Pyrrole Plasma Polymer-Coated Electrospun Scaffolds for Neural Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Fibrous Composite Scaffolds
2.1.1. Electrospinning
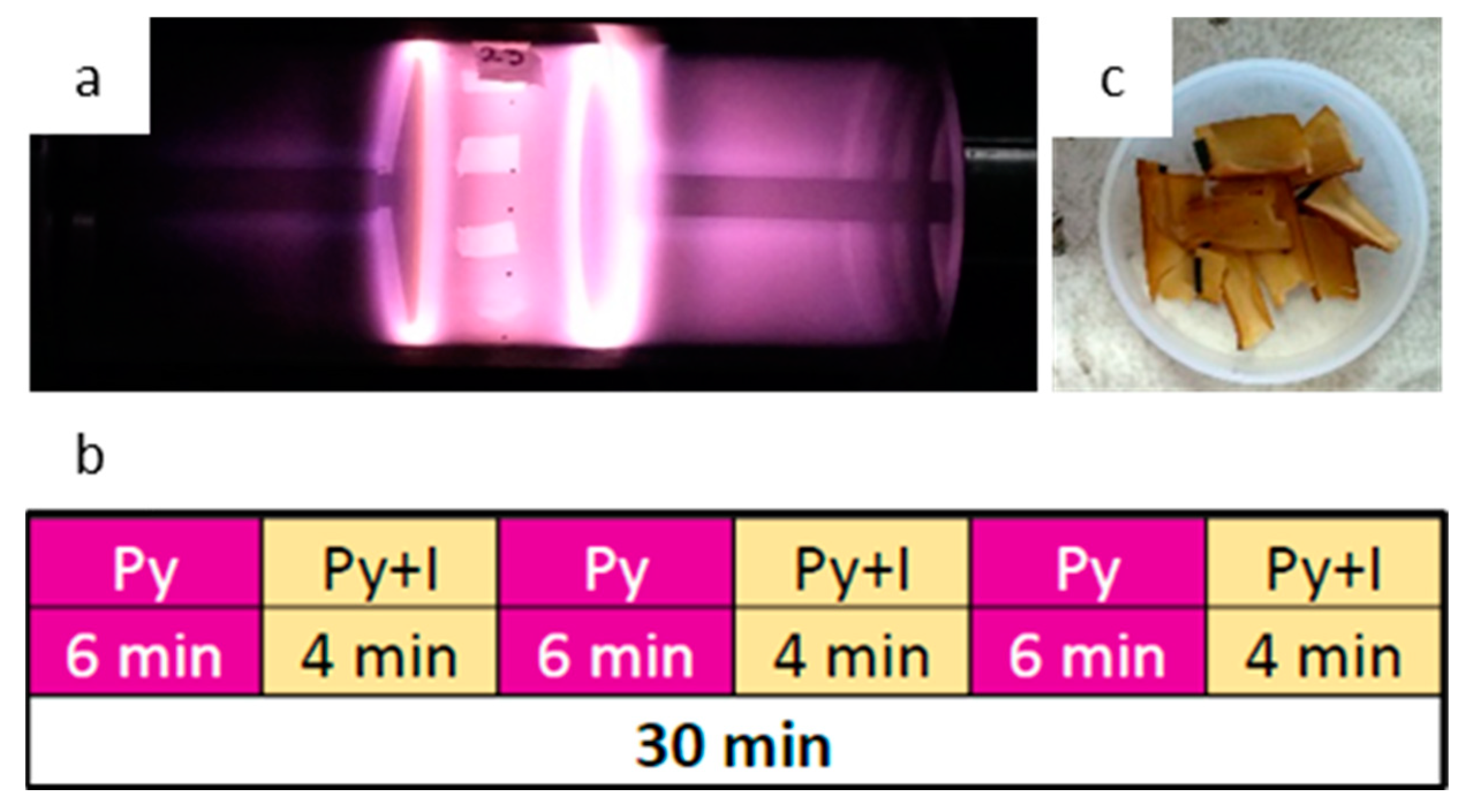
2.1.2. Pyrrole Plasma Polymer Coating
2.2. Characterization of the Scaffolds
2.2.1. Physicochemical Characterization
2.2.2. Morphology and Microstructure
2.2.3. Biological Characterization
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. Morphological Characterization
3.3. Biological Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doblado, L.R.; Martínez-Ramos, C.; Pradas, M.M. Biomaterials for neural tissue engineering. Front. Nanotechnol. 2021, 3, 21. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Biol. 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- Hanumantharao, S.N.; Rao, S. Multi-functional electrospun nanofibers from polymer blends for scaffold tissue engineering. Fibers 2019, 7, 66. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials 2020, 11, 21. [Google Scholar] [CrossRef]
- Kakoria, A.; Sinha-Ray, S. A review on biopolymer-based fibers via electrospinning and solution blowing and their applications. Fibers 2018, 6, 45. [Google Scholar] [CrossRef]
- Ng, J.-J.; Supaphol, P. Rotating-disk electrospinning: Needleless electrospinning of poly(caprolactone), poly(lactic acid) and poly(vinyl alcohol) nanofiber mats with controlled morphology. J. Polym. Res. 2018, 25, 155. [Google Scholar] [CrossRef]
- Moon, S.; Gil, M.; Lee, K.J. Syringeless electrospinning toward versatile fabrication of nanofiber web. Sci. Rep. 2017, 7, srep41424. [Google Scholar] [CrossRef]
- Yan, X.; Yu, M.; Ramakrishna, S.; Russell, S.J.; Long, Y.-Z. Advances in portable electrospinning devices for in situ delivery of personalized wound care. Nanoscale 2019, 11, 19166–19178. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.-K. Electrospinning nanofibers for therapeutics delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, Y.; Chen, B.; Dai, J. Scaffolds for Spinal Cord Injury Repair: From Proof of Concept to First In-Human Studies and Clinical Trials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 603–619. [Google Scholar]
- Garrudo, F.F.; Mikael, P.E.; Rodrigues, C.A.; Udangawa, R.W.; Paradiso, P.; Chapman, C.A.; Hoffman, P.; Colaço, R.; Cabral, J.M.; Morgado, J.; et al. Polyaniline-polycaprolactone fibers for neural applications: Electroconductivity enhanced by pseudo-doping. Mater. Sci. Eng. C 2021, 120, 111680. [Google Scholar] [CrossRef]
- Hurtado, A.; Cregg, J.; Wang, H.B.; Wendell, D.F.; Oudega, M.; Gilbert, R.J.; McDonald, J.W. Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers. Biomaterials 2011, 32, 6068–6079. [Google Scholar] [CrossRef]
- Schaub, N.J.; Le Beux, C.; Miao, J.; Linhardt, R.J.; Alauzun, J.G.; Laurencin, D.; Gilbert, R.J. The effect of surface modification of aligned poly-L-lactic acid electrospun fibers on fiber degradation and neurite extension. PLoS ONE 2015, 10, e0136780. [Google Scholar] [CrossRef]
- Sudwilai, T.; Ng, J.J.; Boonkrai, C.; Israsena, N.; Chuangchote, S.; Supaphol, P. Polypyrrole-coated electrospun poly(lactic acid) fibrous scaffold: Effects of coating on electrical conductivity and neural cell growth. J. Biomater. Sci. Polym. Ed. 2014, 25, 1240–1252. [Google Scholar] [CrossRef]
- Alves, C.M.; Yang, Y.; Marton, D.; Carnes, D.L.; Ong, J.L.; Sylvia, V.L.; Dean, D.D.; Reis, R.L.; Agrawal, C.M. Plasma surface modification of poly(D,L-lactic acid) as a tool to enhance protein adsorption and the attachment of different cell types. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87B, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Arora, M.; Kumar, M.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Niu, Y.; Stadler, F.J.; Fu, M. Biomimetic electrospun tubular PLLA/gelatin nanofiber scaffold promoting regeneration of sciatic nerve transection in SD rat. Mater. Sci. Eng. C 2021, 121, 111858. [Google Scholar] [CrossRef] [PubMed]
- Faweett, J.W.; Keynes, R.J. Peripheral nerve regeneration. Annu. Rev. Neurosci. 1990, 13, 43–60. [Google Scholar] [CrossRef]
- Imani, F.; Karimi-Soflou, R.; Shabani, I.; Karkhaneh, A. PLA electrospun nanofibers modified with polypyrrole-grafted gelatin as bioactive electroconductive scaffold. Polymer 2021, 218, 123487. [Google Scholar] [CrossRef]
- Mozaffari, A.; Gashti, M.P.; Mirjalili, M.; Parsania, M. Argon and argon–oxygen plasma surface modification of gelatin nanofibers for tissue engineering applications. Membranes 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Sultana, T.; Park, M.; Lee, B.-T. Fibroblast cell derived extracellular matrix containing electrospun scaffold as a hybrid biomaterial to promote in vitro endothelial cell expansion and functionalization. Mater. Sci. Eng. C 2021, 120, 111659. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Tarasi, R.; Sedghi, R.; Moghimi, A.; Niknejad, H. Fabrication of nanochitosan incorporated polypyrrole/alginate conducting scaffold for neural tissue engineering. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Gajendiran, M.; Choi, J.; Kim, S.-J.; Kim, K.; Shin, H.; Koo, H.-J.; Kim, K. Conductive biomaterials for tissue engineering applications. J. Ind. Eng. Chem. 2017, 51, 12–26. [Google Scholar] [CrossRef]
- Rinoldi, C.; Lanzi, M.; Fiorelli, R.; Nakielski, P.; Zembrzycki, K.; Kowalewski, T.; Urbanek, O.; Grippo, V.; Jezierska-Woźniak, K.; Maksymowicz, W.; et al. Three-dimensional printable conductive semi-interpenetrating polymer network hydrogel for neural tissue applications. Biomacromolecules 2021, 22, 3084–3098. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Jafarkhani, M.; Salehi, Z.; Nematian, T. Preparation and characterization of chitosan/graphene oxide composite hydrogels for nerve tissue engineering. Mater. Today Proc. 2018, 5, 15620–15628. [Google Scholar] [CrossRef]
- Shafiee, A.; Kehtari, M.; Zarei, Z.; Soleimani, M.; Varshochian, R.; Ahmadi, A.; Atyabi, F.; Dinarvand, R. An in situ hydrogel-forming scaffold loaded by PLGA microspheres containing carbon nanotube as a suitable niche for neural differentiation. Mater. Sci. Eng. C 2021, 120, 111739. [Google Scholar] [CrossRef] [PubMed]
- Jarrin, S.; Cabré, S.; Dowd, E. The potential of biomaterials for central nervous system cellular repair. Neurochem. Int. 2021, 144, 104971. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sánchez, M.G.; Islas-Arteaga, N.C.; Raya-Rivera, A.M.; Esquiliano-Rendon, D.R.; Morales-Corona, J.; Uribe-Juarez, O.E.; Vivar-Velázquez, F.I.; Ortiz-Vázquez, G.P.; Olayo, R. Effect of a plasma synthesized polypyrrole coverage on polylactic acid/hydroxyapatite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2021, 109, 2199–2211. [Google Scholar] [CrossRef]
- Flores-Sánchez, M.G.; Raya-Rivera, A.M.; Esquiliano-Rendon, D.R.; Ontiveros-Nevares, P.G.; Islas-Arteaga, N.C.; Morales-Corona, J.; Olayo, R. Scaffolds of polylactic acid/hydroxyapatite coated by plasma with polypyrrole-iodine for the generation of neo-tissue–bone in vivo: Study in rabbit. Int. J. Polym. Mater. 2017, 67, 427–437. [Google Scholar] [CrossRef]
- Islas-Arteaga, N.C.; Rivera, A.R.; Rendon, D.R.E.; Morales-Corona, J.; Ontiveros-Nevares, P.G.; Sánchez, M.G.F.; Mojica-Cardoso, C.; Olayo, R. Electrospun scaffolds with surfaces modified by plasma for regeneration of articular cartilage tissue: A pilot study in rabbit. Int. J. Polym. Mater. 2018, 68, 1089–1098. [Google Scholar] [CrossRef]
- Cortés-Ortiz, E.; Olayo-Valles, R.; Rodríguez-Talavera, R.; González-Torres, M.; Vargas-Muñoz, S.; Olayo, R.; Godinez-Fernández, R.; Juárez, O.E.U.; Morales-Corona, J. Plasma functionalized scaffolds of polyhydroxybutyrate electrospun fibers for pancreatic beta cell cultures. Front. Mater. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Cruz, Y.; Muñoz, E.; Gomez-Pachón, E.Y.; Morales-Corona, J.; Olayo-Lortia, J.; Olayo, R.; Olayo-Valles, R. Electrospun PCL-protein scaffolds coated by pyrrole plasma polymerization. J. Biomater. Sci. Polym. Ed. 2019, 30, 832–845. [Google Scholar] [CrossRef]
- Zuñiga-Aguilar, E.; Olayo, R.; Ramirez-Fernandez, O.; Morales, J.; Godínez, R. Nerve cells culture from lumbar spinal cord on surfaces modified by plasma pyrrole polymerization. J. Biomater. Sci. Polym. Ed. 2014, 25, 729–747. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mejía, L.; Morales, J.; Cruz, G.J.; Olayo, M.-G.; Olayo, R.; Diaz-Ruiz, A.; Ríos, C.; Mondragon-Lozano, R.; Sanchez-Torres, S.; Morales-Guadarrama, A.; et al. Functional recovery in spinal cord injured rats using polypyrrole/iodine implants and treadmill training. J. Mater. Sci. Mater. Electron. 2015, 26, 1–11. [Google Scholar] [CrossRef]
- Mondragon-Lozano, R.; Ríos, C.; Roldan-Valadez, E.; Cruz, G.J.; Olayo, M.G.; Olayo, R.; Salgado-Ceballos, H.; Morales, J.; Mendez-Armenta, M.; Alvarez-Mejia, L.; et al. Delayed injection of polypyrrole doped with iodine particle suspension after spinal cord injury in rats improves functional recovery and decreased tissue damage evaluated by 3.0 Tesla in vivo magnetic resonance imaging. Spine J. 2017, 17, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Fabela-Sánchez, O.; Salgado–Ceballos, H.; Medina-Torres, L.; Alvarez-Mejía, L.; Sanchez-Torres, S.; Mondragón-Lozano, R.; Morales-Guadarrama, A.; Diaz-Ruiz, A.; Olayo, M.-G.; Cruz, G.J.; et al. Effect of the combined treatment of albumin with plasma synthesised pyrrole polymers on motor recovery after traumatic spinal cord injury in rats. J. Mater. Sci. Mater. Electron. 2017, 29, 13. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, S.; Díaz-Ruíz, A.; Ríos, C.; Olayo, M.G.; Cruz, G.J.; Olayo, R.; Morales, J.; Mondragon-Lozano, R.; Fabela, O.; Orozco-Barrios, C.; et al. Recovery of motor function after traumatic spinal cord injury by using plasma-synthesized polypyrrole/iodine application in combination with a mixed rehabilitation scheme. J. Mater. Sci. Mater. Med. 2020, 31, 1–18. [Google Scholar] [CrossRef]
- Olayo, R.; Ríos, C.; Salgado-Ceballos, H.; Cruz, G.J.; Morales, J.; Olayo, M.G.; Alcaraz-Zubeldia, M.; Alvarez-Mejía, L.; Mondragon-Lozano, R.; Morales, A.; et al. Tissue spinal cord response in rats after implants of polypyrrole and polyethylene glycol obtained by plasma. J. Mater. Sci. Mater. Electron. 2008, 19, 817–826. [Google Scholar] [CrossRef]
- Cruz, G.J.; Mondragon-Lozano, R.; Díaz-Ruíz, A.; Manjarrez, J.; Olayo, R.; Salgado-Ceballos, H.; Olayo, M.-G.; Morales, J.; Alvarez-Mejía, L.; Morales, A.; et al. Plasma polypyrrole implants recover motor function in rats after spinal cord transection. J. Mater. Sci. Mater. Electron. 2012, 23, 2583–2592. [Google Scholar] [CrossRef]
- Morales-Guadarrama, A.; Salgado-Ceballos, H.; Grijalva, I.; Morales, J.; Ríos, C.; Cruz, G.J.; Diaz-Ruiz, A.; Olayo, M.-G.; Alvarez-Mejía, L.; Mondragón-Lozano, R.; et al. Spinal cord injury of rhesus monkey implanted with PPy/I plasma polymer, MRI study. In Proceedings of the VI Latin American Congress on Biomedical Engineering CLAIB 2014, Paraná, Argentina, 29–31 October 2014; pp. 174–177. [Google Scholar]
- Hosono, K.; Matsubara, I.; Murayama, N.; Shin, W.; Izu, N.; Kanzaki, S. Structure and properties of plasma polymerized and 4-ethylbenzenesulfonic acid-doped polypyrrole films. Thin Solid Films 2003, 441, 72–75. [Google Scholar] [CrossRef]
- Cruz, G.; Morales, J.; Olayo, R. Films obtained by plasma polymerization of pyrrole. Thin Solid Films 1999, 342, 119–126. [Google Scholar] [CrossRef]
- Colin, E.; Olayo, M.; Cruz, G.; Carapia, L.; Morales, J.; Olayo, R. Affinity of amine-functionalized plasma polymers with ionic solutions similar to those in the human body. Prog. Org. Coat. 2009, 64, 322–326. [Google Scholar] [CrossRef]
- Kumar, S.; Nakamura, K.; Nishiyama, S.; Ishii, S.; Noguchi, H.; Kashiwagi, K.; Yoshida, Y. Optical and electrical characterization of plasma polymerized pyrrole films. J. Appl. Phys. 2003, 93, 2705–2711. [Google Scholar] [CrossRef]
- Cruz, G.; Morales, J.; Castillo-Ortega, M.; Olayo, R. Synthesis of polyaniline films by plasma polymerization. Synth. Met. 1997, 88, 213–218. [Google Scholar] [CrossRef]
- Fonseca, L.M.M.; Medeiros, M.J.; Góes, F.S.N.; Zamberlan-Amorim, N.E.; Scochi, C.G.S. Evaluation of the digital learning object taking care of the sensory environment in neonatal units: Noise, light and handling. Procedia Soc. Behav. Sci. 2012, 46, 509–514. [Google Scholar] [CrossRef][Green Version]
- Baji, A.; Mai, Y.-W.; Wong, S.-C.; Abtahi, M.; Chen, P. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Hamprecht, B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Adv. Appl. Microbiol. 1977, 49, 99–170. [Google Scholar] [CrossRef]
- Israël, M.; Lesbats, B.; Synguelakis, M.; Joliot, A. Acetylcholine accumulation and release by hybrid NG108-15, glioma and neuroblastoma cells—Role of a 16 kDA membrane protein in release. Neurochem. Int. 1994, 25, 103–109. [Google Scholar] [CrossRef]
- Molnar, P.; Hickman, J.J. Modeling of action potential generation in NG108-15 cells. Adv. Struct. Saf. Stud. 2014, 1183, 253–261. [Google Scholar] [CrossRef]
- Tojima, T.; Yamane, Y.; Takahashi, M.; Ito, E. Acquisition of neuronal proteins during differentiation of NG108-15 cells. Neurosci. Res. 2000, 37, 153–161. [Google Scholar] [CrossRef]
- Osorio-Londoño, D.; Godínez-Fernández, J.R.; Acosta-García, M.C.; Morales-Corona, J.; Olayo-González, R. Morphology and viability of nerve cells cultured on plasma polymerized polypyrrole-coated scaffolds. In Proceedings of the VIII Latin American Conference on Biomedical Engineering and XLII National Conference on Biomedical Engineering, Cancún, Mexico, 2–5 October 2019; pp. 652–655. [Google Scholar]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.; van de Vosse, F.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2011, 11, 461–473. [Google Scholar] [CrossRef]
- Sage, D. OrientationJ: A Series of ImageJ Plugins for Directional Image Analysis. (n.d.). Available online: http://bigwww.epfl.ch/demo/orientation/ (accessed on 24 March 2021).
- Stoddart, M.J. (Ed.) Mammalian Cell Viability. Methods and Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 740. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R. 2020. Available online: www.rstudio.com/ (accessed on 7 October 2021).
- Stuard, B.H. Infrared Spectroscopy: Fundamentals and Applications; Analytical Techniques in the Sciences; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- St. Thomas. Spectroscopic Tools. (n.d.). Available online: http://www.science-and-fun.de/tools/ (accessed on 15 March 2021).
- Morales, J.; Olayo, M.G.; Cruz, G.J.; Olayo, R. Plasma polymerization of random polyaniline-polypyrrole-iodine copolymers. J. Appl. Polym. Sci. 2002, 85, 263–270. [Google Scholar] [CrossRef]
- Vasquez-Ortega, M.; Ortega, M.; Morales, J.; Olayo, M.G.; Cruz, G.J.; Olayo, R. Core-shell polypyrrole nanoparticles obtained by atmospheric pressure plasma polymerization. Polym. Int. 2014, 63, 2023–2029. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, W.-C.; Tong, B.; Yang, R.-J. Crystallization, mechanical and flame-retardant properties of poly(lactic acid) composites with DOPO and DOPO-POSS. Chin. J. Polym. Sci. 2018, 36, 871–879. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Chen, S.; Qin, J.; Chen, X.; Zhou, D.; Wu, H. Synthesis, characterization, and crystallization behaviors of poly(D-lactic acid)-based triblock copolymer. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Hara, S.; Watanabe, S.; Takahashi, K.; Shimizu, S.; Ikake, H. Preparation of crystallites for oriented poly(lactic acid) films using a casting method under a magnetic field. Polymers 2018, 10, 1083. [Google Scholar] [CrossRef]
- Qin, L.; Qiu, J.; Liu, M.; Ding, S.; Shao, L.; Lü, S.; Zhang, G.; Zhao, Y.; Fu, X. Mechanical and thermal properties of poly(lactic acid) composites with rice straw fiber modified by poly(butyl acrylate). Chem. Eng. J. 2011, 166, 772–778. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Zhang, W.; Li, J.; Huang, S.; Meng, Y.; Christiansen, J.D.C.; Yu, D.; Wu, Z.; Jiang, S. Thermal strain-induced cold crystallization of amorphous poly(lactic acid). CrystEngComm 2016, 18, 3237–3246. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Lins, L.C.; Wianny, F.; Livi, S.; Dehay, C.; Duchet-Rumeau, J.; Gérard, J.-F. Effect of polyvinylidene fluoride electrospun fiber orientation on neural stem cell differentiation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2376–2393. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Martín-Pat, G.E.; Rodriguez-Fuentes, N.; Cervantes-Uc, J.M.; Rosales-Ibáñez, R.; Carrillo-Escalante, H.J.; Ku-Gonzalez, A.F.; Avila-Ortega, A.; Hernandez-Sanchez, F. Effect of different exposure times on physicochemical, mechanical and biological properties of PGS scaffolds treated with plasma of iodine-doped polypyrrole. J. Biomater. Appl. 2020, 35, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications —A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Elsawy, M.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Wickman, G.R.; Julian, L.; Mardilovich, K.; Schumacher, S.E.; Munro, J.; Rath, N.; Al Zander, S.; Mleczak, A.; Sumpton, D.; Morrice, N.; et al. Blebs produced by actin–myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 2013, 20, 1293–1305. [Google Scholar] [CrossRef]
- Tixeira, R.; Caruso, S.; Paone, S.; Baxter, A.A.; Atkin-Smith, G.K.; Hulett, M.D.; Poon, I.K.H. Defining the morphologic features and products of cell disassembly during apoptosis. Apoptosis 2017, 22, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Serratos, I.N.; Olayo, R.; Millan-Pacheco, C.; Morales-Corona, J.; Vicente-Escobar, J.O.; Soto-Estrada, A.M.; Córdoba-Herrera, J.G.; Uribe, O.; Gómez-Quintero, T.; Arroyo-Ornelas, M.Á.; et al. Modeling integrin and plasma-polymerized pyrrole interactions: Chemical diversity relevance for cell regeneration. Sci. Rep. 2019, 9, 7009. [Google Scholar] [CrossRef]
- Assoian, R.K. Anchorage-dependent cell cycle progression. J. Cell Biol. 1997, 136, 1–4. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Yamada, K.M.; Galbraith, J.A. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science 2007, 315, 992–995. [Google Scholar] [CrossRef] [PubMed]
- ATCC. NG108-15 (ATCC ® HB-12317 TM. 2020. Available online: https://www.atcc.org (accessed on 7 October 2021).
- Delcroix, G.J.-R.; Schiller, P.C.; Benoit, J.-P.; Montero-Menei, C.N. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials 2010, 31, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Daud, M.F.; Pawar, K.; Claeyssens, F.; Ryan, A.; Haycock, J.W. An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials 2012, 33, 5901–5913. [Google Scholar] [CrossRef] [PubMed]














| Scaffold | 16.7° Peak Intensity | 19° Peak Intensity | Degree of Crystallinity |
|---|---|---|---|
| rPLA | - | - | 0.07 |
| aPLA | - | - | 0.14 |
| rPLA+pPPy | 629 | - | 0.06 |
| aPLA+pPPy | 1821 | 401 | 0.18 |
| rPLA+pPPy/I | 1669 | 411 | 0.16 |
| aPLA+pPPy/I | 3728 | 410 | 0.19 |
| Scaffolds | PLA | PLA+pPPy | PLA+pPPy/I |
|---|---|---|---|
| Tg (°C) | 60 | 56.5 | 60 |
| Tcc (°C) | 87.44 | 121.04 | 106.73 |
| Tm (°C) | 167.58 | 159.47 | 163.25 |
| Tm1 (°C) | - | 153.65 | 158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Londoño, D.M.; Godínez-Fernández, J.R.; Acosta-García, M.C.; Morales-Corona, J.; Olayo-González, R.; Morales-Guadarrama, A. Pyrrole Plasma Polymer-Coated Electrospun Scaffolds for Neural Tissue Engineering. Polymers 2021, 13, 3876. https://doi.org/10.3390/polym13223876
Osorio-Londoño DM, Godínez-Fernández JR, Acosta-García MC, Morales-Corona J, Olayo-González R, Morales-Guadarrama A. Pyrrole Plasma Polymer-Coated Electrospun Scaffolds for Neural Tissue Engineering. Polymers. 2021; 13(22):3876. https://doi.org/10.3390/polym13223876
Chicago/Turabian StyleOsorio-Londoño, Diana María, José Rafael Godínez-Fernández, Ma. Cristina Acosta-García, Juan Morales-Corona, Roberto Olayo-González, and Axayácatl Morales-Guadarrama. 2021. "Pyrrole Plasma Polymer-Coated Electrospun Scaffolds for Neural Tissue Engineering" Polymers 13, no. 22: 3876. https://doi.org/10.3390/polym13223876
APA StyleOsorio-Londoño, D. M., Godínez-Fernández, J. R., Acosta-García, M. C., Morales-Corona, J., Olayo-González, R., & Morales-Guadarrama, A. (2021). Pyrrole Plasma Polymer-Coated Electrospun Scaffolds for Neural Tissue Engineering. Polymers, 13(22), 3876. https://doi.org/10.3390/polym13223876








