacterial and Anti-Inflammatory Properties for Acute Bacterial Rhinosinusitis with Micro Invasive Treatment. Adv. Healthc. Mater. 2020, 9, 2001032. [Google Scholar] [CrossRef]
- Banerjee, S.L.; Samanta, S.; Sarkar, S.; Singha, N.K. A self-healable and antifouling hydrogel based on PDMS centered ABA tri-block copolymer polymersomes: A potential material for therapeutic contact lenses. J. Mater. Chem. B 2020, 8, 226–243. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration during Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Shi, Y.; Wang, B.; Xue, W.; Zhang, Y. Transforming sustained release into on-demand release: Self-healing guanosine–borate supramolecular hydrogels with multiple responsiveness for Acyclovir delivery. Biomater. Sci. 2020, 8, 6190–6203. [Google Scholar] [CrossRef]
- Yu, B.; Zhan, A.; Liu, Q.; Ye, H.; Huang, X.; Shu, Y.; Yang, Y.; Liu, H. A designed supramolecular cross-linking hydrogel for the direct, convenient, and efficient administration of hydrophobic drugs. Int. J. Pharm. 2020, 578, 119075. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Dai, Z.; Jiang, F.; Tian, J.; Zhang, W. A super-stretchable, self-healing and injectable supramolecular hydrogel constructed by a host–guest crosslinker. Biomater. Sci. 2020, 8, 3359–3369. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Paul, A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.L.; Elmotasem, H.; Salama, A.A.A. Colchicine mesoporous silica nanoparticles/hydrogel composite loaded cotton patches as a new encapsulator system for transdermal osteoarthritis management. Int. J. Biol. Macromol. 2020, 164, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-assembled hydrogels utilizing polymer–nanoparticle interactions. Nat. Commun. 2015, 6, 6295. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels with Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Tong, Z.; Jin, L.; Oliveira, J.M.; Reis, R.L.; Zhong, Q.; Mao, Z.; Gao, C. Adaptable hydrogel with reversible linkages for regenerative medicine: Dynamic mechanical microenvironment for cells. Bioact. Mater. 2021, 6, 1375–1387. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Tortorella, I.; Bazzucchi, M.; Porcellati, S.; Emiliani, C.; Martino, S. Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions. Int. J. Mol. Sci. 2019, 20, 5337. [Google Scholar] [CrossRef] [Green Version]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Cooke, M.E.; Rosenzweig, D.H. The rheology of direct and suspended extrusion bioprinting. APL Bioeng. 2021, 5, 011502. [Google Scholar] [CrossRef]
- Farhat, W.; Hasan, A.; Lucia, L.; Becquart, F.; Ayoub, A.; Kobeissy, F. Hydrogels for Advanced Stem Cell Therapies: A Biomimetic Materials Approach for Enhancing Natural Tissue Function. IEEE Rev. Biomed. Eng. 2019, 12, 333–351. [Google Scholar] [CrossRef]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.G.; Robey, P.G. Human Pluripotent Stem Cell Culture: Considerations for Maintenance, Expansion, and Therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Mushtaq, M.; Kovalevska, L.; Darekar, S.; Abramsson, A.; Zetterberg, H.; Kashuba, V.; Klein, G.; Arsenian-Henriksson, M.; Kashuba, E. Cell stemness is maintained upon concurrent expression of RB and the mitochondrial ribosomal protein S18-2. Proc. Natl. Acad. Sci. USA 2020, 117, 15673–15683. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Li, Y.; Chen, Q.; Fu, L.; Tao, L.; Wei, Y. Injectable and Self-Healing Chitosan Hydrogel Based on Imine Bonds: Design and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, T.-C.; Tao, L.; Hsieh, F.-Y.; Wei, Y.; Chiu, I.-M.; Hsu, S. An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System. Adv. Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef]
- Madl, C.M.; Heilshorn, S.C. Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche. Annu. Rev. Biomed. Eng. 2018, 20, 21–47. [Google Scholar] [CrossRef]
- Yue, B. Biology of the Extracellular Matrix. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491–497. [Google Scholar] [CrossRef]
- Pettignano, A.; Häring, M.; Bernardi, L.; Tanchoux, N.; Quignard, F.; Díaz Díaz, D. Self-healing alginate–gelatin biohydrogels based on dynamic covalent chemistry: Elucidation of key parameters. Mater. Chem. Front. 2017, 1, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Sisso, A.M.; Boit, M.O.; DeForest, C.A. Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. Part A 2020, 108, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Guterman, T.; Adadi, N.; Yadid, M.; Brosh, T.; Adler-Abramovich, L.; Dvir, T.; Gazit, E. A Self-Healing, All-Organic, Conducting, Composite Peptide Hydrogel as Pressure Sensor and Electrogenic Cell Soft Substrate. ACS Nano 2019, 13, 163–175. [Google Scholar] [CrossRef]
- Chakraborty, P.; Oved, H.; Bychenko, D.; Yao, Y.; Tang, Y.; Zilberzwige-Tal, S.; Wei, G.; Dvir, T.; Gazit, E. Nanoengineered Peptide-Based Antimicrobial Conductive Supramolecular Biomaterial for Cardiac Tissue Engineering. Adv. Mater. 2021, 33, 2008715. [Google Scholar] [CrossRef]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst. Nanoeng. 2020, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Daly, A.C.; Davidson, M.D.; Burdick, J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Anthony, D.F.; Shiels, P.G. Exploiting paracrine mechanisms of tissue regeneration to repair damaged organs. Transplant. Res. 2013, 2, 10. [Google Scholar] [CrossRef]
- Smith, L.R.; Cho, S.; Discher, D.E. Stem Cell Differentiation is Regulated by Extracellular Matrix Mechanics. Physiology 2018, 33, 16–25. [Google Scholar] [CrossRef]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Yang, H.; Jiang, X.; Yang, B.; Zhu, K.; Lai, N.C.-H.; Huang, C.; Chang, C.; Bian, L.; Zhang, L. Injectable chitin hydrogels with self-healing property and biodegradability as stem cell carriers. Carbohydr. Polym. 2021, 256, 117574. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, X.; Park, S.; Jin, X.; Ma, P.X. Rapid Self-Integrating, Injectable Hydrogel for Tissue Complex Regeneration. Adv. Healthc. Mater. 2015, 4, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Chen, X.; Li, R.; Feng, Q.; Bian, L. Multivalent Host–Guest Hydrogels as Fatigue-Resistant 3D Matrix for Excessive Mechanical Stimulation of Encapsulated Cells. Chem. Mater. 2017, 29, 8604–8610. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Lee, S.S.; Hsu, E.L.; Mendoza, M.; Ghodasra, J.; Nickoli, M.S.; Ashtekar, A.; Polavarapu, M.; Babu, J.; Riaz, R.M.; Nicolas, J.D.; et al. Gel Scaffolds of BMP-2-Binding Peptide Amphiphile Nanofibers for Spinal Arthrodesis. Adv. Healthc. Mater. 2015, 4, 131–141. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Basu, P.; Sukul, M.; Mano, J.F.; Reseland, J.E. Injectable biomaterials for dental tissue regeneration. Int. J. Mol. Sci. 2020, 21, 3442. [Google Scholar] [CrossRef]
- Yan, S.; Wang, W.; Li, X.; Ren, J.; Yun, W.; Zhang, K.; Li, G.; Yin, J. Preparation of mussel-inspired injectable hydrogels based on dual-functionalized alginate with improved adhesive, self-healing, and mechanical properties. J. Mater. Chem. B 2018, 6, 6377–6390. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, S.; Wang, X.; Zhang, Y.; Chen, L.; Zhang, L. Noncompressible Hemostasis and Bone Regeneration Induced by an Absorbable Bioadhesive Self-Healing Hydrogel. Adv. Funct. Mater. 2021, 31, 2009189. [Google Scholar] [CrossRef]
- Lü, S.; Bai, X.; Liu, H.; Ning, P.; Wang, Z.; Gao, C.; Ni, B.; Liu, M. An injectable and self-healing hydrogel with covalent cross-linking: In vivo for cranial bone repair. J. Mater. Chem. B 2017, 5, 3739–3748. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Zhu, W.; Xu, Z.; Fuchs, S.; Hilborn, J.; Zhu, L.; Ma, Q.; Wang, Y.; Weng, X.; et al. Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Adv. Funct. Mater. 2017, 27, 1–14. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, J.; Zhang, K.; Yao, H.; Zheng, N.; Zheng, L.; Wang, J.; Wei, K.; Xiao, X.; Qin, L.; et al. Dynamic and Cell-Infiltratable Hydrogels as Injectable Carrier of Therapeutic Cells and Drugs for Treating Challenging Bone Defects. ACS Cent. Sci. 2019, 5, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A New Self-Healing Hydrogel Containing hucMSC-Derived Exosomes Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 1047. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, Y.; Wang, Z.; Yang, X.; Yu, X.; Lu, Y.; Deng, L.; Cui, W. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Mater. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Dai, Z.; Cao, H.; Li, J.; Zhang, W. Sustained protein therapeutics enabled by self-healing nanocomposite hydrogels for non-invasive bone regeneration. Biomater. Sci. 2020, 8, 682–693. [Google Scholar] [CrossRef]
- Chen, B.; Liang, Y.; Zhang, J.; Bai, L.; Xu, M.; Han, Q.; Han, X.; Xiu, J.; Li, M.; Zhou, X.; et al. Synergistic enhancement of tendon-to-bone healing via anti-inflammatory and pro-differentiation effects caused by sustained release of Mg2+/curcumin from injectable self-healing hydrogels. Theranostics 2021, 11, 5911–5925. [Google Scholar] [CrossRef]
- Lin, S.-H.; Papadakis, C.M.; Kang, J.-J.; Lin, J.-M.; Hsu, S. Injectable Phenolic-Chitosan Self-Healing Hydrogel with Hierarchical Micelle Architectures and Fast Adhesiveness. Chem. Mater. 2021, 33, 3945–3958. [Google Scholar] [CrossRef]
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-printed self-healing hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Nadgorny, M.; Collins, J.; Xiao, Z.; Scales, P.J.; Connal, L.A. 3D-printing of dynamic self-healing cryogels with tuneable properties. Polym. Chem. 2018, 9, 1684–1692. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Cong, Y.; Osi, A.R.; Zhou, Y.; Huang, F.; Zaccaria, R.P.; Chen, J.; Wang, R.; Fu, J. Direct 3D Printed Biomimetic Scaffolds Based on Hydrogel Microparticles for Cell Spheroid Growth. Adv. Funct. Mater. 2020, 30, 1910573. [Google Scholar] [CrossRef]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized Hydrogel: Self-Healing Properties of Supramolecular Hydrogels Formed by Polymerization of Host-Guest-Monomers that Contain Cyclodextrins and Hydrophobic Guest Groups. Adv. Mater. 2013, 25, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi V. K., A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. https://doi.org/10.3390/polym13213782
Devi V. K. A, Shyam R, Palaniappan A, Jaiswal AK, Oh T-H, Nathanael AJ. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers. 2021; 13(21):3782. https://doi.org/10.3390/polym13213782
Chicago/Turabian StyleDevi V. K., Anupama, Rohin Shyam, Arunkumar Palaniappan, Amit Kumar Jaiswal, Tae-Hwan Oh, and Arputharaj Joseph Nathanael. 2021. "Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications" Polymers 13, no. 21: 3782. https://doi.org/10.3390/polym13213782
APA StyleDevi V. K., A., Shyam, R., Palaniappan, A., Jaiswal, A. K., Oh, T.-H., & Nathanael, A. J. (2021). Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers, 13(21), 3782. https://doi.org/10.3390/polym13213782