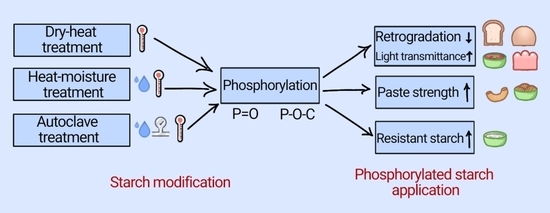
Effect of Thermal Pretreatments on Phosphorylation of Corypha umbraculifera L. Stem Pith Starch: A Comparative Study Using Dry-Heat, Heat-Moisture and Autoclave Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Talipot Starch
2.3. Phosphorylation of NTS
2.4. Thermal Modifications of NTS
2.5. Pretreatment of PTS with Thermal Treatments
2.6. Phosphorous Content and Degree of Crosslinking
2.7. Amylose Content
2.8. SEM
2.9. XRD and RC
2.10. FT-IR
2.11. Swelling Power and Solubility
2.12. Light Transmittance
2.13. Pasting Properties
2.14. Textural Properties
2.15. Dynamic Rheology
2.16. In Vitro Digestibility
2.17. Statistical Analysis
3. Result and Discussions
3.1. Degree of Crosslinking and Amylose Content
3.2. Morphology Analysis
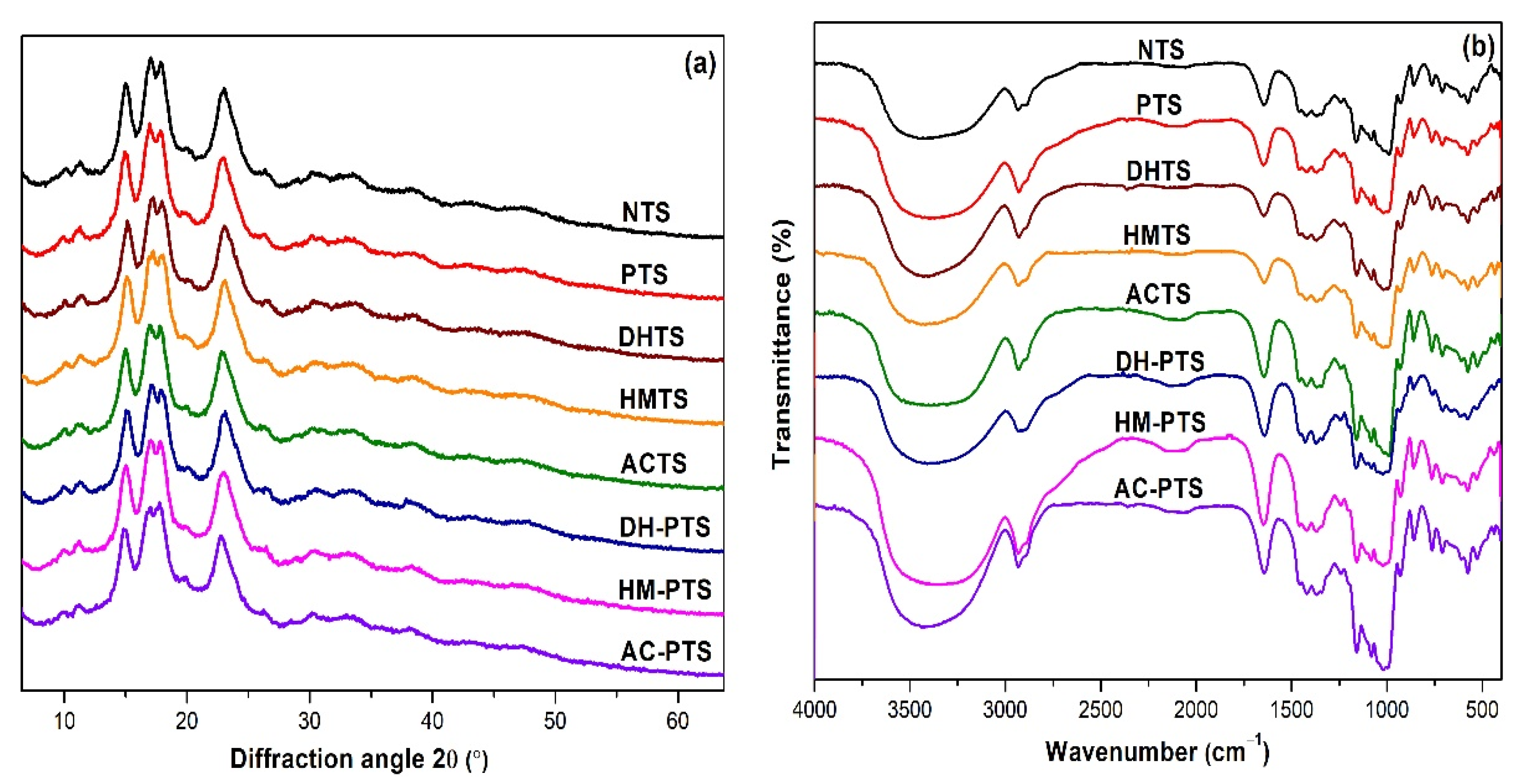
3.3. XRD and RC Analysis
3.4. FT-IR Spectral Analysis
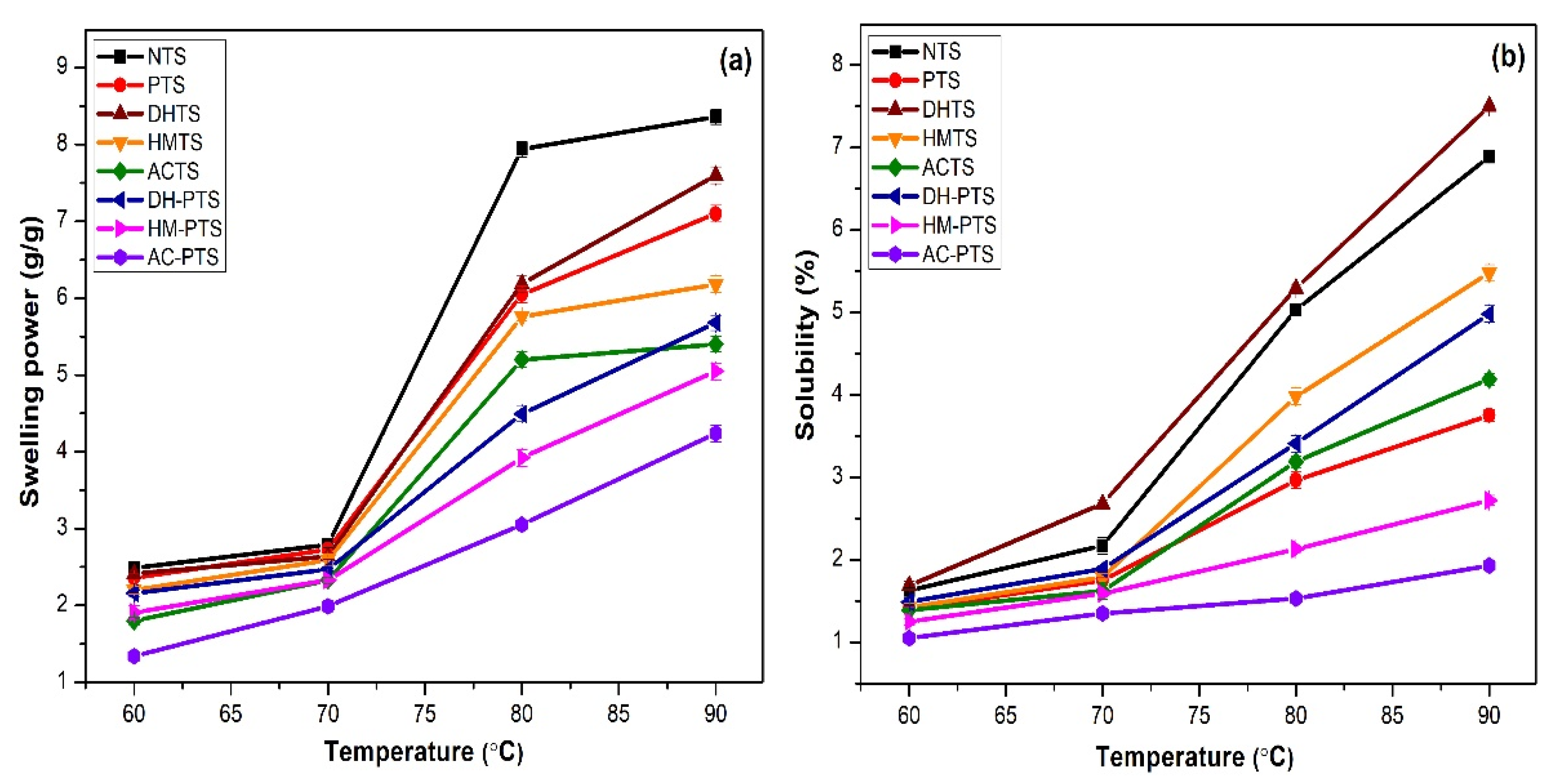
3.5. Swelling Power and Solubility
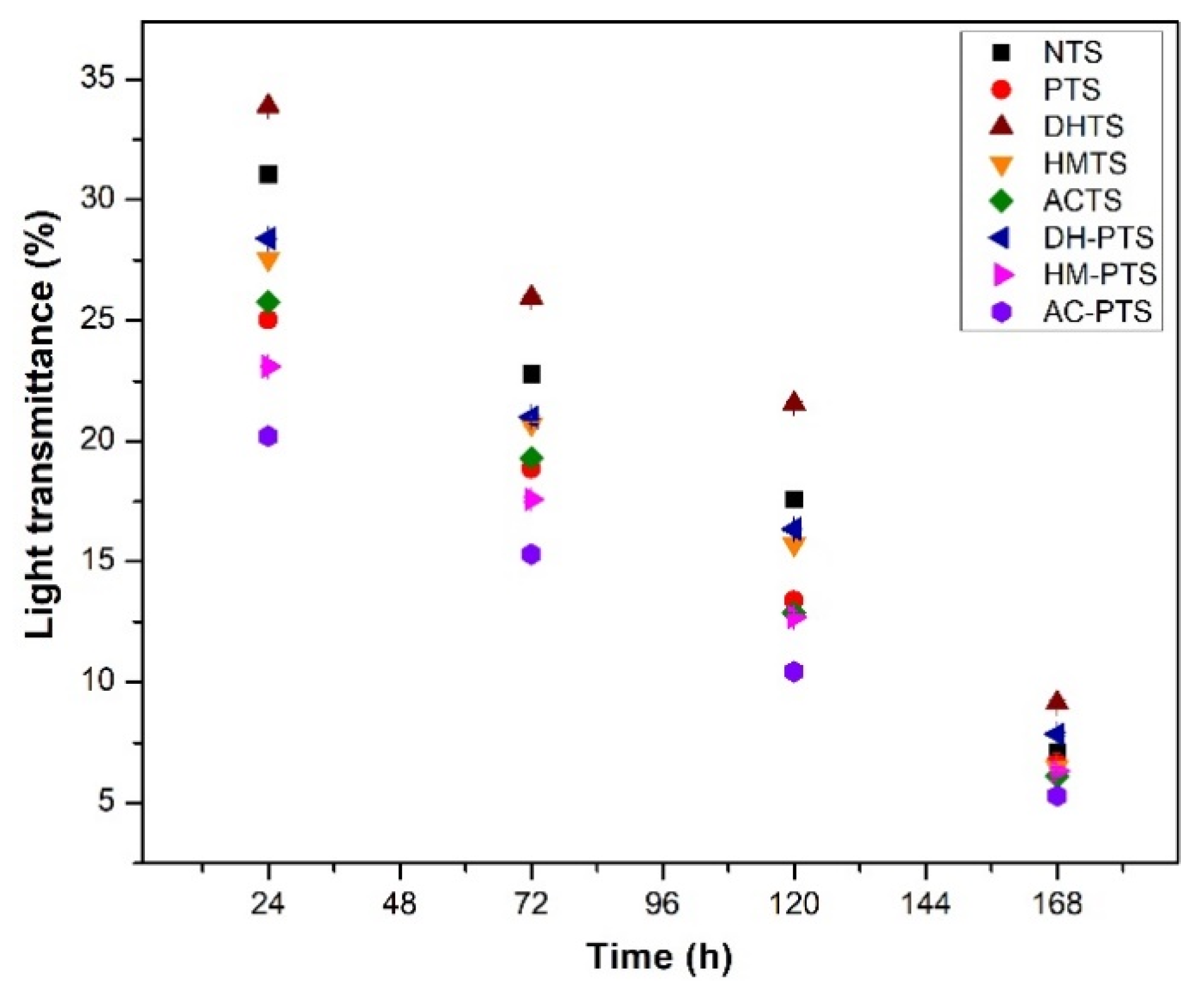
3.6. Light Transmittance
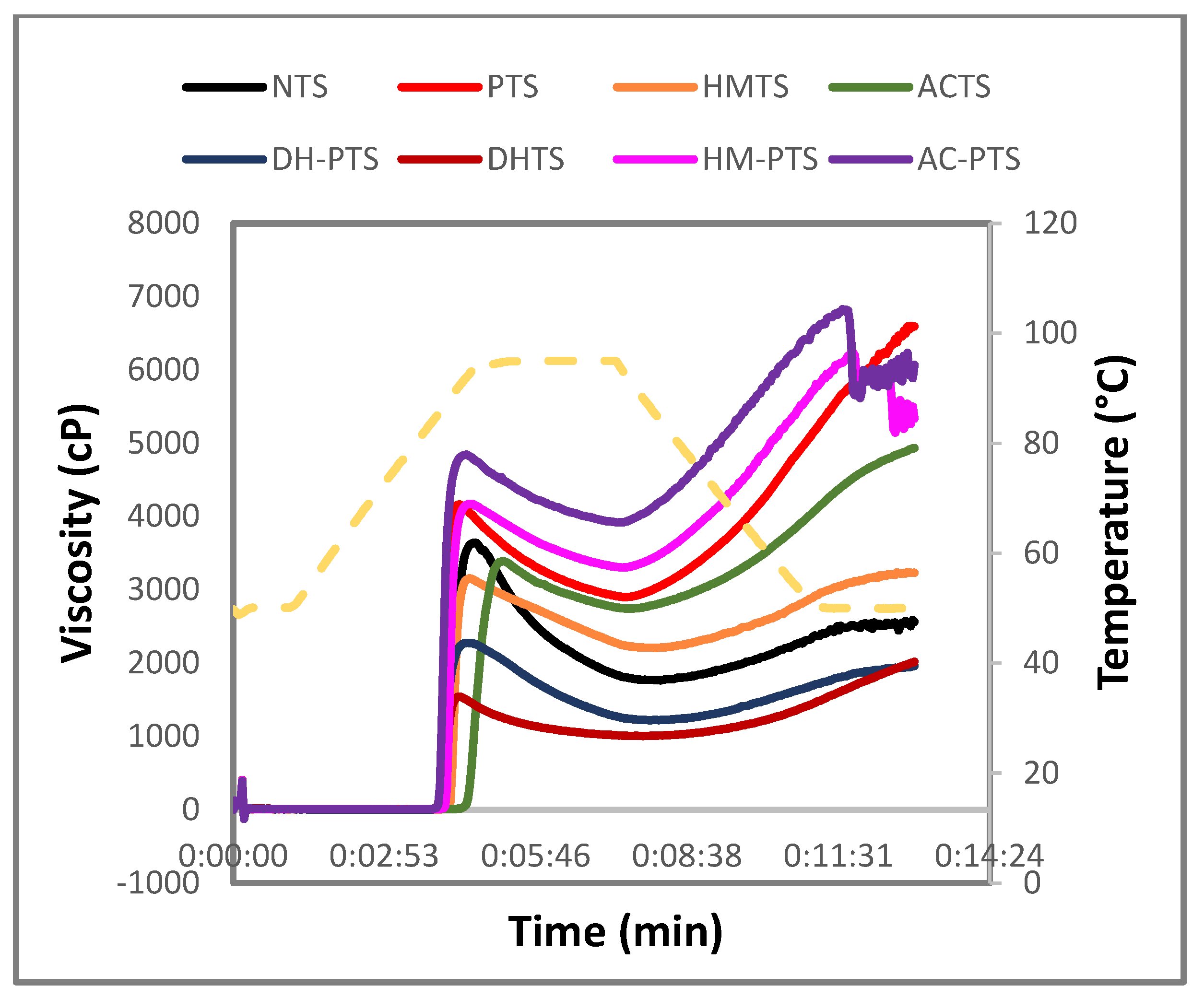
3.7. Pasting Properties
3.8. Textural Properties
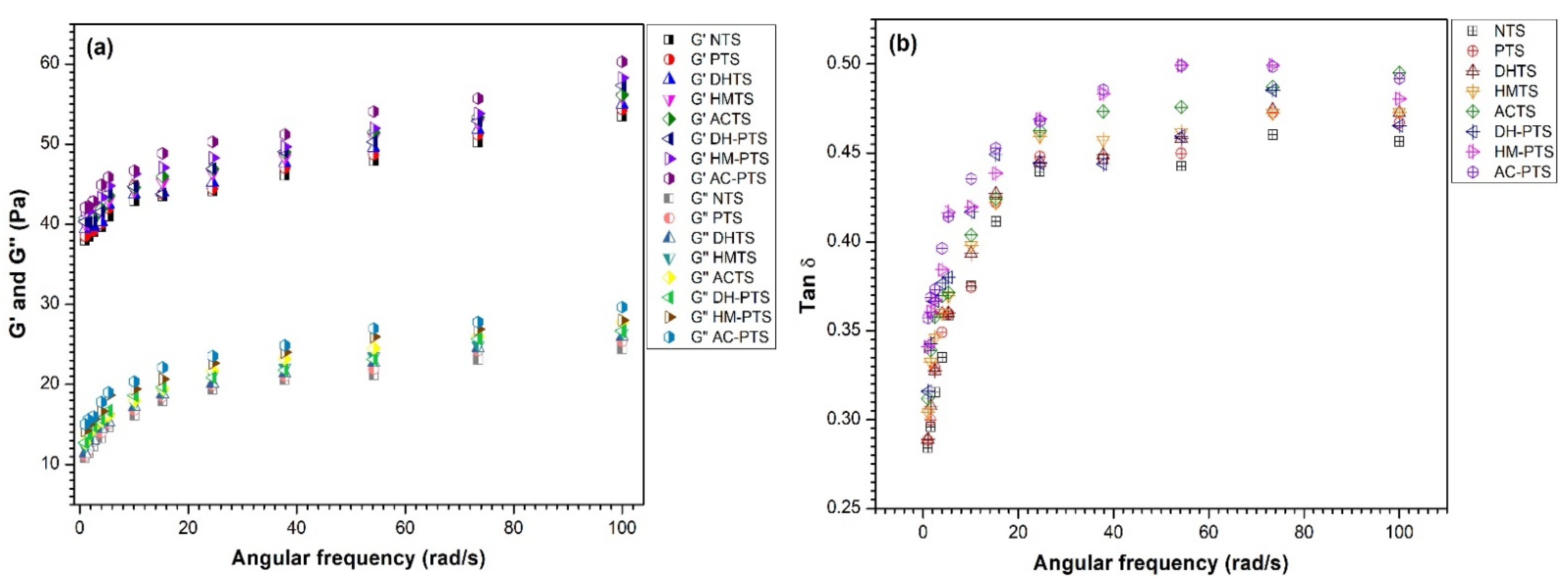
3.9. Dynamic Rheology
3.10. In Vitro Digestibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aaliya, B.; Sunooj, K.V.; Lackner, M. Biopolymer composites: A review. Int. J. Biobased Plast. 2021, 3, 40–84. [Google Scholar] [CrossRef]
- Muhammed, N.; Sunooj, K.V.; Aaliya, B.; Sudheesh, C.; George, J. Physico-chemical, functional, morphological, thermal properties and digestibility of Talipot palm (Corypha umbraculifera L.) flour and starch grown in Malabar region of South India. J. Food Meas. Charact. 2020, 14, 1601–1613. [Google Scholar] [CrossRef]
- George, J.; Nair, S.G.; Kumar, R.; Semwal, A.; Sudheesh, C.; Basheer, A.; Sunooj, K.V. A new insight into the effect of starch nanocrystals in the retrogradation properties of starch. Food Hydrocoll. Health 2021, 1, 100009. [Google Scholar] [CrossRef]
- Sudheesh, C.; Aaliya, B.; Sunooj, K.V. Role of Starch in Gluten-Free Breads, in: Gluten-Free Bread Technol; Springer: Cham, Switzerland, 2021; pp. 155–181. [Google Scholar] [CrossRef]
- Rożnowski, J.; Juszczak, L.; Szwaja, B.; Przetaczek-Rożnowska, I. Effect of Esterification Conditions on the Physicochemical Properties of Phosphorylated Potato Starch. Polymers 2021, 13, 2548. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Polachini, T.C.; Norwood, E.-A.; Le-Bail, P.; Le-Bail, A. Thermal technologies to enhance starch performance and starchy products. Curr. Opin. Food Sci. 2021, 40, 72–80. [Google Scholar] [CrossRef]
- Keppler, S.; Bakalis, S.; Leadley, C.; Fryer, P. A systematic study of the residence time of flour in a vibrating apparatus used for thermal processing. Innov. Food Sci. Emerg. Technol. 2015, 33, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Maniglia, B.C.; Lima, D.C.; Júnior, M.D.M.; Oge, A.; Le-Bail, P.; Augusto, P.E.; Le-Bail, A. Dry heating treatment: A potential tool to improve the wheat starch properties for 3D food printing application. Food Res. Int. 2020, 137, 109731. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 2482–2505. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and potential applications: A review of the modification of starches by heat-moisture treatment (HMT). Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Pinto, V.Z.; Vanier, N.L.; Klein, B.; Zavareze, E.D.R.; Elias, M.C.; Gutkoski, L.C.; Helbig, E.; Dias, A.R.G. Physicochemical, crystallinity, pasting and thermal properties of heat-moisture-treated pinhão starch. Starch-Stärke 2012, 64, 855–863. [Google Scholar] [CrossRef]
- Zheng, M.; Xiao, Y.; Yang, S.; Liu, H.; Liu, M.; Yaqoob, S.; Xu, X.; Liu, J. Effects of heat–moisture, autoclaving, and microwave treatments on physicochemical properties of proso millet starch. Food Sci. Nutr. 2020, 8, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Soler, A.; Velazquez, G.; Velazquez-Castillo, R.; Morales-Sanchez, E.; Osorio-Diaz, P.; Mendez-Montealvo, G. Retrogradation of autoclaved corn starches: Effect of water content on the resistant starch formation and structure. Carbohydr. Res. 2020, 497, 108137. [Google Scholar] [CrossRef]
- Dong, H.; Vasanthan, T. Amylase resistance of corn, faba bean, and field pea starches as influenced by three different phosphorylation (cross-linking) techniques. Food Hydrocoll. 2019, 101, 105506. [Google Scholar] [CrossRef]
- Dong, H.; Vasanthan, T. Effect of phosphorylation techniques on structural, thermal, and pasting properties of pulse starches in comparison with corn starch. Food Hydrocoll. 2020, 109, 106078. [Google Scholar] [CrossRef]
- Singh, A.V.; Nath, L.K. Synthesis and evaluation of physicochemical properties of cross-linked sago starch. Int. J. Biol. Macromol. 2012, 50, 14–18. [Google Scholar] [CrossRef]
- Park, E.Y.; Ma, J.-G.; Kim, J.; Lee, D.H.; Kim, S.Y.; Kwon, D.-J.; Kim, J.-Y. Effect of dual modification of HMT and crosslinking on physicochemical properties and digestibility of waxy maize starch. Food Hydrocoll. 2018, 75, 33–40. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The uniqueness of palms. Bot. J. Linn. Soc. 2006, 151, 5–14. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Sasidharan, A.; Sabu, S.; Basheer, A.; Navaf, M.; Raghavender, C.; Sinha, S.; George, J. Energetic neutral N2 atoms treatment on the kithul (Caryota urens) starch biodegradable film: Physico-chemical characterization. Food Hydrocoll. 2020, 103, 105650. [Google Scholar] [CrossRef]
- Hazarika, B.J.; Sit, N. Effect of dual modification with hydroxypropylation and cross-linking on physicochemical properties of taro starch. Carbohydr. Polym. 2016, 140, 269–278. [Google Scholar] [CrossRef]
- Marboh, V.; Mahanta, C.L. Physicochemical and rheological properties and in vitro digestibility of heat moisture treated and annealed starch of sohphlang (Flemingia vestita) tuber. Int. J. Biol. Macromol. 2020, 168, 486–495. [Google Scholar] [CrossRef]
- Deka, D.; Sit, N. Dual modification of taro starch by microwave and other heat moisture treatments. Int. J. Biol. Macromol. 2016, 92, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Caruso, J. Determination of phosphorus. Methods Carbohydr. Chem. 1964, 4, 42–46. [Google Scholar]
- Williams, P.C.; Kuzina, F.D.; Hlynka, I. Rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970, 47, 411–420. [Google Scholar]
- Sudheesh, C.; Sunooj, K.V.; Bhavani, B.; Aaliya, B.; Navaf, M.; Akhila, P.P.; Sabu, S.; Sasidharan, A.; Sinha, S.K.; Kumar, S.; et al. Energetic neutral atoms assisted development of kithul (Caryota urens) starch–lauric acid complexes: A characterisation study. Carbohydr. Polym. 2020, 250, 116991. [Google Scholar] [CrossRef]
- Navaf, M.; Sunooj, K.V.; Aaliya, B.; Sudheesh, C.; Akhila, P.P.; Sabu, S.; Sasidharan, A.; George, J. Talipot palm (Corypha umbraculifera L.) a nonconventional source of starch: Effect of citric acid on structural, rheological, thermal properties and in vitro digestibility. Int. J. Biol. Macromol. 2021, 182, 554–563. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Anjali, K.; Aaliya, B.; Navaf, M.; Kumar, S.; Sajeevkumar, V.A.; George, J. Effect of lysine incorporation, annealing and heat moisture treatment alone and in combination on the physico-chemical, retrogradation, rheological properties and in vitro digestibility of kithul (Caryota urens L.) starch. Int. J. Food Sci. Technol. 2019, 55, 2391–2398. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Majzoobi, M.; Beparva, P.; Farahnaky, A.; Badii, F. Effects of malic acid and citric acid on the functional properties of native and cross-linked wheat starches. Starch-Stärke 2013, 66, 491–495. [Google Scholar] [CrossRef]
- Gou, M.; Wu, H.; Saleh, A.S.; Jing, L.; Liu, Y.; Zhao, K.; Su, C.; Zhang, B.; Jiang, H.; Li, W. Effects of repeated and continuous dry heat treatments on properties of sweet potato starch. Int. J. Biol. Macromol. 2019, 129, 869–877. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. Recent advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2020, 344, 128700. [Google Scholar] [CrossRef]
- Ge, X.; Shen, H.; Su, C.; Zhang, B.; Zhang, Q.; Jiang, H.; Li, W. The improving effects of cold plasma on multi-scale structure, physicochemical and digestive properties of dry heated red adzuki bean starch. Food Chem. 2021, 349, 129159. [Google Scholar] [CrossRef]
- Sun, Q.; Gong, M.; Li, Y.; Xiong, L. Effect of dry heat treatment on the physicochemical properties and structure of proso millet flour and starch. Carbohydr. Polym. 2014, 110, 128–134. [Google Scholar] [CrossRef]
- Zhou, D.; Ma, Z.; Yin, X.; Hu, X.; Boye, J.I. Structural characteristics and physicochemical properties of field pea starch modified by physical, enzymatic, and acid treatments. Food Hydrocoll. 2019, 93, 386–394. [Google Scholar] [CrossRef]
- Rafiq, S.I.; Singh, S.; Saxena, D.C. Effect of heat-moisture and acid treatment on physicochemical, pasting, thermal and morphological properties of Horse Chestnut (Aesculus indica) starch. Food Hydrocoll. 2016, 57, 103–113. [Google Scholar] [CrossRef]
- Zou, J.; Xu, M.; Tang, W.; Wen, L.; Yang, B. Modification of structural, physicochemical and digestive properties of normal maize starch by thermal treatment. Food Chem. 2019, 309, 125733. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2018, 271, 102–108. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyser (RVA) as a Tool for Measuring Starch-Related Physiochemical Properties in Cereals: A Review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Aaliya, B.; Navaf, M.; Sunooj, K.V. Dough Handling Properties of Gluten-Free Breads. In Gluten-Free Bread Technology; Springer: Cham, Switzerland, 2021; pp. 49–70. [Google Scholar] [CrossRef]
- Lima, D.C.; Maniglia, B.C.; Junior, M.D.M.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Dual-process of starch modification: Combining ozone and dry heating treatments to modify cassava starch structure and functionality. Int. J. Biol. Macromol. 2020, 167, 894–905. [Google Scholar] [CrossRef]
- Pepe, L.S.; Moraes, J.; Albano, K.M.; Telis, V.R.; Franco, C.M. Effect of heat-moisture treatment on the structural, physicochemical, and rheological characteristics of arrowroot starch. Food Sci. Technol. Int. 2015, 22, 256–265. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Shoemaker, C.F.; Xu, Z.; Zhu, S.; Zhong, F. Effect of dry heat treatment with xanthan on waxy rice starch. Carbohydr. Polym. 2013, 92, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hao, Y.; Chen, Y.; Gao, Q. Effects of dry heat treatment on the structure and physicochemical properties of waxy potato starch. Int. J. Biol. Macromol. 2019, 132, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, M.; Faridah, D.N.; Lioe, H.N. Structural changes to starch after acid hydrolysis, debranching, autoclaving-cooling cycles, and heat moisture treatment (HMT): A review. Starch-Stärke 2017, 70, 1–13. [Google Scholar] [CrossRef]

| Samples | P (%) | DC | Amylose (%) | RC (%) |
|---|---|---|---|---|
| NTS | 0.018 ± 0.03 a | – | 28.81 ± 0.01 f | 16.97 ± 0.02 d |
| PTS | 0.058 ± 0.03 b | 0.00298 a | 25.23 ± 0.03 e | 16.70 ± 0.10 d |
| DHTS | 0.018 ± 0.02 a | – | 23.39 ± 0.08 d | 13.57 ± 0.10 c |
| HMTS | 0.016 ± 0.07 a | – | 25.19 ± 0.17 e | 15.82 ± 0.20 f |
| ACTS | 0.016 ± 0.10 a | – | 31.02 ± 0.10 g | 14.20 ± 0.03 e |
| DH-PTS | 0.069 ± 0.05 c | 0.00361 b | 19.26 ± 0.10 a | 10.60 ± 0.02 a |
| HM-PTS | 0.076 ± 0.05 d | 0.00398 c | 20.54 ± 0.06 b | 13.51 ± 0.04 c |
| AC-PTS | 0.080 ± 0.01 e | 0.00418 d | 22.75 ± 0.11 c | 12.12 ± 0.01 b |
| Samples | Hardness (N) | Adhesiveness (Nmm) | Springiness (mm) | Cohesiveness | Gumminess (Nmm) |
|---|---|---|---|---|---|
| NTS | 45.72 ± 0.03 a | −16.26 ± 0.10 a | 0.90 ± 0.10 a | 0.46 ± 0.11 a | 21.03 ± 0.09 a |
| PTS | 82.42 ± 0.12 e | −11.38 ± 0.20 e | 0.96 ± 0.03 d | 0.54 ± 0.04 d | 44.50 ± 0.03 e |
| DHTS | 51.72 ± 0.02 b | −15.23 ± 0.12 b | 0.92 ± 0.12 b | 0.52 ± 0.11 b | 26.89 ± 0.30 b |
| HMTS | 55.29 ± 0.09 c | −15.21 ± 0.11 c | 0.93 ± 0.11 c | 0.53 ± 0.10 c | 29.30 ± 0.09 c |
| ACTS | 68.26 ± 0.10 d | −13.97 ± 0.02 d | 0.96 ± 0.20 d | 0.56 ± 0.02 e | 38.22 ± 0.03 d |
| DH-PTS | 90.83 ± 0.11 f | −9.38 ± 0.07 f | 1.00 ± 0.09 e | 0.62 ± 0.19 f | 56.32 ± 0.10 f |
| HM-PTS | 97.29 ± 0.18 g | −8.49 ± 0.20 g | 1.00 ± 0.01 e | 0.63 ± 0.13 g | 61.29 ± 0.15 g |
| AC-PTS | 113.14 ± 0.11 h | −7.62 ± 0.08 h | 1.03 ± 0.06 f | 0.66 ± 0.01 h | 74.67 ± 0.03 h |
| Samples | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| NTS | 29.44 ± 0.05 f | 33.85 ± 0.11 d | 36.71 ± 0.02 b |
| PTS | 20.62 ± 0.14 b | 35.01 ± 0.31 e | 44.37 ± 0.13 e |
| DHTS | 35.20 ± 0.03 g | 30.65 ± 0.08 a | 34.15 ± 0.09 a |
| HMTS | 27.71 ± 0.16 e | 33.31 ± 0.02 c | 38.98 ± 0.18 c |
| ACTS | 22.54 ± 0.11 d | 35.38 ± 0.02 e | 42.08 ± 0.15 d |
| DH-PTS | 27.93 ± 0.12 e | 32.97 ± 0.27 b | 39.10 ± 0.10 c |
| HM-PTS | 21.70 ± 0.02 c | 33.86 ± 0.10 d | 44.44 ± 0.05 e |
| AC-PTS | 15.91 ± 0.17 a | 36.01 ± 0.18 f | 48.08 ± 0.03 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aaliya, B.; Sunooj, K.V.; Rajkumar, C.B.S.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; George, J.; Lackner, M. Effect of Thermal Pretreatments on Phosphorylation of Corypha umbraculifera L. Stem Pith Starch: A Comparative Study Using Dry-Heat, Heat-Moisture and Autoclave Treatments. Polymers 2021, 13, 3855. https://doi.org/10.3390/polym13213855
Aaliya B, Sunooj KV, Rajkumar CBS, Navaf M, Akhila PP, Sudheesh C, George J, Lackner M. Effect of Thermal Pretreatments on Phosphorylation of Corypha umbraculifera L. Stem Pith Starch: A Comparative Study Using Dry-Heat, Heat-Moisture and Autoclave Treatments. Polymers. 2021; 13(21):3855. https://doi.org/10.3390/polym13213855
Chicago/Turabian StyleAaliya, Basheer, Kappat Valiyapeediyekkal Sunooj, Chillapalli Babu Sri Rajkumar, Muhammed Navaf, Plachikkattu Parambil Akhila, Cherakkathodi Sudheesh, Johnsy George, and Maximilian Lackner. 2021. "Effect of Thermal Pretreatments on Phosphorylation of Corypha umbraculifera L. Stem Pith Starch: A Comparative Study Using Dry-Heat, Heat-Moisture and Autoclave Treatments" Polymers 13, no. 21: 3855. https://doi.org/10.3390/polym13213855
APA StyleAaliya, B., Sunooj, K. V., Rajkumar, C. B. S., Navaf, M., Akhila, P. P., Sudheesh, C., George, J., & Lackner, M. (2021). Effect of Thermal Pretreatments on Phosphorylation of Corypha umbraculifera L. Stem Pith Starch: A Comparative Study Using Dry-Heat, Heat-Moisture and Autoclave Treatments. Polymers, 13(21), 3855. https://doi.org/10.3390/polym13213855