Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Production of the Multilayer Films
2.3. ATR-FTIR Analyses
2.4. Thermal Characterization
2.5. Scanning Electron Microscopy (SEM)
2.6. Oxygen Transmission Rate Measurements
2.7. Evaluation of Wettability, Surface Energies and the Work of Adhesion
2.8. Evaluation of the Seal Strength
2.9. Tensile Tests
2.10. Statistical Analysis methodology
3. Results
3.1. FTIR-ATR Analysis
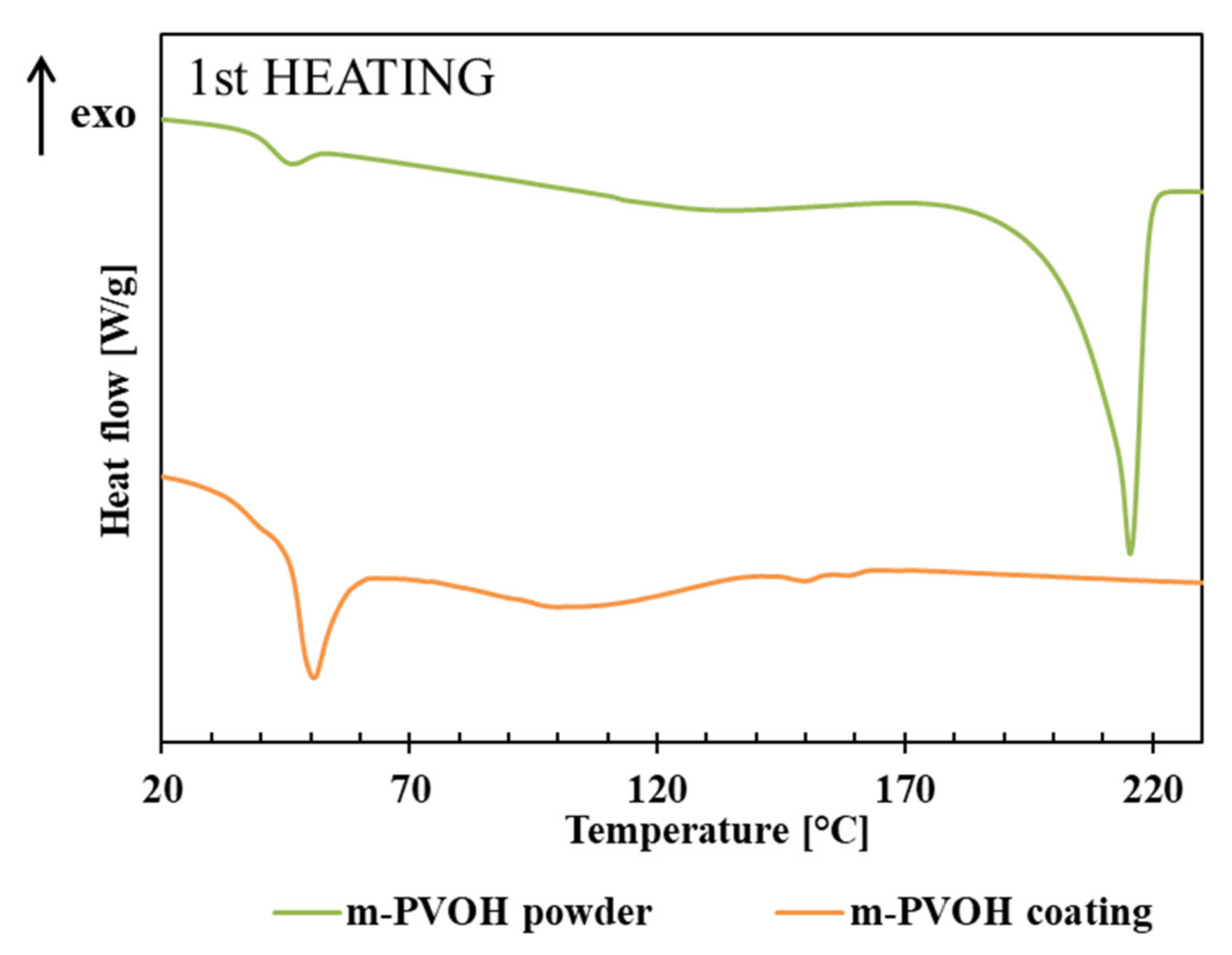
3.2. Thermal Transition and Crystallinity
3.3. SEM Analyses
3.4. Oxygen Barrier Properties
3.5. Evaluation of Wettability, Surface Energies and the Work of Adhesion
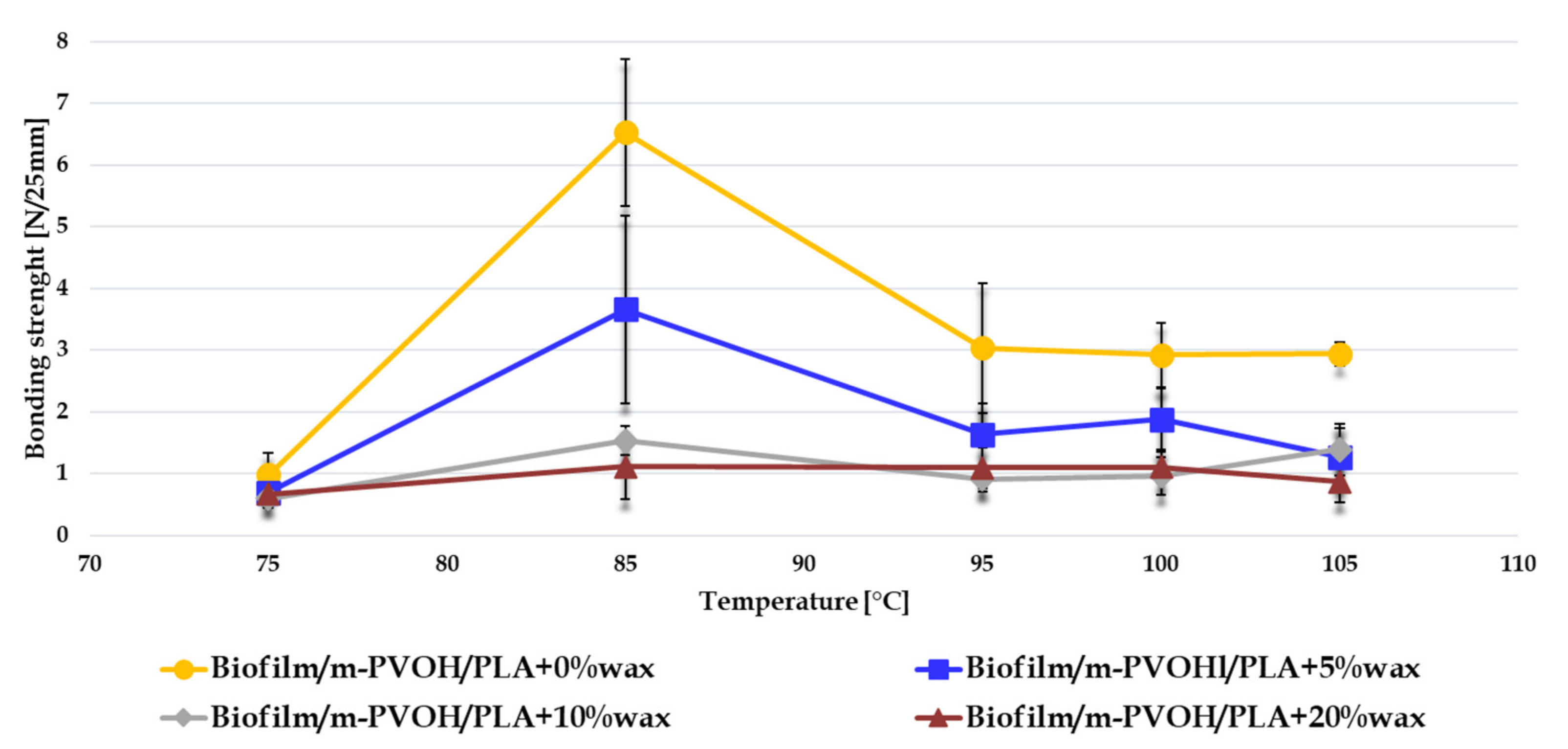
3.6. Evaluation of Seal Strength
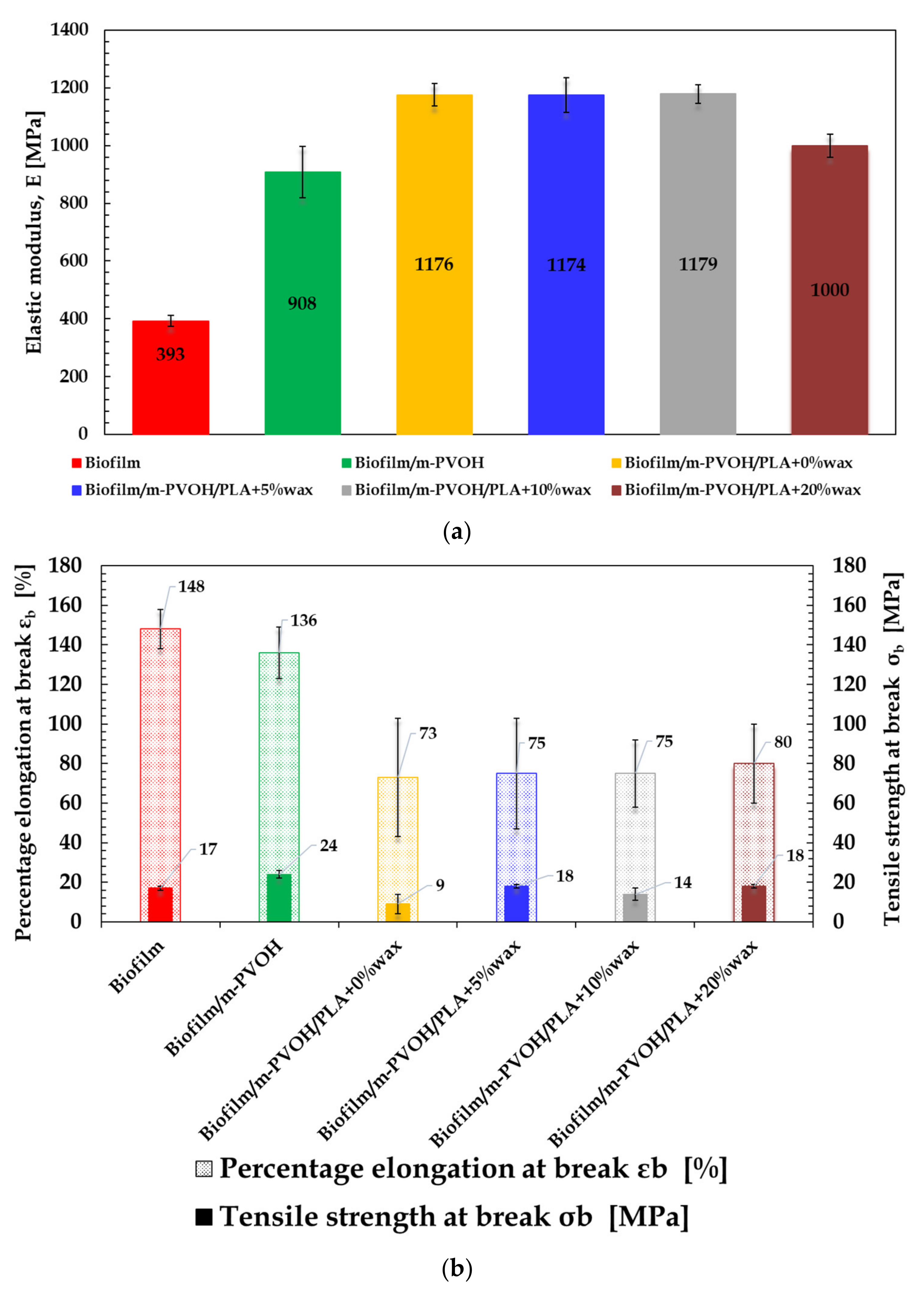
3.7. Tensile Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Riechers, M.; Fanini, L.; Apicella, A.; Galván, C.B.; Blondel, E.; Espiña, B.; Kefer, S.; Keroullé, T.; Klun, K.; Pereira, T.R.; et al. Plastics in Our Ocean as Transdisciplinary Challenge. Mar. Pollut. Bull. 2021, 164, 112051. [Google Scholar] [CrossRef] [PubMed]
- Flexible Plastic Packaging Market by Material Type (Oil Base Polymers, Bio-Plastics), Product (Pouches, Bags & Sacks, Tubes), End Use (Food, Beverages, Personal Care) & Region—Forecast to 2021–2031. Available online: https://www.futuremarketinsights.com/reports/flexible-plastic-packaging-market (accessed on 6 February 2022).
- PlasticsEurope. Plastics-the Facts 2021 An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf (accessed on 17 January 2022).
- Aliotta, L.; Gigante, V.; Coltelli, M.; Cinelli, P.; Lazzeri, A. Evaluation of Mechanical and Interfacial Properties of Bio-Composites Based on Poly (Lactic Acid) with Natural Cellulose Fibers. Int. J. Mol. Sci. 2019, 20, 960. [Google Scholar] [CrossRef] [Green Version]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439862421. [Google Scholar]
- George, J.; Aaliya, B.; Sunooj, K.V.; Kumar, R. An overview of higher barrier packaging using nanoadditives. In Nanotechnology-Enhanced Food Packaging; Wiley: Hoboken, NJ, USA, 2022; pp. 235–264. [Google Scholar]
- Zhong, W.; Wei, B.; Wang, Y.; Bai, Y. Effects of the Cross-Linking Structures of Polyacrylate Coating on PET Films on Oxygen Permeability. Polym. Bull. 2022, 1–6. [Google Scholar] [CrossRef]
- Nasri, Y.; Benaniba, M.T.; Bouquey, M. Elaboration and Characterization of Polymers Used in Flexible Multilayer Food Packaging. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Pietrosanto, A.; Scarfato, P.; di Maio, L.; Nobile, M.R.; Incarnato, L. Evaluation of the Suitability of Poly (Lactide)/Poly (Butylene-Adipate-Co-Terephthalate) Blown Films for Chilled and Frozen Food Packaging Applications. Polymers 2020, 12, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, B.A. The Science and Technology of Flexible Packaging, 1st ed.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 259–308. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; di Maio, L.; Incarnato, L. Transport Properties of Multilayer Active PET Films with Different Layers Configuration. React. Funct. Polym. 2018, 127, 29–37. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; di Maio, L.; Garofalo, E.; Incarnato, L. Evaluation of performance of PET packaging films based on different copolyester O2 scavengers. Proc. AIP Conf. Proc. 2018, 1981, 020130. [Google Scholar]
- Apicella, A.; Scarfato, P.; di Maio, L.; Incarnato, L. Oxygen Absorption Data of Multilayer Oxygen Scavenger-Polyester Films with Different Layouts. Data Brief. 2018, 19, 1530–1536. [Google Scholar] [CrossRef]
- Guerritore, M.; Olivieri, F.; Castaldo, R.; Avolio, R.; Cocca, M.; Errico, M.E.; Galdi, M.R.; Carfagna, C.; Gentile, G. Recyclable-by-Design Mono-Material Flexible Packaging with High Barrier Properties Realized through Graphene Hybrid Coatings. Resour. Conserv. Recycl. 2022, 179, 106126. [Google Scholar] [CrossRef]
- Demirgöz, D.; Elvira, C.; Mano, J.F.; Cunha, A.M.; Piskin, E.; Reis, R.L. Chemical Modification of Starch Based Biodegradable Polymeric Blends: Effects on Water Uptake, Degradation Behaviour and Mechanical Properties. Polym. Degrad. Stab. 2000, 70, 161–170. [Google Scholar] [CrossRef]
- Gigante, V.; Panariello, L.; Coltelli, M.B.; Danti, S.; Obisesan, K.A.; Hadrich, A.; Staebler, A.; Chierici, S.; Canesi, I.; Lazzeri, A.; et al. Liquid and Solid Functional Bio-Based Coatings. Polymers 2021, 13, 3640. [Google Scholar] [CrossRef] [PubMed]
- Apicella, A.; Scarfato, P.; di Maio, L.; Incarnato, L. Sustainable Active PET Films by Functionalization with Antimicrobial Bio-Coatings. Front. Mater. 2019, 6, 243. [Google Scholar] [CrossRef]
- Ye, M.; Neetoo, H.; Chen, H. Control of Listeria Monocytogenes on Ham Steaks by Antimicrobials Incorporated into Chitosan-Coated Plastic Films. Food Microbiol. 2008, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Torlak, E.; Sert, D. Antibacterial Effectiveness of Chitosan-Propolis Coated Polypropylene Films against Foodborne Pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef]
- Panariello, L.; Vannozzi, A.; Morganti, P.; Coltelli, M.B.; Lazzeri, A. Biobased and Eco-Compatible Beauty Films Coated with Chitin Nanofibrils, Nanolignin and Vitamin e. Cosmetics 2021, 8, 27. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of Chitin Nano-Whiskers on the Antibacterial and Physicochemical Properties of Maize Starch Films. Carbohydr. Polym. 2016, 147, 372–378. [Google Scholar] [CrossRef]
- Andrade-Del Olmo, J.; Pérez-Álvarez, L.; Hernáez, E.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Antibacterial Multilayer of Chitosan and (2-Carboxyethyl)- β-Cyclodextrin onto Polylactic Acid (PLLA). Food Hydrocoll. 2019, 88, 228–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Wang, S.; Cao, Q.; Li, Y.; Zhou, J.; Zhu, B.W. Functional Food Packaging for Reducing Residual Liquid Food: Thermo-Resistant Edible Super-Hydrophobic Coating from Coffee and Beeswax. J. Colloid Interface Sci. 2019, 533, 742–749. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, T.; Zhang, J. Superhydrophobic Coatings with High Repellency to Daily Consumed Liquid Foods Based on Food Grade Waxes. J. Colloid Interface Sci. 2018, 515, 255–263. [Google Scholar] [CrossRef]
- Manrich, A.; Moreira, F.K.V.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H.C. Hydrophobic Edible Films Made up of Tomato Cutin and Pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, S.; Garcia-Valdez, O.; Kennedy, E.; Champagne, P.; Cunningham, M.; Grunlan, J. Crosslinkable-Chitosan-Enabled Moisture-Resistant Multilayer Gas Barrier Thin Film. Macromol. Rapid Commun. 2019, 40, 1800853. [Google Scholar] [CrossRef]
- Schmid, M.; Sängerlaub, S.; Wege, L.; Stäbler, A. Properties of Transglutaminase Crosslinked Whey Protein Isolate Coatings and Cast Films. Packag. Technol. Sci. 2014, 27, 799–817. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey Protein Layer Applied on Biodegradable Packaging Film to Improve Barrier Properties While Maintaining Biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of Whey-Protein-Coated Films and Laminates as Novel Recyclable Food Packaging Materials with Excellent Barrier Properties. Int. J. Polym. Sci. 2012, 2012, 562381. [Google Scholar] [CrossRef] [Green Version]
- Bugnicourt, E.; Schmid, M.; Nerney, O.M.; Wildner, J.; Smykala, L.; Lazzeri, A.; Cinelli, P. Processing and Validation of Whey-Protein-Coated Films and Laminates at Semi-Industrial Scale as Novel Recyclable Food Packaging Materials with Excellent Barrier Properties. Adv. Mater. Sci. Eng. 2013, 2013, 496207. [Google Scholar] [CrossRef] [Green Version]
- Weizman, O.; Dotan, A.; Nir, Y.; Ophir, A. Modified Whey Protein Coatings for Improved Gas Barrier Properties of Biodegradable Films. Polym. Adv. Technol. 2017, 28, 261–270. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl Cellulose/Cellulose Nanocrystals Immobilized Silver Nanoparticles as an Effective Coating to Improve Barrier and Antibacterial Properties of Paper for Food Packaging Applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of Modified Cellulose Nanocrystals on the Barrier and Migration Properties of PLA Nano-Biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef]
- Rampazzo, R.; Alkan, D.; Gazzotti, S.; Ortenzi, M.A.; Piva, G.; Piergiovanni, L. Cellulose Nanocrystals from Lignocellulosic Raw Materials, for Oxygen Barrier Coatings on Food Packaging Films. Packag. Technol. Sci. 2017, 30, 645–661. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Yu, Q.; Fang, L.; Wang, J.; Wu, T.; Song, P. All-Organic Multilayer Coatings for Advanced Poly(Lactic Acid) Films with High Oxygen Barrier and Excellent Antifogging Properties. ACS Appl. Polym. Mater. 2019, 1, 3470–3476. [Google Scholar] [CrossRef]
- Idris, A.; Muntean, A.; Mesic, B.; Lestelius, M.; Javed, A. Oxygen Barrier Performance of Poly (Vinyl Alcohol) Coating Films with Different Induced Crystallinity and Model Predictions. Coatings 2021, 11, 1253. [Google Scholar] [CrossRef]
- Schmid, M.; Benz, A.; Stinga, C.; Samain, D.; Zeyer, K.P. Fundamental Investigations Regarding Barrier Properties of Grafted PVOH Layers. Int. J. Polym. Sci. 2012, 2013, 637837. [Google Scholar] [CrossRef]
- Suhag, A.; Biswas, K.; Singh, S.; Kulshreshtha, A. Crosslinking Effect on Polyvinyl Alcohol Resin for Barrier Properties of Barrier Biaxial Orientation Films. Prog. Org. Coat. 2022, 163, 106662. [Google Scholar] [CrossRef]
- Shen, Z.; Rajabi-Abhari, A.; Oh, K.; Yang, G.; Youn, H.J.; Lee, H.L. Improving the Barrier Properties of Packaging Paper by Polyvinyl Alcohol Based Polymer Coating-Effect of the Base Paper and Nanoclay. Polymers 2021, 13, 1334. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl Alcohol: A Review of Research Status and Use of Polyvinyl Alcohol Based Nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Tian, H.; Yan, J.; Rajulu, A.V.; Xiang, A.; Luo, X. Fabrication and Properties of Polyvinyl Alcohol/Starch Blend Films: Effect of Composition and Humidity. Int. J. Biol. Macromol. 2017, 96, 518–523. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Pérez-Cataluña, A.; Ekiz, H.I.; Sanchez, G.; López-Rubio, A. Biodegradable Active Food Packaging Structures Based on Hybrid Cross-Linked Electrospun Polyvinyl Alcohol Fibers Containing Essential Oils and Their Application in the Preservation of Chicken Breast Fillets. Food Packag. Shelf Life 2021, 27, 100613. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of Polyvinyl Alcohol on the Physicochemical Properties of Biodegradable Starch Films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent Advances in Starch, Polyvinyl Alcohol Based Polymer Blends, Nanocomposites and Their Biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Nyflött, Å.; Meriçer, Ç.; Minelli, M.; Moons, E.; Järnström, L.; Lestelius, M.; Baschetti, M.G. The Influence of Moisture Content on the Polymer Structure of Polyvinyl Alcohol in Dispersion Barrier Coatings and Its Effect on the Mass Transport of Oxygen. J. Coat. Technol. Res. 2017, 14, 1345–1355. [Google Scholar] [CrossRef]
- Schmid, M.; Sängerlaub, S.; Miesbauer, O.; Jost, V.; Werthan, J.; Stinga, C.; Samain, D.; Stramm, C.; Noller, K.; Müller, K. Water Repellence and Oxygen and Water Vapor Barrier of PVOH-Coated Substrates before and after Surface Esterification. Polymers 2014, 6, 2764–2783. [Google Scholar] [CrossRef] [Green Version]
- Apicella, A.; Scarfato, P.; D’Arienzo, L.; Garofalo, E.; di Maio, L.; Incarnato, L. Antimicrobial biodegradable coatings based on LAE for food packaging applications. Proc. AIP Conf. Proc. 2018, 1981, 020010. [Google Scholar]
- Li, H.; Cui, R.; Peng, L.; Cai, S.; Li, P.; Lan, T. Preparation of Antibacterial Cellulose Paper Using Layer-by-Layer Assembly for Cooked Beef Preservation at Ambient Temperature. Polymers 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.L.; Vale, A.C.; Sousa, M.P.; Barbosa, A.M.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial Bioadhesive Layer-by-Layer Coatings for Orthopedic Applications. J. Mater. Chem. B 2016, 4, 5385–5393. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Fang, F.; Zhang, X.; Ding, X.; Wang, Y.; Chen, L.; Tian, X. Flame-Retardant, Electrically Conductive and Antimicrobial Multifunctional Coating on Cotton Fabric via Layer-by-Layer Assembly Technique. RSC Adv. 2016, 6, 27669–27676. [Google Scholar] [CrossRef]
- Katagiri, K.; Shishijima, Y.; Koumoto, K.; Inumaru, K. Preparation of PH-Responsive Hollow Capsules via Layer-by-Layer Assembly of Exfoliated Layered Double Hydroxide Nanosheets and Polyelectrolytes. J. Nanosci. Nanotechnol. 2017, 18, 110–115. [Google Scholar] [CrossRef]
- Kuraray Europe GmbH. Exceval™—Attractive Protection for Your Food. Available online: https://www.kuraray-poval.com/fileadmin/technical_information/brochures/poval/Kuraray_Exceval_attractive_protection_for_your_food_engl.pdf (accessed on 4 February 2022).
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van Der Waals and Polar Interactions in Macroscopic Systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. The Role of van Der Waals Forces and Hydrogen Bonds in “Hydrophobic Interactions” between Biopolymers and Low Energy Surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- Zonder, L.; McCarthy, S.; Rios, F.; Ophir, A.; Kenig, S. Viscosity Ratio and Interfacial Tension as Carbon Nanotubes Distributing Factors in Melt-Mixed Blends of Polyamide 12 and High-Density Polyethylene. Adv. Polym. Technol. 2014, 33, 21427. [Google Scholar] [CrossRef]
- Ebnesajjad, S.; Landrock, A.H. Adhesives Technology Handbook, 3rd ed.; William Andrew Publishing: Norwich, NY, USA, 2015. [Google Scholar] [CrossRef]
- Nakhaei, M.; Naderi, K.; Nasrekani, A.A.; Timm, D. Chemical, rheological, and moisture resistance properties of warm mix asphalt modified with polyethylene-wax and ethylene-bis-stearamide additives. In Proceedings of the Transportation Research Board 97th Annual Meeting, Washington, DC, USA, 7–11 January 2018. [Google Scholar]
- Vuluga, Z.; Corobea, M.C.; Elizetxea, C.; Ordonez, M.; Ghiurea, M.; Raditoiu, V.; Nicolae, C.A.; Florea, D.; Iorga, M.; Somoghi, R.; et al. Morphological and Tribological Properties of PMMA/Halloysite Nanocomposites. Polymers 2018, 10, 816. [Google Scholar] [CrossRef] [Green Version]
- Meaurio, E.; López-Rodríguez, N.; Sarasua, J.R. Infrared Spectrum of Poly(l-lactide): Application to Crystallinity Studies. Macromolecules 2006, 39, 9291–9301. [Google Scholar] [CrossRef]
- Falqi, F.H.; Bin-Dahman, O.A.; Hussain, M.; Al-Harthi, M.A. Preparation of Miscible PVA/PEG Blends and Effect of Graphene Concentration on Thermal, Crystallization, Morphological, and Mechanical Properties of PVA/PEG (10wt%) Blend. Int. J. Polym. Sci. 2018, 2018, 8527693. [Google Scholar] [CrossRef] [Green Version]
- Kuraray Europe GmbH. Basic Physical Properties of PVOH Resin. Available online: https://www.kuraray.eu/fileadmin/product_groups/polyvinylalcohol/downloads/kuraray_poval_basic_physical_properties_web.pdf (accessed on 17 January 2022).
- Benjasirimongkol, P.; Ueda, K.; Higashi, K.; Sriamornsak, P.; Moribe, K. An Insight into Stabilization Mechanism of a Solid Dispersion of Indomethacin/Partially Hydrolyzed Polyvinyl Alcohol Prepared by Hot–Melt Extrusion. Chem. Pharm. Bull. 2018, 66, 859–865. [Google Scholar] [CrossRef] [Green Version]
- Fong, R.J.; Robertson, A.; Mallon, P.E.; Thompson, R.L. The Impact of Plasticizer and Degree of Hydrolysis on Free Volume of Poly (vinyl alcohol) Films. Polymers 2018, 10, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, M.; Bojda, J.; Piorkowska, E.; Murariu, M.; Bonnaud, L.; Dubois, P. The Effect of Halloysite Nanotubes and N, N’-Ethylenebis (Stearamide) on Morphology and Properties of Polylactide Nanocomposites with Crystalline Matrix. Polym. Test. 2017, 64, 83–91. [Google Scholar] [CrossRef]
- Pietrosanto, A.; Scarfato, P.; di Maio, L.; Incarnato, L. Development of Eco-Sustainable PBAT-Based Blown Films and Performance Analysis for Food Packaging Applications. Materials 2020, 13, 5395. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent Updates on the Barrier Properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A Review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef] [Green Version]
- Turco, R.; Ortega-Toro, R.; Tesser, R.; Mallardo, S.; Collazo-Bigliardi, S.; Chiralt Boix, A.; Malinconico, M.; Rippa, M.; Di Serio, M.; Santagata, G. Poly (Lactic Acid)/Thermoplastic Starch Films: Effect of Cardoon Seed Epoxidized Oil on Their Chemicophysical, Mechanical, and Barrier Properties. Coatings 2019, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Thiyagu, T.T.; Sai Prasanna Kumar, J.V.; Gurusamy, P.; Sathiyamoorthy, V.; Maridurai, T.; Arun Prakash, V.R. Effect of cashew shell biomass synthesized cardanol oil green compatibilizer on flexibility, barrier, thermal, and wettability of PLA/PBAT biocomposite films. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; de la Rosa-Ramírez, H.; López-Martínez, J.; Arrieta, M.P. Improvement of PBAT Processability and Mechanical Performance by Blending with Pine Resin Derivatives for Injection Moulding Rigid Packaging with Enhanced Hydrophobicity. Polymers 2020, 12, 2891. [Google Scholar] [CrossRef] [PubMed]
- de C.D. Nunes, E.; de Souza, A.G.; dos S. Rosa, D. Effect of the Joncryl® ADR Compatibilizing Agent in Blends of Poly (Butylene Adipate-Co-Terephthalate)/Poly (Lactic Acid). Macromol. Symp. 2019, 383, 1800035. [Google Scholar] [CrossRef] [Green Version]
- Luque-Agudo, V.; Romero-Guzmán, D.; Fernández-Grajera, M.; González-Martín, L.; Gallardo-Moreno, A.M. Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability. Coatings 2019, 9, 814. [Google Scholar] [CrossRef] [Green Version]
- Hejda, F.; Solar, P.; Kousal, J. Surface free energy determination by contact angle measurements—A comparison of various approaches. In Proceedings of the WDS’10 Contributed Papers, Part III; Faculty of Mathematics and Physycs, Ed.; Charles University: Prague, Czech Republic, 2010; pp. 25–30. [Google Scholar]
- Karbowiak, T.; Debeaufort, F.; Voilley, A. Importance of Surface Tension Characterization for Food, Pharmaceutical and Packaging Products: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 391–407. [Google Scholar] [CrossRef]
- Lindner, M.; Rodler, N.; Jesdinszki, M.; Schmid, M.; Sängerlaub, S. Surface Energy of Corona Treated PP, PE and PET Films, Its Alteration as Function of Storage Time and the Effect of Various Corona Dosages on Their Bond Strength after Lamination. J. Appl. Polym. Sci. 2018, 135, 45842. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of Bioplastics: Routes and Benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Ilhan, I.; ten Klooster, R.; Gibson, I. Effects of Process Parameters and Solid Particle Contaminants on the Seal Strength of Low-Density Polyethylene-Based Flexible Food Packaging Films. Packag. Technol. Sci. 2021, 34, 413–421. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Najarzadeh, Z.; Ajji, A. Development of High Performance Sealable Films Based on Biodegradable/Compostable Blends. Ind. Crops Prod. 2015, 72, 206–213. [Google Scholar] [CrossRef]
- Barbaro, G.; Galdi, M.R.; di Maio, L.; Incarnato, L. Effect of BOPET Film Surface Treatments on Adhesion Performance of Biodegradable Coatings for Packaging Applications. Eur. Polym. J. 2015, 68, 80–89. [Google Scholar] [CrossRef]
- Arruda, L.C.; Magaton, M.; Bretas, R.E.; Ueki, M.M. Influence of chain extender on mechanical, thermal and morphological properties of blown films of PLA/PBAT blends. Polym. Test. 2015, 43, 27–37. [Google Scholar] [CrossRef]
- Su, S.; Duhme, M.; Kopitzky, R. Uncompatibilized Pbat/Pla Blends: Manufacturability, Miscibility and Properties. Materials 2020, 13, 4897. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Wu, J.-S.; Huang, C.-C.; Lin, L.-S. Morphological, Thermal, Barrier and Mechanical Properties of LDPE/EVOH Blends in Extruded Blown Films. J. Polym. Res. 2004, 11, 75–83. [Google Scholar] [CrossRef]
- Kashyap, S.; Pratihar, S.K.; Behera, S.K. Strong and Ductile Graphene Oxide Reinforced PVA Nanocomposites. J. Alloys Compd. 2016, 684, 254–260. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Ramy-Ratiarison, R.; Paint, Y.; Raquez, J.; Dubois, P. Recent advances in production of poly (lactic acid) (PLA) nanocomposites: A versatile method to tune crystallization properties of PLA. Nanocomposites 2015, 1, 71–82. [Google Scholar] [CrossRef] [Green Version]

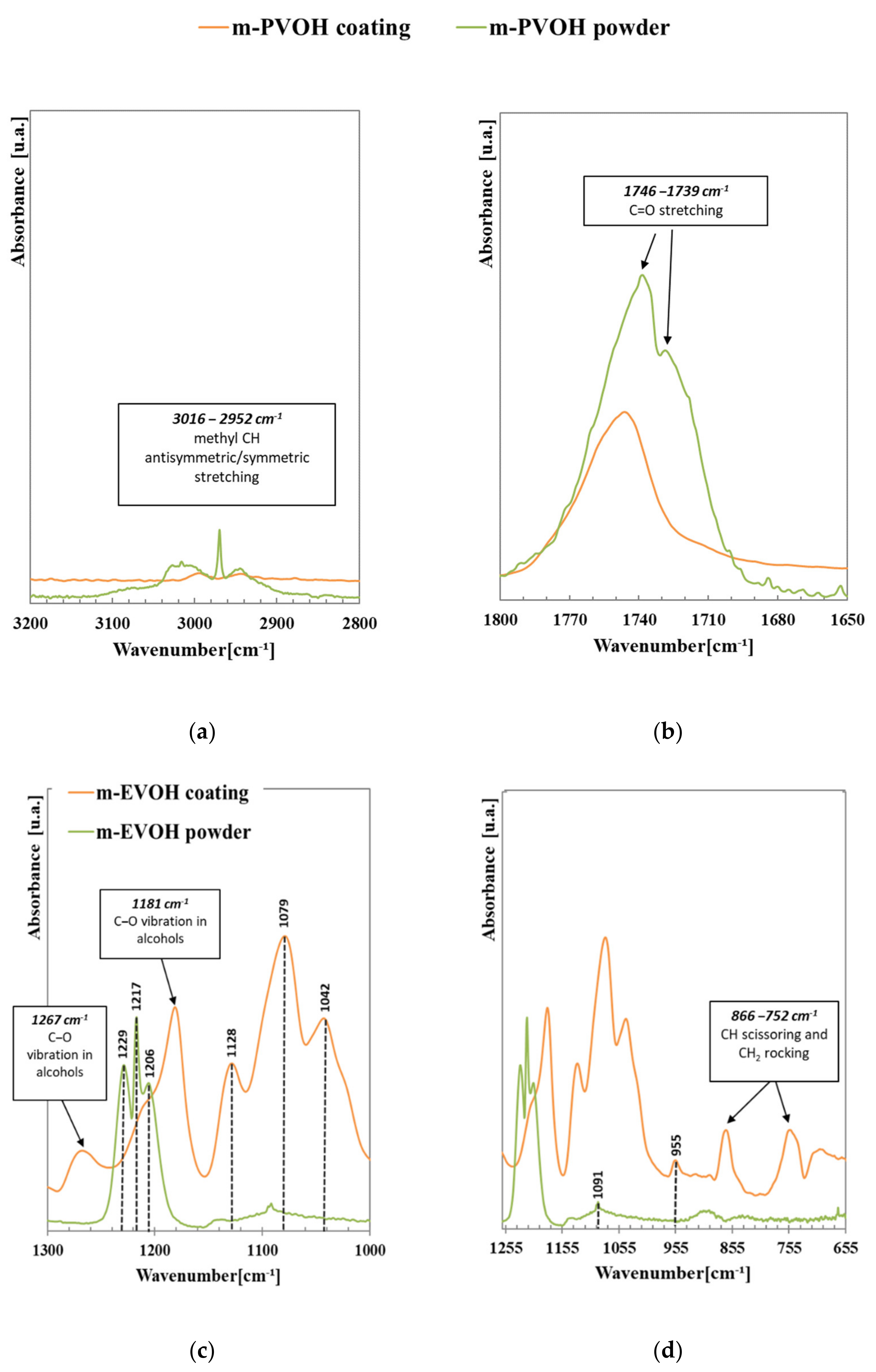



| Sample Film | Wax Concentration (w/w PLA) | Thickness of the First Coating (μm) | Thickness of the Second Coating (μm) | Total Thickness (μm) |
|---|---|---|---|---|
| Biofilm | 0 | - | - | 22 ± 2 |
| Biofilm/m-PVOH | 0 | 5 ± 1 | - | 27 ± 3 |
| Biofilm/m-PVOH/PLA + 0% wax | 0 | 5 ± 1 | 6 ± 1 | 33 ± 4 |
| Biofilm/m-PVOH/PLA + 5% wax | 5 | 5 ± 1 | 6 ± 1 | 33 ± 4 |
| Biofilm/m-PVOH/PLA + 10% wax | 10 | 5 ± 1 | 7 ± 2 | 34 ± 4 |
| Biofilm/m-PVOH/PLA + 20% wax | 20 | 5 ± 1 | 8 ± 2 | 35 ± 5 |
| Sample | First Heating | Cooling | ||||
|---|---|---|---|---|---|---|
| Tg (°C) | DHmrel (J/g) | Tm1 (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | |
| m-PVOH powder | 42.7 | 1.8 | 215.4 | 70.4 | 150.7 | 45.2 |
| m-PVOH coating | 39.8 | 4.9 | n.d. | n.d. | n.d. | n.d. |
| First Heating | Cooling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Tg1 (°C) | DHmrel (J/g) | Tm1 (°C) | DHm1 (J/g) | Tm2 (°C) | DHm2 (J/g) | Tm3 (°C) | DHm3 (J/g) | Tm4 (°C) | DHm4 (J/g) | Tc1 (°C) | ΔHc1 (J/g) |
| Wax powder | n.d. | n.d. | 69.5 | 7.7 | 87.7 | 2.1 | 104.4 | 8.5 | 146.7 | 119.8 | 140.8 | 121.7 |
| PLA + 0% wax coating | 60.1 | 3.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| PLA + 5% wax coating | 60.8 | 4.2 | n.d. | n.d. | n.d. | n.d. | 109.6 | 3.03 | 145.9 | 6.84 | 127.0 | 7.3 |
| PLA + 10% wax coating | 59.4 | 2.5 | n.d. | n.d. | n.d. | n.d. | 110.7 | 3.96 | 145.8 | 9.99 | 132.1 | 10.7 |
| PLA + 20% wax coating | 60.1 | 3.6 | n.d. | n.d. | n.d. | n.d. | 110.2 | 7.7 | 145.6 | 20.0 | 139.5 | 20.4 |
| Sample Films | |
|---|---|
| Biofilm | 2200 ± 83.6 |
| Biofilm/m-PVOH | 8.14 ± 1.04 |
| Biofilm/m-PVOH/PLA + 0% wax | 10.0 ± 1.28 |
| Biofilm/m-PVOH/PLA + 5% wax | 9.39 ± 1.71 |
| Biofilm/m-PVOH/PLA + 10% wax | 9.41 ± 1.24 |
| Biofilm/m-PVOH/PLA + 20% wax | 8.58 ± 2.42 |
| Sample Films | |
|---|---|
| m-PVOH | 0.047 |
| PLA (semicrystalline) | 3.36–15.0 (23 °C/50% or 0%) |
| PBAT | 62.0 (23 °C/50%) |
| PBS | 5.28 (23 °C/50%) 8.64 (20 °C/90%) |
| PHA | 0.20 (23 °C/85%) 2.16 (23 °C/0%) 5.84 (25 °C/80%) |
| PCL | 50.5 (25 °C/0%) |
| Films Analysed Surface | Static Contact Angle (°) | Surface Energy (mN/m)/Polarity [−] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Disperse-Polar (Owens–Wendt) | Acid-Base (Van Oss–Good) | ||||||||||
| H2O | DM | EG | γsp | γsd | Ps | γS | γsLW | γs+ | γs− | γS | |
| Biofilm | 97.9 ± 2 | 65.9 ± 2 | 80.2 ± 1 | 1.67 | 23.76 | 0.07 | 25.44 | 25.19 | 3.18 | 0.04 | 25.92 |
| m-PVOH | 47.4 ± 2 | 38.3 ± 1 | 39.8 ± 3 | 23.78 | 31.96 | 0.43 | 55.74 | 40.45 | 39.46 | 0.01 | 41.40 |
| PLA + 0% wax | 65.4 ± 1 | 37.5 ± 1 | 52.4 ± 1 | 11.63 | 35.07 | 0.25 | 46.70 | 40.84 | 21.31 | 0.10 | 43.76 |
| PLA + 5% wax | 67.2 ± 1 | 36.2 ± 1 | 52.3 ± 1 | 11.52 | 35.68 | 0.24 | 47.20 | 41.47 | 21.44 | 0.12 | 44.73 |
| PLA + 10% wax | 69.8 ± 1 | 37.0 ± 1 | 54.6 ± 1 | 10.72 | 35.56 | 0.23 | 46.27 | 41.09 | 20.67 | 0.18 | 44.95 |
| PLA + 20% wax | 71.0 ± 1 | 37.1 ± 2 | 56.6 ± 2 | 9.48 | 35.89 | 0.21 | 45.37 | 41.04 | 18.94 | 0.23 | 45.24 |
| Films Interface | Owens-Wendt | Van Oss-Good | ||
|---|---|---|---|---|
| Biofilm/m-PVOH | 67.7 | 13.4 | 66.7 | 0.6 |
| m-PVOH/PLA + 0% wax | 100.2 | 2.2 | 86.0 | −0.8 |
| m-PVOH/PLA + 5% wax | 100.6 | 2.3 | 87.0 | −0.9 |
| m-PVOH/PLA + 10% wax | 99.3 | 2.7 | 87.6 | −1.2 |
| m-PVOH/PLA + 20% wax | 97.8 | 3.3 | 88.2 | −1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apicella, A.; Barbato, A.; Garofalo, E.; Incarnato, L.; Scarfato, P. Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films. Polymers 2022, 14, 935. https://doi.org/10.3390/polym14050935
Apicella A, Barbato A, Garofalo E, Incarnato L, Scarfato P. Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films. Polymers. 2022; 14(5):935. https://doi.org/10.3390/polym14050935
Chicago/Turabian StyleApicella, Annalisa, Antonio Barbato, Emilia Garofalo, Loredana Incarnato, and Paola Scarfato. 2022. "Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films" Polymers 14, no. 5: 935. https://doi.org/10.3390/polym14050935
APA StyleApicella, A., Barbato, A., Garofalo, E., Incarnato, L., & Scarfato, P. (2022). Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films. Polymers, 14(5), 935. https://doi.org/10.3390/polym14050935







