Effect of Egg-Coating Material Properties by Blending Cassava Starch with Methyl Celluloses and Waxes on Egg Quality
Abstract
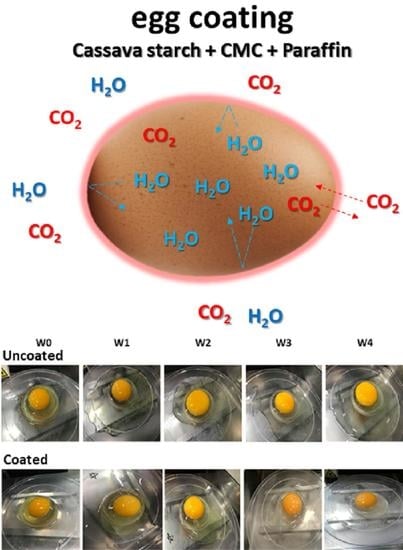
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Coating Solutions
2.2. Coating and Film Preparation
2.3. Tensile Properties of Films
2.4. Measurement of Haugh Unit
2.5. Determination of Weight Loss
2.6. Measurement of Contact Angle
2.7. Water Vapor Transmission Rate (WVTR) and Water Vapor Permeability (WVP)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Tensile Properties of Films
3.2. Haugh Unit
3.3. Weight Loss
3.4. Contact Angle Measurements
3.5. WVTR and WVP of Coating Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lesnierowski, G.; Stangierski, J. What’s new in chicken egg research and technology for human health promotion?—A review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Al-Nasser, A.; Al-Khalaifa, H.; Al-Saffar, A.; Khalil, F.; Al-Bahouh, M.; Ragheb, G.; Al-Haddad, A.; Mashaly, M. Overview of chicken taxonomy and domestication. World’s Poult. Sci. J. 2007, 63, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Yüceer, M.; Caner, C. Antimicrobial lysozyme-chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 2014, 94, 153–162. [Google Scholar] [CrossRef]
- Kodsangma, A.; Homsaard, N.; Nadon, S.; Rachtanapun, P.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Effect of sodium benzoate and chlorhexidine gluconate on a bio-thermoplastic elastomer made from thermoplastic starch-chitosan blended with epoxidized natural rubber. Carbohydr. Polym. 2020, 242, 116421. [Google Scholar] [CrossRef]
- Chi, K.; Wang, H.; Catchmark, J.K. Sustainable-bases barrier coatings for packaging applications. Food Hydrocoll. 2020, 103, 105696. [Google Scholar] [CrossRef]
- Leksawasdi, N.; Chaiyaso, T.; Rachtanapun, P.; Thanakkasaranee, S.; Jantrawut, P.; Ruksiriwanich, W.; Seesuriyachan, P.; Phimolsiripol, Y.; Techapun, C.; Sommano, S.R.; et al. Corn starch reactive blending with latex from natural rubber using Na+ ions augmented carboxymethyl cellulose as a crosslinking agent. Sci. Rep. 2021, 11, 19250. [Google Scholar] [CrossRef] [PubMed]
- Suriyatem, R.; Noikang, N.; Kankam, T.; Jantanasakulwong, K.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Physical properties of carboxymethyl cellulose from palm bunch and bagasse agricultural wastes: Effect of delignification with hydrogen peroxide. Polymers 2020, 12, 1505. [Google Scholar] [CrossRef]
- Tantala, J.; Rachtanapun, C.; Tongdeesoontorn, W.; Jantanasakulwong, K.; Rachtanapun, P. Moisture Sorption Isotherms and Prediction Models of Carboxymethyl Chitosan Films from Different Sources with Various Plasticizers. Adv. Mater. Sci. Eng. 2019, 2019, 4082439. [Google Scholar] [CrossRef] [Green Version]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, characterization, and application of carboxymethyl cellulose from asparagus stalk end. Polymers 2021, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; et al. Carboxymethyl bacterial cellulose from nata de coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Kodsangma, A.; Homsaard, N.; Nadon, S.; Jantrawut, P.; Ruksiriwanich, W.; Seesuriyachan, P.; Leksawasdi, N.; Phimolsiripol, Y.; Chaiyaso, T.; et al. Thermoplastic mung bean starch/natural rubber/sericin blends for improved oil resistance. Int. J. Biol. Macromol. 2021, 188, 283–289. [Google Scholar] [CrossRef]
- Chaiyaso, T.; Rachtanapun, P.; Thajai, N.; Kiattipornpithak, K.; Jantrawut, P.; Ruksiriwanich, W.; Seesuriyachan, P.; Leksawasdi, N.; Phimolsiripol, Y.; Techapun, C.; et al. Sericin cocoon bio-compatibilizer for reactive blending of thermoplastic cassava starch. Sci. Rep. 2021, 11, 19945. [Google Scholar] [CrossRef] [PubMed]
- Kaewsalud, T.; Yakul, K.; Jantanasakulwong, K.; Tapingkae, W.; Watanabe, M.; Chaiyaso, T. Biochemical characterization and application of thermostable-alkaline keratinase from Bacillus halodurans SW-X to valorize chicken feather wastes. Waste Biomass Valorization 2021, 12, 3951–3964. [Google Scholar] [CrossRef]
- Yakul, K.; Kaewsalud, T.; Techapun, C.; Seesuriyachan, P.; Jantanasakulwong, K.; Watanabe, M.; Takenaka, S.; Chaiyaso, T. Enzymatic valorization process of yellow cocoon waste for production of antioxidative sericin and fibroin film. J. Chem. Technol. Biotechnol. 2021, 96, 953–962. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Masavang, S.; Mahe, J.; Sommano, S.; Ruksiriwanich, W.; Brachais, C.H.; Chambin, O.; Jantrawut, P. Mango (cv. Nam Dokmai) peel as a source of pectin and its potential use as a film-forming polymer. Food Hydrocoll. 2020, 102, 105611. [Google Scholar] [CrossRef]
- Junmahasathien, T.; Panraksa, P.; Protiarn, P.; Hormdee, D.; Noisombut, R.; Kantrong, N.; Jantrawut, P. Preparation and evaluation of metronidazole-loaded pectin films for potentially targeting a microbial infection associated with periodontal disease. Polymers 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiwarit, T.; Ruksiriwanich, W.; Jantanasakulwong, K.; Jantrawut, P. Use of orange oil loaded pectin films as antibacterial material for food packaging. Polymers 2018, 10, 1144. [Google Scholar] [CrossRef] [Green Version]
- Wongkaew, M.; Sommano, S.R.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango peel pectin by microwave-assisted extraction and its use as fat replacement in dried chinese sausage. Foods 2020, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.G.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Surayot, U.; Wang, J.; Seesuriyachan, P.; Kuntiya, A.; Tabarsa, M.; Lee, Y.; Kim, J.K.; Park, W.J.; You, S.G. Exopolysaccharides from lactic acid bacteria: Structural analysis, molecular weight effect on immunomodulation. Int. J. Biol. Macromol. 2014, 68, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Surin, S.; You, S.G.; Seesuriyachan, P.; Muangrat, R.; Wangtueai, S.; Jambrak, A.R.; Phongthai, S.; Jantanasakulwong, K.; Chaiyaso, T.; Phimolsiripol, Y. Optimization of ultrasonic-assisted extraction of polysaccharides from purple glutinous rice bran (Oryza sativa L.) and their antioxidant activities. Sci. Rep. 2020, 10, 10410. [Google Scholar] [CrossRef] [PubMed]
- Caner, C. The effect of edible eggshell coatings on egg quality and consumer perception. J. Sci. Food Agric. 2005, 85, 1897–1902. [Google Scholar] [CrossRef]
- Pires, P.G.S.; Machado, G.S.; Franceschi, C.H.; Kindlein, L.; Andretta, I. Rice protein coating in extending the shelf-life of conventional eggs. Poult. Sci. 2019, 98, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.A.; Borges, S.V.; Dias, M.V.; Piccoli, R.H.; Fassani, E.J.; da Silva, E.M.C. Impact of whey protein isolate/sodium montmorillonite/sodium metabisulfite coating on the shelf life of fresh eggs during storage. LWT Food Sci. Technol. 2021, 139, 110611. [Google Scholar] [CrossRef]
- Akpinar, G.C.; Canogullari, S.; Baylan, M.; Alasahan, S.; Aygun, A. The use of propolis extract for the storage of quail eggs. J. Appl. Poult. Res. 2015, 24, 427–435. [Google Scholar] [CrossRef]
- Copur, G.; Camci, O.; Sahinler, N.; Gul, A. The effect of propolis egg shell coatings on interior egg quality. Eur. Poult. Sci. 2008, 72, 35–40. [Google Scholar]
- Caner, C.; Yüceer, M. Efficacy of various protein-based coating on enhancing the shelf life of fresh eggs during storage. Poult. Sci. 2015, 94, 1665–1677. [Google Scholar] [CrossRef]
- Meyer, R.; Spencer, J.V. The effect of various coatings on shell strength and egg quality. Poult. Sci. 1973, 52, 703–711. [Google Scholar] [CrossRef]
- Wong, Y.C.; Herald, T.J.; Hachmeister, K.A. Evaluation of mechanical and barrier properties of protein coatings on shell eggs. Poult. Sci. 1996, 75, 417–422. [Google Scholar] [CrossRef]
- Homsaard, N.; Kodsangma, A.; Jantrawut, P.; Rachtanapun, P.; Leksawasdi, N.; Phimolsiripol, Y.; Seesuriyachan, P.; Chaiyaso, T.; Sommano, S.R.; Rohindra, D.; et al. Efficacy of cassava starch blending with gelling agents and palmoil coating in improving egg shelf life. Int. J. Food Sci. Technol. 2020, 56, 3655–3661. [Google Scholar] [CrossRef]
- Torrico, D.D.; Wardy, W.; Carabante, K.M.; Pujols, K.D.; Xu, Z.; No, H.K.; Prinyawiwatkul, W. Quality of eggs coated with oil–chitosan emulsion: Combined effects of emulsifier types, initial albumen quality, and storage. LWT Food Sci. Technol. 2014, 57, 35–41. [Google Scholar] [CrossRef]
- Wardy, W.; Torrico, D.D.; Jirangrat, W.; No, H.K.; Saalia, F.K.; Prinyawiwatkul, W. Chitosan-soybean oil emulsion coating affects physico-functional and sensory quality of eggs during storage. LWT Food Sci. Technol. 2011, 44, 2349–2355. [Google Scholar] [CrossRef]
- Nongtaodum, S.; Jangchud, A.; Jangchud, K.; Dhamvithee, P.; No, H.K.; Prinyawiwatkul, W. Oil coating affects internal quality and sensory acceptance of selected attributes of raw eggs during storage. J. Food Sci. 2013, 78, S329–S335. [Google Scholar] [CrossRef] [PubMed]
- Cindric, I.J.; Zeiner, M.; Steffan, I. Trace elemental characterization of edible oils by ICP–AES and GFAAS. Microchem. J. 2007, 85, 136–139. [Google Scholar] [CrossRef]
- Morsy, M.K.; Sharoba, A.M.; Khalaf, H.H.; El-Tanahy, H.H.; Cutter, C.N. Efficacy of antimicrobial pullulan-based coating to improve internal quality and shelf-life of chicken eggs during storage. J. Food Sci. 2015, 80, M1066–M1074. [Google Scholar] [CrossRef]
- Yüceer, M.; Aday, M.S.; Caner, C. Ozone treatment of shell eggs to preserve functional quality and enhance shelf life during storage. J. Sci. Food Agric. 2016, 96, 2755–2763. [Google Scholar] [CrossRef]
- Biladeau, A.M.; Keener, K.M. The effects of edible coatings on chicken egg quality under refrigerated storage. Poult. Sci. 2009, 88, 1266–1274. [Google Scholar] [CrossRef]
- Caner, C. Whey protein isolate coating and concentration effects on egg shelf life. J. Sci. Food Agric. 2005, 85, 2143–2148. [Google Scholar] [CrossRef]
- Jones, D.R.; Ward, G.E.; Regmi, P.; Karcher, D.M. Impact of egg handling and conditions during extended storage on egg quality. Poult. Sci. 2018, 97, 716–723. [Google Scholar] [CrossRef]
- Kim, S.H.; No, H.K.; Kim, S.D.; Prinyawiwatkul, W. Effect of plasticizer concentration and solvent types on shelf-life of eggs coated with chitosan. J. Food Sci. 2006, 71, S349–S353. [Google Scholar] [CrossRef]
- Suppakul, P.; Jutakorn, K.; Bangchokedee, Y. Efficacy of cellulose-based coating on enhancing the shelf life of fresh eggs. J. Food Eng. 2010, 98, 207–213. [Google Scholar] [CrossRef]
- Wu, C.; Qi, H.; Chen, W.; Huang, C.; Su, C.; Li, W.; Hou, S. Preparation and evaluation of a Carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi 2007, 127, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayorinde, J.O.; Odeniyi, M.A.; Balogun-Agbaje, O. Formulation and evaluation of oral dissolving films of amlodipine besylate using blends of starches with hydroxypropyl methyl cellulose. Polym. W Med. 2016, 46, 45–51. [Google Scholar] [CrossRef]
- Jantanasakulwong, K.; Wongsuriyasak, S.; Rachtanapun, P.; Seesuriyachan, P.; Chaiyaso, T.; Leksawasdi, N.; Techapun, C. Mechanical properties improvement of thermoplastic corn starch and polyethylene-grafted-maleicanhydride blending by Na+ ions neutralization of carboxymethyl cellulose. Int. J. Biol. Macromol. 2018, 120, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; Mohamed, N.H.; Rostom, M.; Al-Lohedon, H.A.; Abdullah, M.M.S. New hydrophobic silica nanoparticles capped with petroleum paraffin wax embedded in epoxy networks as multifunctional steel epoxy coatings. Prog. Org. Coat. 2019, 128, 99–111. [Google Scholar] [CrossRef]
- Pires, P.G.D.S.; Bavaresco, C.; Pires, P.D.D.S.; Cardinal, K.M.; Leuven, A.F.R.; Andretta, I. Development of an innovation green coating to reduce egg losses. Clean. Eng. Technol. 2021, 2, 100065. [Google Scholar] [CrossRef]
- Sun, R.; Song, G.; Zhang, H.; Zhang, H.; Chi, Y.; Ma, Y.; Li, H.; Bai, S.; Zhang, X. Effect of basil essential oil and beeswax incorporation on the physical, structural, and antibacterial properties of chitosan emulsion based coating for eggs preservation. LWT 2021, 150, 112020. [Google Scholar] [CrossRef]
- Thajai, N.; Jantanasakulwong, K.; Rachtanapun, P.; Jantrawut, P.; Kiattipornpithak, K.; Kanthiya, T.; Punyodom, W. Effect of chlorhexidine gluconate on mechanical and anti-microbial properties of thermoplastic cassava starch. Carbohydr. Polym. 2021, 275, 118690. [Google Scholar] [CrossRef]
- Wang, B.; Wei, W.; Aputexiakere, J.; Li, Y.; Ma, H. Surface decontamination of whole eggs using pulsed light technology and shelf life study of combined pulsed light and vaseline coating during room temperature storage. Food Control 2021, 108411. [Google Scholar] [CrossRef]
- Menzel, C.; Andersson, M.; Andersson, R.; Vázquez-Gutiérrez, J.L.; Daniel, G.; Langton, M.; Gällstedt, M.; Koch, K. Improved material properties of solution-cast starch films: Effect of varying amylopectin structure and amylose content of starch from genetically modified potatoes. Carbohydr. Polym. 2015, 130, 388–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monira, K.N.; Salahuddin, M.; Miah, G. Effect of breed and holding period on egg quality characteristics of chicken. Int. J. Poult. Sci. 2003, 2, 261–263. [Google Scholar]
- Gomez, G.N.; Florez, J.F.; Mendoza, J.S.; Cervera, C.P.; Pizarro, R.A. Development and characterization of dual-modified yam (Dioscorea rotundata) starch-based films. Heliyon 2021, 7, e06644. [Google Scholar] [CrossRef]
- Su, J.F.; Huang, Z.; Yuan, X.Y.; Wang, X.Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Yadollahi, M.; Namazi, H.; Barkhordari, S. Preparation and properties of carboxymethyl cellulose/layered double hydroxide bionanocomposite films. Carbohydr. Polym. 2014, 108, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Okiki, P.; Ahmed, O. Preservation of quality of table eggs using vegetable oil and shea butter. Int. Lett. Nat. Sci. 2017, 63, 27–33. [Google Scholar] [CrossRef]
- Ezazi, A.; Javadi, A.; Jafarizadeh-Malmiri, H.J.; Mirzaei, H. Development of a chitosan-propolis extract edible coating formulation based on physico-chemical attributes of hens’ eggs: Optimization and characteristics edible coating of egg using chitosan and propolis. Food Biosci. 2021, 40, 100894. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Ferreira, C.O.; Nunes, C.A.; Delgadillo, I.; Lopes-da-Silva, J.A. Characterization of chitosan-whey protein films at acid pH. Food Res. Int. 2009, 42, 807–813. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Rachtanapun, P. Water vapour permeability and sorption isotherms of cassava starch based films blended with gelatin and carboxymethylcellulose. Asian J. Food Agro Ind. 2009, 2, 501–514. [Google Scholar]
- Valencia-Chamorro, S.A.; Pérez-Gago, M.B.; del Río, M.Á.; Palou, L. Effect of antifungal hydroxypropyl methylcellulose (HPMC)-lipid edible composite coatings on postharvest decay development and quality attributes of cold-stored “Valencia” oranges. Postharvest Biol. Technol. 2009, 54, 72–79. [Google Scholar] [CrossRef]





| Formulation | Composition (w/v%) | |||||
|---|---|---|---|---|---|---|
| CS | Glycerol | CMC | HPMC | Beeswax | Paraffin | |
| CS/HPMC/Beeswax3 | 6 | 2 | - | 1 | 3 | - |
| CS/CMC/Paraffin3 | 6 | 2 | 1 | - | - | 3 |
| CS/CMC/Paraffin0.5 | 6 | 2 | 1 | - | - | 0.5 |
| CS/CMC/Beeswax0.5 | 6 | 2 | 1 | - | 0.5 | - |
| CS/HPMC/Paraffin0.5 | 6 | 2 | - | 1 | - | 0.5 |
| CS/CMC/Paraffin(L)0.5 | 6 | 2 | 1 | - | - | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachtanapun, P.; Homsaard, N.; Kodsangma, A.; Leksawasdi, N.; Phimolsiripol, Y.; Phongthai, S.; Khemacheewakul, J.; Seesuriyachan, P.; Chaiyaso, T.; Chotinan, S.; et al. Effect of Egg-Coating Material Properties by Blending Cassava Starch with Methyl Celluloses and Waxes on Egg Quality. Polymers 2021, 13, 3787. https://doi.org/10.3390/polym13213787
Rachtanapun P, Homsaard N, Kodsangma A, Leksawasdi N, Phimolsiripol Y, Phongthai S, Khemacheewakul J, Seesuriyachan P, Chaiyaso T, Chotinan S, et al. Effect of Egg-Coating Material Properties by Blending Cassava Starch with Methyl Celluloses and Waxes on Egg Quality. Polymers. 2021; 13(21):3787. https://doi.org/10.3390/polym13213787
Chicago/Turabian StyleRachtanapun, Pornchai, Nattagarn Homsaard, Araya Kodsangma, Noppol Leksawasdi, Yuthana Phimolsiripol, Suphat Phongthai, Julaluk Khemacheewakul, Phisit Seesuriyachan, Thanongsak Chaiyaso, Suwit Chotinan, and et al. 2021. "Effect of Egg-Coating Material Properties by Blending Cassava Starch with Methyl Celluloses and Waxes on Egg Quality" Polymers 13, no. 21: 3787. https://doi.org/10.3390/polym13213787
APA StyleRachtanapun, P., Homsaard, N., Kodsangma, A., Leksawasdi, N., Phimolsiripol, Y., Phongthai, S., Khemacheewakul, J., Seesuriyachan, P., Chaiyaso, T., Chotinan, S., Jantrawut, P., Ruksiriwanich, W., Wangtueai, S., Sommano, S. R., Tongdeesoontorn, W., & Jantanasakulwong, K. (2021). Effect of Egg-Coating Material Properties by Blending Cassava Starch with Methyl Celluloses and Waxes on Egg Quality. Polymers, 13(21), 3787. https://doi.org/10.3390/polym13213787