Photochemical Stability of a Cotton Fabric Surface Dyed with a Reactive Triphenodioxazine Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Color Modifications Measurements
2.2.2. Ultraviolet-Visible (UV-Vis) Absorption Spectra
2.2.3. Irradiation
2.2.4. Near Infrared Chemical Imaging Spectroscopy (NIR-CI)
2.2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results
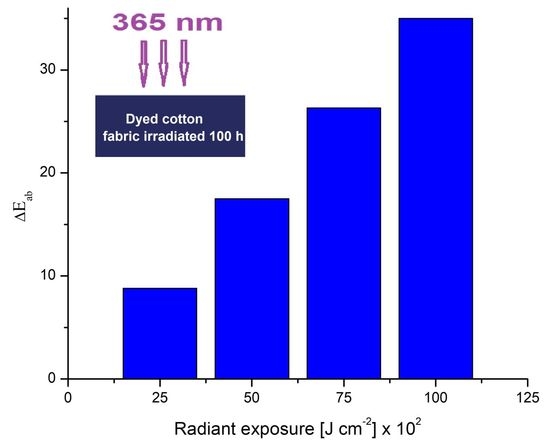
3.1. Color Modification Studies
3.2. Study of the Variation of Color Intensity as a Function of Irradiation Time and Different Dye Concentrations
3.3. Quantitative Spectrophotometric Analysis in UV-Vis for the Reactive Dye RB_204
3.4. Structural Modification Studies by FTIR
3.5. Spectrophotometric Determination of the Concentration and Amount of Extracted Dye
3.5.1. Results Obtained on Non-Irradiated Samples in a Neutral Medium
3.5.2. Results Obtained on Irradiated Samples by Extraction in Alkaline Medium
3.5.3. Results Obtained on Irradiated Samples by Extraction in Acid Medium
3.6. Structural Modifications Studies by NIR-CI Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and Dyes: Developments and Applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.M. Developments in the chemistry and their application process. Color. Technol. 2014, 130, 382–412. [Google Scholar] [CrossRef]
- Ristić, I.; Zdravkavić, A.; Mičić, A.; Nicolič, D.M.; Ristić, N. Ecological alternatives to conventional dyeing of cotton with reactive dyeis. Zasta Mater. 2020, 61, 60–68. [Google Scholar]
- Klemola, K.; Pearson, J.; Lindstrom-Seppä, P. Toxicity of reactives dyes and dyed fabrics with HaCaT citotoxicity test. AUTEXT Res. J. 2007, 7, 217–223. [Google Scholar]
- Letašiová, S.; Medved’ová, A.; Šovčíková, A.; Dušinská, M.; Volkovová, K.; Mosoiu, C.; Bartonová, A. Bladder cancer, a review of the environmental risk factors. Environ. Health 2012, 11, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Suryavathi, V.; Sharma, S.; Sharma, S.; Saxena, P.; Pandey, S.; Grover, R.; Kumar, S.; Sharma, K.P. Acute toxicity of textile dye effluents (untreated and treated) of Sanganer on male reproductive systems of albino rats and mice. Reprod. Toxicol. 2005, 19, 547–556. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Wu, M.-T.; Lee, J.C.-M.; Cheng, T.-Y. Isothermal adsorption properties for the adsorption and removal of reactive Blue 221 dye from aqueous solutions by cross-linked β-chitosan glycan as acid-resistant adsorbent. Polymers 2018, 10, 1328. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.A.S. The dyeing of cellulosic fibres with reactive dyes. Adv. Colour Sci. Technol. 1998, 1, 1–11. [Google Scholar]
- Renfrew, A.H.M. Reactive Dyes for cellulose: Replacement of anthraquinone blues by triphenodioxazines. Rev. Prog. Coloration Relat. Top. 1985, 15, 15. [Google Scholar] [CrossRef]
- Taylor, J.A. Recent developments in reactive dyes. Rev. Prog. Coloration Relat. Top. 2000, 30, 93–107. [Google Scholar] [CrossRef]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Kanetkar, V.R.; Shankarling, G.S.; Patil, S. Recent developments in reactive dyes-Part III: New chromophoric and reactive systems introduced. Colourage 2001, 48, 49–52. [Google Scholar]
- Fang, K.; Shu, D.; Liu, X.; Cai, Y.; An, F.; Zhang, X. Reactive pad-steam dyeing of cotton fabric modified with cationic P(St-BA-VBT) nanospheres. Polymers 2018, 10, 564. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Du, S.; Yan, S.; Yu, X.; Zhang, Z.; Zhang, S. Salt-free dyeing of modified cotton through graft polymerization with highly enhanced dye fixation and good strength properties. Polymers 2020, 12, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusic, H.; Koprivanac, N.; Bozic, A.L. Environmental aspects on the photodegradation of reactive triazine dyes in aqueous media. J. Photochem. Photobiol. A Chem. 2013, 252, 131–144. [Google Scholar] [CrossRef]
- Chatterjee, D.; Rupini Patnam, V.; Sikdar, A.; Joshi, P.; Misra, R.; Rao, N.N. Kinetics of the decoloration of reactive dyes over visible light-irradiated TiO2 semiconductor photocatalyst. J. Hazard. Mater. 2008, 156, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.R.; Pons, M.N.; Zahraa, O. Photocatalytic degradation of three azo dyes using immobilized TiO2 nanoparticles on glass plates activated by UV light irradiation: Influence of dye molecular structure. J. Hazard. Mater. 2009, 168, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Rosu, L.; Rosu, D.; Gavat, C.-C.; Varganici, C.-D. Photochemical stability of cellulose textile surfaces painted with some reactive azo-triazine dyes. J. Mater. Sci. 2014, 49, 4469–4480. [Google Scholar] [CrossRef]
- Dong, X.; Xing, T.; Chen, G. Durable antipilling modification of cotton fabric with chloropyrimidine compounds. Polymers 2019, 11, 1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolut. Technol. 2011, 8, 15–28. [Google Scholar] [CrossRef]
- Williams, R.S. Chapter 7: Weathering of wood. In Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005; p. 151. [Google Scholar]
- Huang, X.; Kocaefe, D.; Kocaefe, Y.; Boluk, Y.; Pichette, A. A spectrometric and chemical study on color modification of heat-treated wood during artificial weathering. Appl. Surf. Sci. 2012, 258, 5360–5369. [Google Scholar] [CrossRef]
- McKellar, J.F. The photo-oxidation of an aromatic amine studied by flash photolysis. Proc. R. Soc. Lond. A 1965, 287, 363–380. [Google Scholar]
- Wojnárovits, L.; Takács, E. Irradiation treatment of azo dye containing wastewater: An overview. Rad. Phys. Chem. 2008, 77, 225–244. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, R.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Osipov, V.Y.; Romanov, N.M.; Kogane, K.; Touhara, H.; Hattori, Y.; Takai, K. Intrinsic infrared absorption for carbon–fluorine bonding in fluorinated nanodiamond. Mendeleev Commun. 2020, 30, 84–87. [Google Scholar] [CrossRef]
- Cocca, M.; Arienzo, L.D.; Orazio, L.D. Effects of different artificial agings on structure and properties of Whatman paper samples. ISRN Mat. Sci. 2011, 7, 863083. [Google Scholar] [CrossRef] [Green Version]
- Hon, N.S. Fundamental degradation processes relevant to solar irradiation of cellulose: ESR studies. J. Macromol. Sci. Pure Appl. Chem. 1976, 10, 1175–1192. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.-D.; Rosu, D. Theoretical aspects regarding polymer photochemistry. In Photochemical Behavior of Multicomponent Polymeric-Based Materials; Rosu, D., Visakh, P.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Bhatt, I.A.; Adeel, S.; Siddique, S.; Abbas, M. Effect of UV radiation on the dyeing of cotton fabric with reactive blue 13. J. Saudi Chem. Soc. 2014, 18, 606–609. [Google Scholar] [CrossRef] [Green Version]
- Ghaffar, A.; Adeel, S.; Habib, N.; Jalal, F.; Haq, A.; Munir, B.; Ahmad, A.; Jahangeer, M.; Jamil, Q. Effects of Microwave Radiation on Cotton Dyeing with Reactive Blue 21 Dye. Pol. J. Environ. Stud. 2019, 28, 1–5. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Aromatic and heteroaromatic rings. In Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 261–288. [Google Scholar]
- Patil, S.; Choudhary, A.; Sekar, N.; Pipliya, P.; Rathod, P. Synthesis and optical properties of Near-Infrared (NIR) absorbing azo dyes. Curr. Trends Fash. Technol. Text. Eng. 2019, 4, 555649. [Google Scholar] [CrossRef]
- Salvador, M.A.; Almeida, P.; Reis, L.V.; Santos, P. Near-infrared delocalized cationic azo dyes. Dyes Pigment. 2009, 82, 118–123. [Google Scholar] [CrossRef]
- Li, M.; Han, G.; Jiang, W.; Zhou, C.; Zhang, Y.; Wang, S.; Su, J.; Li, X. Rapid identification of plant- and chemical-dyed cotton fabrics using the near-infrared technique. Text. Res. J. 2020, 90, 2275–2283. [Google Scholar] [CrossRef]
- Liu, Y.; Chris Delhom, C.; Campbell, B.T.; Martin, V. Application of near infrared spectroscopy in cotton fiber micronaire measurement. Inf. Process. Agric. 2016, 3, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, J.; Beck, K. NIR Characterization and measurement of the cotton content of dyed blend fabrics. Text. Res. J. 2009, 79, 675–686. [Google Scholar] [CrossRef]
- Rodgers, J.; Ghosh, S. NIR analysis of textiles. In Handbook of Near-Infrared Analysis, 3rd ed.; Burns, D., Ciurczak, E., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Varganici, C.-D.; Rosu, L.; Mocanu (Paduraru), O.-M.; Rosu, D. Influence of poly (vinyl alcohol) on cellulose photochemical stability in cryogels during UV irradiation. J. Photochem. Photobiol. A Chem. 2015, 297, 20–30. [Google Scholar] [CrossRef]

















| Name | Reactive Blue_204 (RB_204) |
|---|---|
| Structural formula |  |
| Molecular formula | C42H28O20N14S6Cl2F2Na6 |
| Molecular weight (Mw) | 1487.97 g/mol |
| λmax | 260 nm; 636 nm |
| IUPAC name | Hexasodium-6,13-dichloro-3,10-bis((4-(2,5-disulfonatoanilino)-6-fluoro-1,3,5- triazin-2-ylamino)prop-3-ylamino)-5,12-dioxa-7,14-diazapentacene-4,11-disulfonate |
| CAS name | 4,11-Triphenodioxazinedisulfonic-acid,6,13-dichloro-3,10-bis((3-((4-((2,5-disulfo- phenyl) amino)-6-fluoro-1,3,5-triazin-2yl)amino) propyl) amino)-, hexasodium salt |
| Conc. | Irradiation Time (h) | Radiant Exposure (J cm−2 × 102) | L*/(STD) | a*/(STD) | b*/(STD) | ΔE*/(STD) | ΔL*/(STD) | Δa*/(STD) | Δb*/(STD) |
|---|---|---|---|---|---|---|---|---|---|
| 1% | 0 | 0 | 34.5/(0.34) | 14.4/(1.64) | −33.2/(1.86) | - | - | - | - |
| 25 | 8.8 | 37.0/(0.75) | 12.7/(0.99) | −30.8/(0.53) | 3.9/(1.00) | 2.5/(0.50) | −1.7/(0.64) | 2.4/(0.24) | |
| 50 | 17.5 | 37.3/(1.11) | 1.1/(1.01) | −27.5/1.21) | 7.2/(0.85) | 2.8/(0.47) | −3.3/(0.58) | 5.7/(0.58) | |
| 75 | 26.3 | 35.9/(0.95) | 8.3/(0.81) | −24.3/(1.17) | 10.9/(0.90) | 1.4/(0.43) | −6.1 (0.61) | 8.9/(0.79) | |
| 100 | 35.0 | 35.1/(1.05) | 7.6/(0.78) | −21.0/(1.10) | 14.0/(0.98) | 0.6/(0.11) | −6.8 (0.50) | 12.2 (0.8) | |
| 3% | 0 | 0 | 23.3/(0.85 | 17.6/(0.51) | −34.2/(0.89) | - | - | - | - |
| 25 | 8.8 | 26.8/(0.76 | 14.3/(0.68) | −31.2/(0.78) | 5.7/(0.82) | 3.5/(0.31) | −3.3/(0.31) | 3.0/(0.42) | |
| 50 | 17.5 | 27.2/(0.68) | 13.9/(0.71) | −29.9/(0.86) | 6.9/(0.89) | 3.9/(0.35) | −3.7/(0.72) | 4.3/(0.31) | |
| 75 | 26.3 | 24.6/(0.62 | 13.7/(0.43) | −28.7/(1.21) | 7.0/(0.76) | 3.0/(0.33) | −3.9/(0.68) | 5.5/(0.27) | |
| 100 | 35.0 | 23.7/(0.57) | 13.0/(0.57) | −25.4/(1.05) | 9.9/(0.95) | 0.4/(0.1) | −4.6/(0.55) | 8.8/(0.50) | |
| 5% | 0 | 0 | 19.7/(0.62) | 16.6/(0.44) | −32.9/(0.79) | - | - | - | - |
| 25 | 8.9 | 19.0/(0.51) | 14.1/(0.42) | −29.7/(0.87) | 4.1/(0.71) | −0.7/(0.1) | −2.5/(0.67) | 3.2/(0.6) | |
| 50 | 17.5 | 18.4/(0.58) | 10.8/(0.54) | −28.7/(0.86 | 7.3/(0.65) | −1.3 (0.23) | −5.8/(0.63) | 4.2/(0.44) | |
| 75 | 26.3 | 17.9/(0.65) | 8.9/(0.65) | −27.8/(0.95) | 9.1/(0.38) | −1.8 (0.21) | −7.7/(0.78) | 5.1/(0.30) | |
| 100 | 35.0 | 20.2/(0.49) | 8.4/(0.71) | −27.1/(0.93) | 10.1/(0.24) | 0.51 (0.1) | −8.2/(0.28) | 5.8/(0.26) |
| Concentration | Irradiation Time (h) | K/S Values | Discoloration Degree (%) |
|---|---|---|---|
| 1% | 0 | 2.24 | 0 |
| 25 | 1.71 | 23.661 | |
| 50 | 1.58 | 29.464 | |
| 100 | 1.31 | 41.518 | |
| 3% | 0 | 4.24 | 0 |
| 25 | 3.70 | 12.736 | |
| 50 | 3.41 | 19.575 | |
| 100 | 2.89 | 31.840 | |
| 5% | 0 | 5.31 | 0 |
| 25 | 4.46 | 16.008 | |
| 50 | 4.39 | 17.326 | |
| 100 | 3.82 | 28.060 |
| Concentration | Irradiation Time (h) | Dye Mass Variation During Irradiation (μg dye/g Dyed Fiber) |
|---|---|---|
| 1% | 0 | 5140 |
| 25 | 3924 | |
| 50 | 3626 | |
| 100 | 3006 | |
| 3% | 0 | 24,489 |
| 25 | 21,370 | |
| 50 | 19,695 | |
| 100 | 16,692 | |
| 5% | 0 | 39,892 |
| 25 | 33,506 | |
| 50 | 32,980 | |
| 100 | 28,698 |
| Group Type | Group Structure | Frequency | Current FTIR Frequency in the Studied Dye | |
|---|---|---|---|---|
| Nonirradiated | Irradiated 100 h | |||
| aryl sulfonate | Ar–SO3− | 1230–1120 1080–1025 | 1062 1087 | 1064 1086 |
| aryl-chlorine | Ar–Cl | 850–700 | 708 | 708 |
| fluoro-triazine |  | 1344 | ||
| dioxazine |  | 2100 | 2115 | 2115 |
| triazine |  | 1550 1410 | 1533 | - |
| alkyl-NH-aryl | –H2C–NH–Ar | 3450 | ||
| propylene | –CH2–CH2–CH2– | 740 | ||
| etheric |  | 1020–1075 1200–1275 | 1032 1240 | - 1025 1044 1239 |
| (alkyl)2NH- | –(CH2)2N– | 3310–3350 | 3334 | 3334 |
| Number of Determinations | Mass of Sample (g) | Absorbance | Extraction Time (h) | Extracted Dye Concentration (μg/mL) | Amount of Dye Extracted (μg/g Sample) |
|---|---|---|---|---|---|
| 1 | 0.1200 | 0.028 | 1 | 0.326 | 67.916 |
| 2 | 0.1158 | 0.036 | 2 | 0.420 | 90.674 |
| 3 | 0.1140 | 0.051 | 3 | 0.594 | 130.263 |
| 4 | 0.1128 | 0.075 | 4 | 0.874 | 193.706 |
| 5 | 0.1160 | 0.111 | 5 | 1.294 | 278.879 |
| 6 | 0.1130 | 0.153 | 6 | 1.783 | 394.469 |
| 7 | 0.1080 | 0.197 | 7 | 2.296 | 531.481 |
| 8 | 0.1120 | 0.241 | 8 | 2.809 | 627.009 |
| Number of Determinations | Mass of Sample (g) | Absorbance | Irradiation Time (h) | Extracted Dye Concentration (μg/mL) | Amount of Dye Extracted (μg/g Sample) |
|---|---|---|---|---|---|
| 1 | 0.1043 | 0.084 | 1 | 0.979 | 234.660 |
| 2 | 0.1050 | 0.100 | 2 | 1.166 | 277.619 |
| 3 | 0.1055 | 0.120 | 3 | 1.399 | 331.517 |
| 4 | 0.1047 | 0.145 | 4 | 1.690 | 403.534 |
| 5 | 0.1060 | 0.170 | 5 | 1.981 | 467.217 |
| 6 | 0.1061 | 0.206 | 6 | 2.401 | 565.740 |
| 7 | 0.1040 | 0.235 | 7 | 2.739 | 658.413 |
| 8 | 0.1053 | 0.283 | 8 | 3.298 | 783.000 |
| Number of Determinations | Mass of Sample (g) | Absorbance | Irradiation Time (h) | Extracted Dye Concentration (μg/mL) | Amount of Dye Extracted (μg/g Sample) |
|---|---|---|---|---|---|
| 1 | 0.1083 | 0.037 | 1 | 0.431 | 59.695 |
| 2 | 0.1100 | 0.059 | 2 | 0.688 | 93.818 |
| 3 | 0.1060 | 0.074 | 3 | 0.862 | 121.981 |
| 4 | 0.1072 | 0.092 | 4 | 1.072 | 150.000 |
| 5 | 0.1091 | 0.112 | 5 | 1.305 | 179.423 |
| 6 | 0.1077 | 0.135 | 6 | 1.573 | 219.081 |
| 7 | 0.1084 | 0.160 | 7 | 1.865 | 258.072 |
| 8 | 0.1088 | 0.184 | 8 | 2.145 | 295.726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosu, L.; Gavat, C.-C.; Rosu, D.; Varganici, C.-D.; Mustata, F. Photochemical Stability of a Cotton Fabric Surface Dyed with a Reactive Triphenodioxazine Dye. Polymers 2021, 13, 3986. https://doi.org/10.3390/polym13223986
Rosu L, Gavat C-C, Rosu D, Varganici C-D, Mustata F. Photochemical Stability of a Cotton Fabric Surface Dyed with a Reactive Triphenodioxazine Dye. Polymers. 2021; 13(22):3986. https://doi.org/10.3390/polym13223986
Chicago/Turabian StyleRosu, Liliana, Cristian-Catalin Gavat, Dan Rosu, Cristian-Dragos Varganici, and Fanica Mustata. 2021. "Photochemical Stability of a Cotton Fabric Surface Dyed with a Reactive Triphenodioxazine Dye" Polymers 13, no. 22: 3986. https://doi.org/10.3390/polym13223986