Effect of Gamma Irradiation on Enhanced Biological Activities of Exopolysaccharide from Halomonas desertis G11: Biochemical and Genomic Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Growth, nEPS Production, and Gamma Irradiation
2.2. Structural Characterization of Native EPS and Its Gamma-Irradiated Derivatives
2.3. Biological Characterization of Native EPS and Its Gamma-Irradiated Derivatives
2.3.1. In Vitro Antioxidant Activities
DPPH Radical Scavenging Activity (RSA)
ABTS Scavenging Capacity (ABTS-SC)
The Ferric-Reducing Antioxidant Power (FRAP)
2.3.2. In Vivo Antioxidant Activities
Experimental Design
Biochemical Assay
2.4. Antitumor Activities
2.5. Structural Genomic Analyses
2.6. Statistical Analyses
3. Results and Discussion
3.1. Cell Growth and nEPS Synthesis
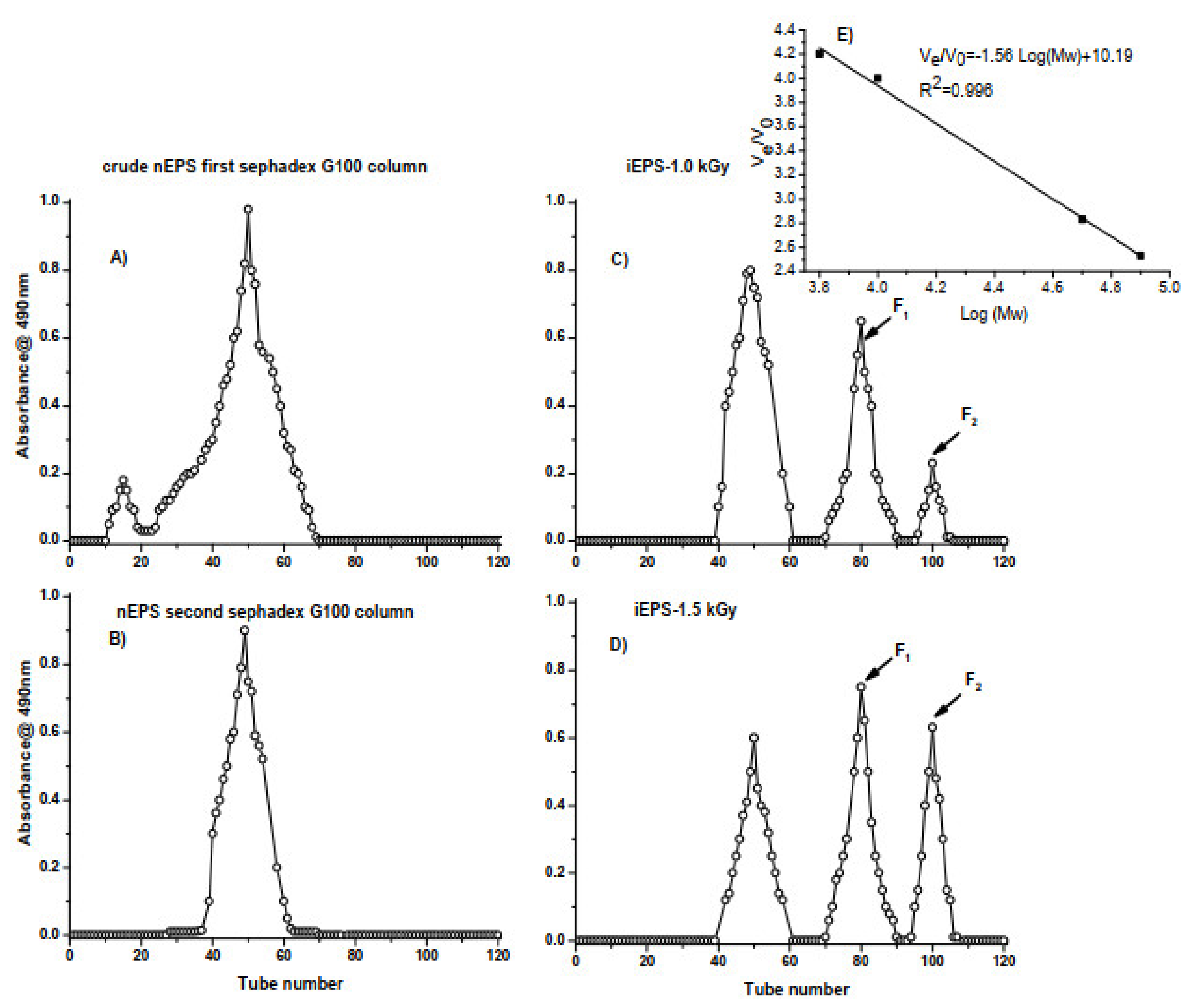
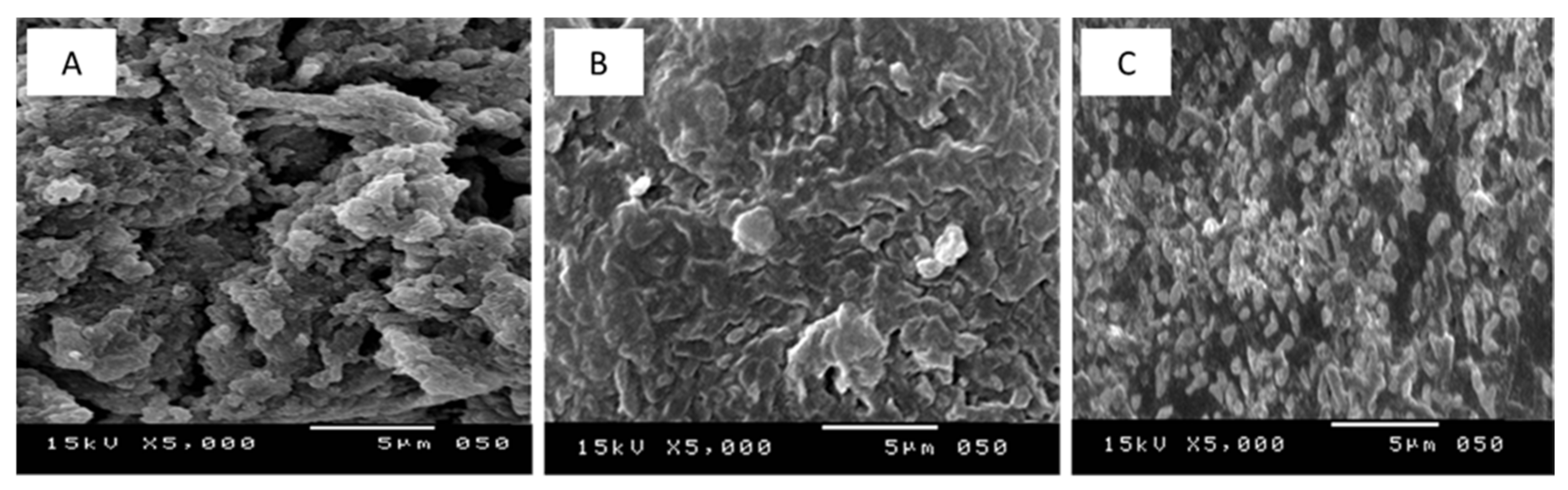
3.2. Structural Characterization of nEPS and Its Gamma-Irradiated Derivatives
3.3. Biological Characterization of nEPS and Its Gamma-Irradiated Derivatives
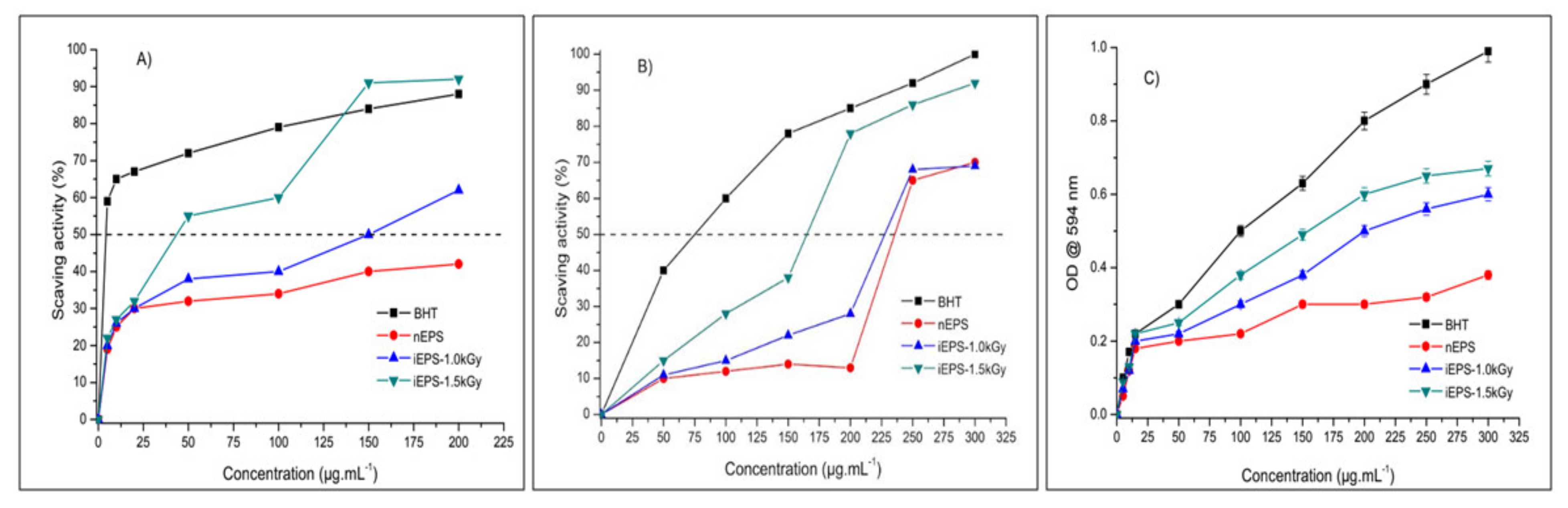
3.3.1. In Vitro Antioxidant Activities
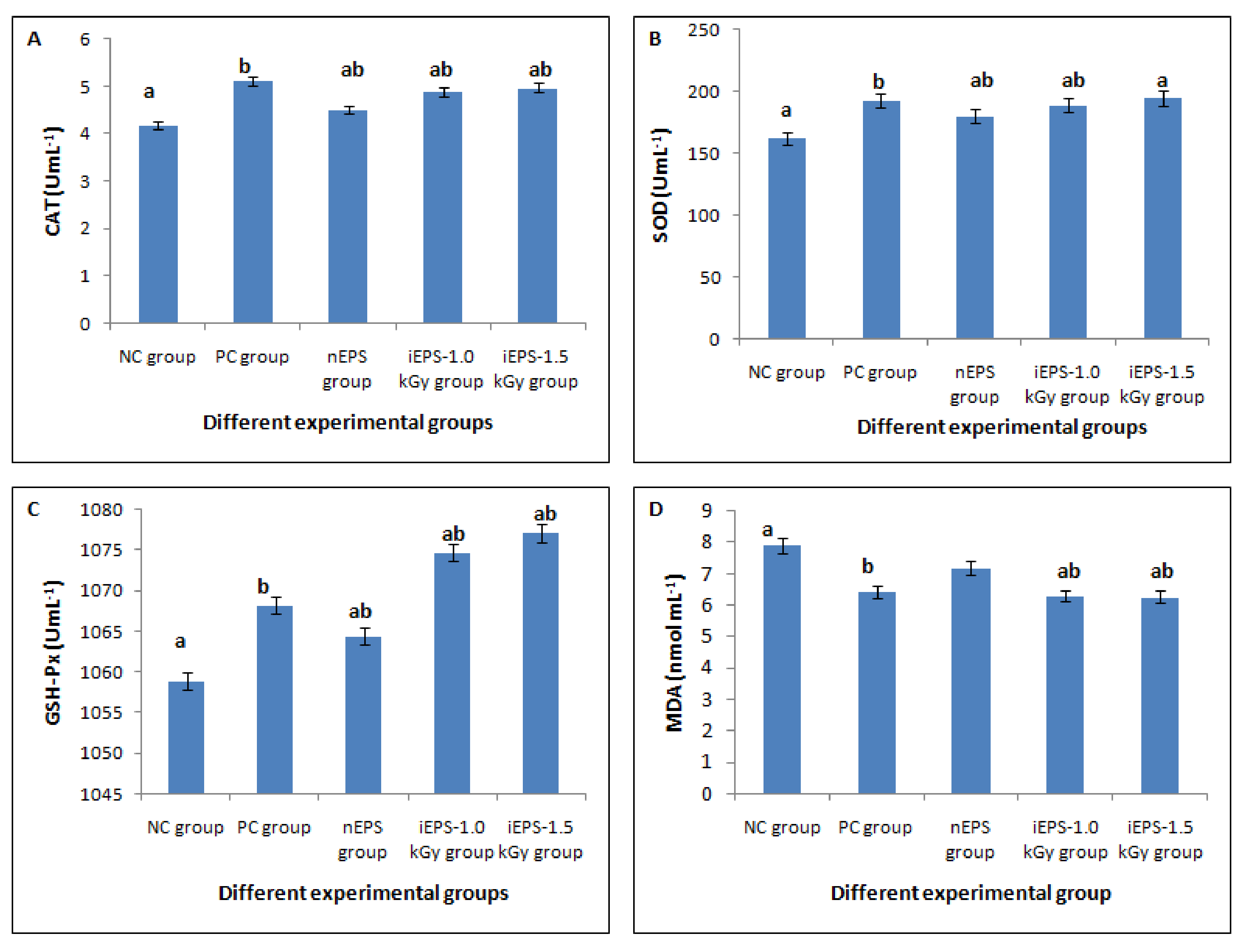
3.3.2. In Vivo Antioxidant Activities
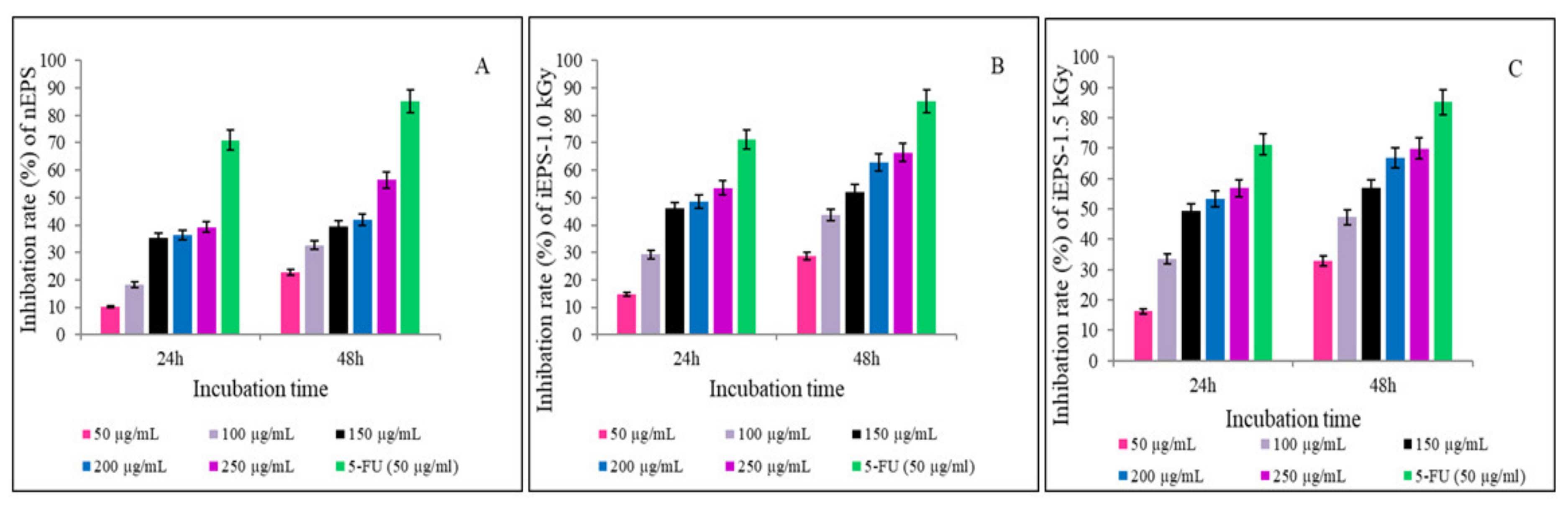
3.3.3. Antitumor Activities of nEPS and iEPSs
3.4. Genes Implicated in nEPS Biosynthesis
3.4.1. Genomic Analyses of the Enzymes Involved in the Nucleotide Sugars Biosynthesis for nEPS Production
3.4.2. Genomic Analyses Enzymatic Regulation of the Biosynthesis of nEPS
3.4.3. Genomic Analyses of Enzymes Involved in the nEPSExportation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tapan, K.S. Microbial Extracellular Polymeric Substances: Production, Isolation and applications. IOSR J. Pharm. 2012, 2, 276–281. [Google Scholar] [CrossRef]
- Chouchane, H.; Neifar, M.; Raddadi, N.; Fava, F.; Masmoudi, A.S.; Cherif, A. Microbial Exopolysaccharides as Alternative Sources of Dietary Fibers with Interesting Functional and Healthy Properties. In Dietary Fiber: Production Challenges, Food Sources and Health Benefits; Clemens, M.E., Ed.; Nova Publishers: New York, NY, USA, 2015; pp. 159–178. ISBN 978-1-63463-676-6. [Google Scholar]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derk, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 4, 168–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullrich, M. Bacterial Polysaccharides: Current Innovations and Future Trends; Caister Academic Press: Norfolk, UK, 2006. [Google Scholar]
- Tabibloghmany, F.S.; Ehsandoost, E. An Overview of Healthy and Functionality of Exopolysacharides Produced by Lactic Acid Bacteria in the Dairy Industry. Eur. J. Nutr. Food Saf. 2014, 4, 63–86. [Google Scholar] [CrossRef]
- Qin, G.; Shao, T.; Li, P.; Zhou, Y.; Li, Y.; Hong, X.; Wong, G. Preparation and Antitumor Activity of Sulfated Exopolysaccharide from Rhizopus nigricans. J. South. Med. Univ. 2019, 10, 1227–1231. [Google Scholar] [CrossRef]
- Surhio, M.M.; Wang, Y.; Xu, P.; Shah, F.; Li, J.; Ye, M. Antihyperlipidemic and hepatoprotective properties of selenium modified polysaccharide from Lachnum sp. Int. J. Biol. Macromol. 2017, 99, 88–95. [Google Scholar] [CrossRef]
- Chouchane, H.; Mahjoubi, M.; Neifar, M.; Cherif, A. A novel thermally stable heteropolysaccharide based bioflocculant from hydrocarbonoclastic strain Kocuria rosea and its application in dye removal. Environ. Technol. 2018, 39, 859–872. [Google Scholar] [CrossRef]
- Guesmi, S.; Chouchane, H.; Neifar, M.; Hosni, F.; Cherif, A.; Sghaier, H. Radiation-inducible radioprotective exopolysaccharides of Bacillus siamensis CV5 from irradiated roots of Cistancheviolacea to decrease free radical damage produced by ionizing radiation. Int. J. Radiat. Biol. 2019, 95, 1552–1563. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Tao, X.; Wei, H. Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydr. Polym. 2016, 153, 25–33. [Google Scholar] [CrossRef]
- Song, Z.; Liu, X.; Zhang, J.; Yang, Q.; Ren, Z.; Zhang, C.; Liu, M.; Gao, Z.; Zhao, H.; Jia, L. Anti-infammatory and hepatoprotective efects of exopolysaccharides isolated from Pleurotusgeesteranus on alcohol induced liver injury. Sci. Rep. 2018, 8, 10493. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Prajapat, J.B. Food and Health Applications of Exopolysaccharides produced by Lactic acid Bacteria. Adv. Dairy Res. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Chouchane, H.; Najjari, A.; Cherif, H.; Neifar, M.; Sghaier, H.; Ouzari, H.I.; Cherif, A. Carboxymethylated Sulfated Heteroexopolysaccharide from a Haloarchaeal Strain as Potential Biomolecule for Harmless Adjuvant Therapy in Cancer Treatment. J. Chem. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Wang, L.; Li, J.; Fu, R.; Shiming, W.; Zhang, J. In vitro and in vivo anti-inflammatory activity of a succinoglycanRiclin from Agrobacterium sp. ZCC3656. J. Appl. Microbiol. 2019, 127, 1716–1726. [Google Scholar] [CrossRef]
- Biliavska, L.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S. Antiviral Activity of Exopolysaccharides Produced by Lactic Acid Bacteria of the Genera Pediococcus, Leuconostoc and Lactobacillus against Human Adenovirus Type 5. Medicina 2019, 55, 519. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.Y.; Shin, J.S.; Rhee, Y.K.; Cho, C.W.; Lee, M.K.; Hong, H.D.; Lee, K.T. In vitro and in vivo immunostimulatory activity of an exopolysaccharide-enriched fraction from Bacillus subtilis. J. Appl. Microbiol. 2015, 118, 739–752. [Google Scholar] [CrossRef]
- Hou, R.; Chen, J.; Yue, C.; Li, X.; Liu, J.; Gao, Z.; Liu, C.; Lu, Y.; Wang, D.; Li, H.; et al. Modification of lily polysaccharide by selenylation and the immune enhancing activity. Carbohydr. Polym. 2016, 142, 73–81. [Google Scholar] [CrossRef]
- Xiao, L.; Han, S.; Zhou, J.; Dong, M.; Fan, X.; Rui, X.; Chen, X.; Zhang, Q.; Li, W. Preparation, characterization and antioxidant activities of derivatives of exopolysaccharide from Lactobacillus helveticus. Int. J. Biol. Macrom. 2019, 145, 1008–1017. [Google Scholar] [CrossRef]
- Du, B.; Zeng, H.; Yang, Y.; Bian, Z.; Xu, B. Anti-inflammatory activity of polysaccharide from Schizophyllum commune as affected by ultrasonication. Int. J. Biol. Macrom. 2016, 91, 100–105. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V. Enzymatic Transformations Involved in the Biosynthesis of Microbial Exo-polysaccharides Based on the Assembly of Repeat Units. ChemBioChem 2015, 16, 1141–1147. [Google Scholar] [CrossRef]
- De Sousa, B.F.S.; Castellane, T.C.L.; Campanharo, J.C.; de Macedo Lemos, E.G. Rhizobium spp. exopolysaccharides production and xanthan lyase use on its structural modification. Int. J. Biol. Macrom. 2019, 136, 424–435. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; da Cunha, M.A.A.; Barbosa, A.M.; Dekker, R.F.H.; Malfatti, C.R.M. Biological activities of derivatized d-glucans: A review. Int. J. Biol. Macrom. 2015, 72, 588–598. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2019, 487, 107881. [Google Scholar] [CrossRef]
- Calegari, G.C.; Santos, V.A.Q.; Barbosa-Dekker, A.M.; Busso, C.; Dekker, R.F.H.; da Cunha, M.A. ASulfonated (1→ 6)-β-d-glucan (lasiodiplodan): Preparation, characterization and bioactive properties. Food Technol. Biotechnol. 2019, 57, 490–502. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Li, T.; Chen, X.; Jiang, M.; Dong, M. Chemical modification, characterization and bioactivity of a released exopolysaccharide (r-EPS1) from Lactobacillus plantarum. Glycoconj. J. 2014, 32, 17–27. [Google Scholar] [CrossRef]
- Sun, K.; Chen, Y.; Niu, Q.; Zhu, W.; Wang, B.; Li, P.; Ge, X. An exopolysaccharide isolated from a coral-associated fungus and its sulfated derivative activates macrophages. Int. J. Biol. Macrom. 2016, 82, 387–394. [Google Scholar] [CrossRef]
- He, Y.; Ye, M.; Jing, L.; Du, Z.; Surahio, M.; Xu, H.; Li, J. Preparation, characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum. Carbohydr. Polym. 2015, 117, 788–796. [Google Scholar] [CrossRef]
- Byun, E.H.; Kim, J.H.; Sung, N.Y.; Choi, J.I.; Lim, S.T.; Kim, K.H.; Yook, H.S.; Byun, M.W.; Lee, J. Effects of gamma irradiation on the physical and structural properties of b-glucan. Radiat Phys. Chem. 2008, 77, 781–786. [Google Scholar] [CrossRef]
- Kavitake, D.; Techi, M.U.K.; Abid, U.K.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Effect of γ-irradiation on physico-chemical and antioxidant properties of galactan exopolysaccharide from Weissellaconfusa. KR780676. J. Food Sci. Technol. 2019, 56, 1766–1774. [Google Scholar] [CrossRef]
- Shah, A.; Ahmad, M.; Ashwar, B.A.; Gani, A.; Masoodi, F.A.; Wani, I.A.; Wani, S.M.; Gani, A. Effect of γ irradiation on structure and nutraceutical potential of β-d-glucan from barley (Hordeum vulgare). Int. J. Biol. Macromol. 2015, 72, 1168–1175. [Google Scholar] [CrossRef]
- Kume, T.; Nagasawa, N.; Yoshii, F. Utilization of carbohydrates by radiation processing. Radiat. Phys. Chem. 2002, 63, 625–627. [Google Scholar] [CrossRef]
- El Hidri, D.; Guesmi, A.; Najjari, A.; Cherif, H.; Ettoumi, B.; Hamdi, C.; Cherif, A. Cultivation-Dependant Assessment, Diversity, and Ecology of Haloalkaliphilic Bacteria in Arid Saline Systems of Southern Tunisia. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neifar, M.; Chouchane, H.; Najjari, A.; El Hidri, D.; Mahjoubi, M.; Ghedira, K.; Cherif, A. Genome analysis provides insights into crude oil degradation and biosurfactant production by extremely halotolerant Halomonasdesertis G11 isolated from Chott El-Djerid salt-lake in Tunisian desert. Genomics 2019, 111, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, M.F.; Kennedy, J.F. Carbohydrate Analysis, 2nd ed.; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Yao, H.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, characterization and antioxidant activities in vitro of polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, e9077. [Google Scholar] [CrossRef]
- Zhang, G.; He, L.; Hu, M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg. Technol. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Meng, F.; Liu, Z.; Chen, R.; Zhang, M. Antitumor activities of different fractions of polysaccharide purified from Ornithogalum caudatum Ait. Carbohydr. Polym. 2010, 80, 845–851. [Google Scholar] [CrossRef]
- Athmika; Ghate, S.D.; Arun, A.B.; Rao, S.S.; Kumar, S.T.A.; Kandiyil, M.K.; Saptami, K.; Rekha, P.D. Genome analysis of a halophilic bacterium Halomonasmalpeensis YU-PRIM-29T reveals its exopolysaccharide and pigment producing capabilities. Sci. Rep. 2021, 11, 1749. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Zagnitko, O. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- LIamas, I.; Amjres, H.; Mata, J.A.; Quesada, E.; Béjar, V. The potential biotechnological applications of the exopolysaccharide produced by halophilic bacterium Halomonasalmeriensis. Molecules 2012, 17, 7103–7120. [Google Scholar] [CrossRef] [Green Version]
- Arias, S.; Moral, A.; Ferrer, M.R.; Tallon, R.; Quesada, E.; Bejar, V. Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 2003, 7, 319–326. [Google Scholar] [CrossRef]
- Mata, J.A.; Béjar, V.; Llamas, I.; Arias, S.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E. Exopolysaccharides produced by the recently described halophilic bacteria Halomonasventosae and Halomonasanticariensis. Res. Microbiol. 2006, 157, 827–835. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, C.; Srivastava, G.K.; Carranza, D.; Mata, J.A.; LIamas, I.; Santamarina, M.; Quesada, E.; Molina, I.J. An exopolysaccharide produced by the novel halophilic bacterium Halomonasstenophila strain B100 selectively induces apoptosis in human T leukaemia cells. Appl. Microbiol. Biotechnol. 2011, 89, 345–355. [Google Scholar] [CrossRef]
- Quesada, E.; Béjar, V.; Calvo, C. Exopolysaccharide production by Volcaniellaeurihalina. Experientia 1993, 49, 1037–1041. [Google Scholar] [CrossRef]
- Chikkanna, A.; Ghosh, D.; Kishore, A. Expression and characterization of a potential exopolysaccharide from a newly isolated halophilic thermotolerant bacteria Halomonasnitroreducens strain WB1. PeerJ 2018, 6, e4684. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, T.; Morris, G.; Ellis, D.; Mulloy, B.; Aitken, M.D. Production and characterisation of a marine Halomonas surface-active exopolymer. Appl. Microbiol. Biotechnol. 2020, 104, 1063–1076. [Google Scholar] [CrossRef] [Green Version]
- Joulak, I.; Finore, I.; Poli, A.; Abid, Y.; Bkhairia, I.; Nicolaus, B.; Di Donato, P.; Dal Poggetto, G.; Gharsallaoui, A.; Azabou, S. Hetero-exopolysaccharide from the extremely halophilic Halomonassmyrnensis K2: Production, characterization and functional properties in vitro. 3 Biotech 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Gan, L.; Li, X.; Zhang, H.; Zhang, R.; Wang, H.; Xu, Z.; Peng, B.; Tian, Y. Preparation, characterization and functional properties of a novel exopolysaccharide produced by the halophilic strain Halomonassaliphila. Int. J. Biol. Macrom. 2020, 156, 372–380. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; De Zonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2019, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Shi, J. Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob. Proteins 2018, 11, 1132–1142. [Google Scholar] [CrossRef]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharide(s) from Peanibacillus mucilaginosus TKU032. Mar Drugs. 2016, 14, 40. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.K.; Srinivasan, P.; Kim, J.H.; Park, H.J.; Byun, M.W.; Lee, J.W. Comparison of gamma ray and electron beam irradiation on extraction yield, morphological and antioxidant properties of polysaccharides from tamarind seed. Radiat. Phys. Chem. 2009, 78, 605–609. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef]
- Xu, R.; Qian, S.; Ding, X.; Wengeng, G.; Li, P. Chemical characterization and antioxidant activity of an exopolysaccharide fraction isolated from Bifidobacterium animalis RH. Eur. Food Res. Technol. 2011, 232, 231–240. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Zhang, D.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Liu, W.; Song, Y.; Tuo, Y.; Mu, G.; Ma, F. The Effects of Lactobacillus plantarum-12 Crude Exopolysaccharides on the Cell Proliferation and Apoptosis of Human Colon Cancer (HT-29) Cells. Probiotics Antimicrob. Proteins 2020, 13, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides produced by Lactobacillus strains suppress HT-29 cell growth via induction of G0/G1 cell cycle arrest and apoptosis. Oncol. Lett. 2018, 16, 3577–3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diemer, S.K.; Silja Diemer, K.; Svensson, B.; Babol, L.N.; Cockburn, D.; Grijpstra, P.; Dijkhuizen, L.; Folkenberg, D.M.; Garrigues, C.; Ipsen, R.H. Binding Interactions Between α-glucans from Lactobacillus reuteri and Milk Proteins Characterised by Surface Plasmon Resonance. Food Biophys. 2012, 7, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci Rep. 2019, 9, 8268. [Google Scholar] [CrossRef] [Green Version]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr. Polym. 2020, 253, 117308. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; Zaghlol, G.M.; El-Garawani, I.M.; Ahmed, E.F.; Rateb, M.E.; Abdel Moneim, A.E. Ganoderma applanatum secondary metabolites induced apoptosis through different pathways: In vivo and in vitro anticancer studies. Biomed. Pharmacother. 2018, 101, 264–277. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhou, L.; Shi, S.; Wang, Y.; Ni, X.; Xiao, F.; Wang, S.; Li, P.; Ding, K. Oligosaccharide G19 inhibits U-87 MG human glioma cells growth in vitro and in vivo by targeting epidermal growth factor (EGF) and activating p53/p21 signaling. Glycobiology 2014, 24, 748–765. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, X.; Hao, M.; Qu, X.; Hu, T. New advancesin exopolysaccharides production of Streptococcus thermophilus. Arch. Microbiol. 2017, 199, 799–809. [Google Scholar] [CrossRef]
- Dalsing, B.L.; Allen, C. Nitrate assimilation contributes to Ralstonia solanacearum root attachment, stem colonization, and virulence. J. Bacteriol. 2014, 196, 01378–01313. [Google Scholar] [CrossRef] [Green Version]
- Whitney, J.C.; Howell, P.L. Synthase-dependent exopolysaccharide secretion in Gram-negative bacteria. Trends Microbiol. 2013, 21, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Arco, Y.; Llamas, I.; Martinez-Checa, F.; Argandona, M.; Quesada, E.; Del Moral, A. epsABCJ genes are involved in the biosynthesis of the exopolysaccharide mauran produced by Halomonas maura. Microbiology 2005, 151, 2841–2851. [Google Scholar] [CrossRef] [Green Version]



 : glucose used as carbon source,
: glucose used as carbon source,  : fructose 6P,
: fructose 6P,  : dTDP rhamnose, TPR: tetratricopeptide repeat protein, β-barrel: outer-membrane porin. Synthase complex composed of glycosyltransferase (GT) and co-polymerase.
: dTDP rhamnose, TPR: tetratricopeptide repeat protein, β-barrel: outer-membrane porin. Synthase complex composed of glycosyltransferase (GT) and co-polymerase.
 : glucose used as carbon source,
: glucose used as carbon source,  : fructose 6P,
: fructose 6P,  : dTDP rhamnose, TPR: tetratricopeptide repeat protein, β-barrel: outer-membrane porin. Synthase complex composed of glycosyltransferase (GT) and co-polymerase.
: dTDP rhamnose, TPR: tetratricopeptide repeat protein, β-barrel: outer-membrane porin. Synthase complex composed of glycosyltransferase (GT) and co-polymerase.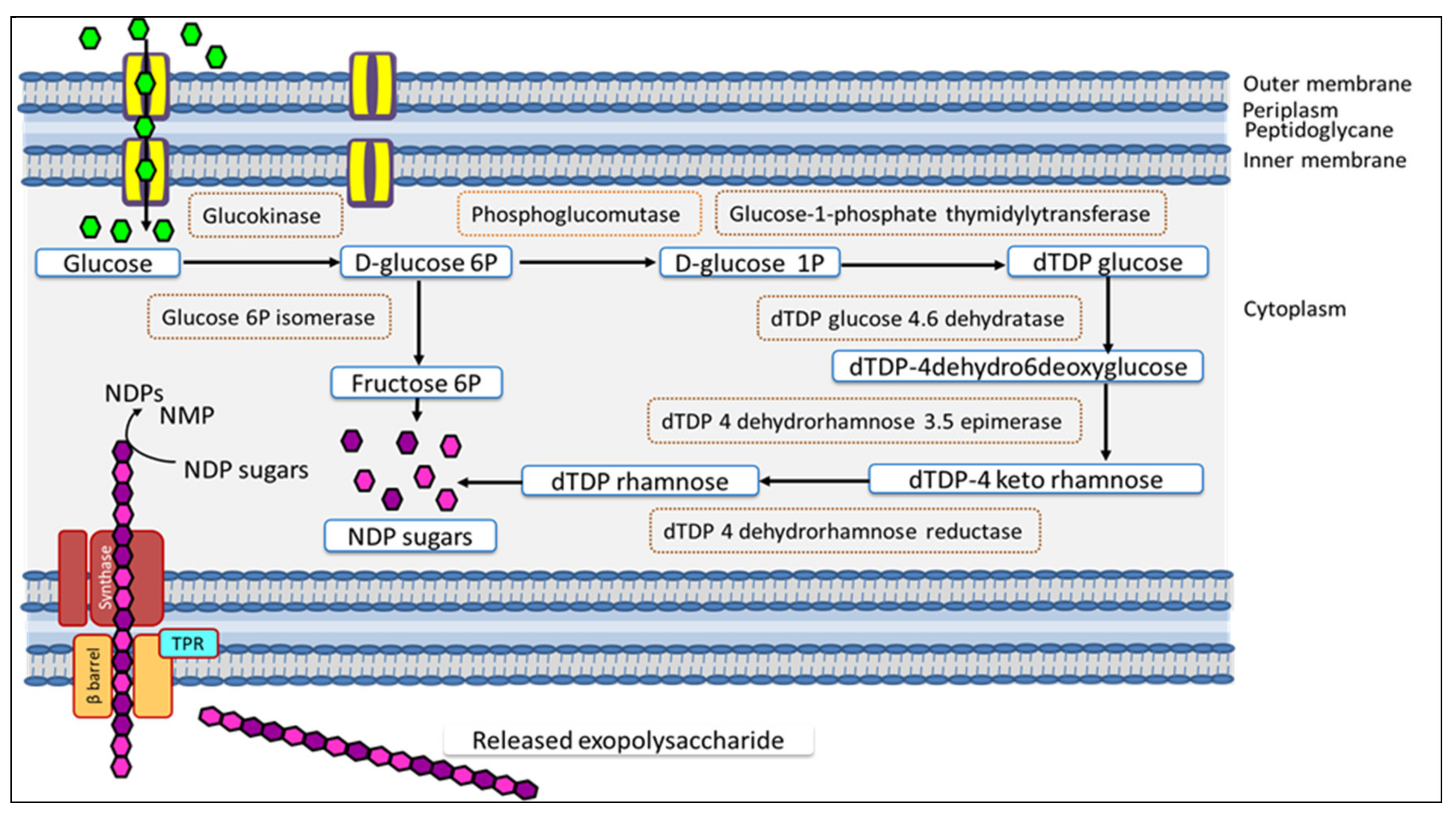
| Acclimatization Period (10 days) | Different groups | Treatment period (14 days) | Blood Collection (One day After Treatment Period) |
| Orally administration of 0.5 mL (once a day/mouse) of | |||
| NC group | saline solution (0.9%) | ||
| PC group | ascorbic acid (400 mg kg−1 body weight) | ||
| nEPS group | native EPS (400 mg kg−1body weight) | ||
| iEPS–1.0 kGy group | 1 kGy irradiated EPS (400 mg kg−1 body weight) | ||
| iEPS–1.5 kGy group | 1.5 kGy irradiated EPS (400 mg kg−1 body weight) | ||
| −10 0 | 14th | 15th |
| Halomonas | Strain | EPS Composition | MM (Daltons) | References | |||
|---|---|---|---|---|---|---|---|
| Carbohydrates | Proteins | UronicAcid | Sulfate | ||||
| H. eurihalina | F2-7 | 37 | 7.5 | ND | 11.2 | ND | [49] |
| A1-12 T | 30.9 | 2.07 | ND | 1.1 | 5.3 × 104 | ||
| H. maura | S-30 | 65 | 2.5 | ND | 6.5 | 4.7 × 106 | [46] |
| H. ventosae | A1-16 | 30.8 | 3.95 | ND | 0.7 | 5.2 × 104 | [47] |
| H. anticarinesis | FP36 | 33.7 | 0.4 | ND | 1.5 | 2 × 104 | |
| H. almeriensis | M8 T | 30.5 | 1.1 | ND | 1.4 | 1.5 × 104 6.3 × 106 | [45] |
| H. nitroreducens | WB1 | 68.9 | 2.2 | ND | ND | 1.3 × 103 5.2 × 106 | [50] |
| Halomonas sp. | MCTG39a | 17.2 | 36.4 | ND | ND | 2.61 × 105 | [51] |
| H. smyrnensis | K2 | 34.47 | 0.75 | 1.01 | ND | 3.96 × 105 | [52] |
| H. saliphila | LCB169 T | 97.6 ± 1.5 | 0 | ND | 0 | 5.13 × 104 | [53] |
| H. malpeensis | YU-PRIM-29 T | 76 | 5 | 50 * | ND | ND | [43] |
| H. Desertis | G11 | 61 | 0 | 17.5 | 10.2 | nEPS: 2.42 × 104 iEPSs: 1.27 × 103 1.78 × 102 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouchane, H.; Boutiti, S.; Ouertani, A.; Hassen, W.; Guesmi, S.; Neifar, M.; Jelassi, H.; Sghaier, H.; Masmoudi, A.S.E.; Cherif, A. Effect of Gamma Irradiation on Enhanced Biological Activities of Exopolysaccharide from Halomonas desertis G11: Biochemical and Genomic Insights. Polymers 2021, 13, 3798. https://doi.org/10.3390/polym13213798
Chouchane H, Boutiti S, Ouertani A, Hassen W, Guesmi S, Neifar M, Jelassi H, Sghaier H, Masmoudi ASE, Cherif A. Effect of Gamma Irradiation on Enhanced Biological Activities of Exopolysaccharide from Halomonas desertis G11: Biochemical and Genomic Insights. Polymers. 2021; 13(21):3798. https://doi.org/10.3390/polym13213798
Chicago/Turabian StyleChouchane, Habib, Sahar Boutiti, Awatef Ouertani, Wafa Hassen, Sihem Guesmi, Mohamed Neifar, Haikel Jelassi, Haïtham Sghaier, Ahmed Salah Eddine Masmoudi, and Ameur Cherif. 2021. "Effect of Gamma Irradiation on Enhanced Biological Activities of Exopolysaccharide from Halomonas desertis G11: Biochemical and Genomic Insights" Polymers 13, no. 21: 3798. https://doi.org/10.3390/polym13213798
APA StyleChouchane, H., Boutiti, S., Ouertani, A., Hassen, W., Guesmi, S., Neifar, M., Jelassi, H., Sghaier, H., Masmoudi, A. S. E., & Cherif, A. (2021). Effect of Gamma Irradiation on Enhanced Biological Activities of Exopolysaccharide from Halomonas desertis G11: Biochemical and Genomic Insights. Polymers, 13(21), 3798. https://doi.org/10.3390/polym13213798






