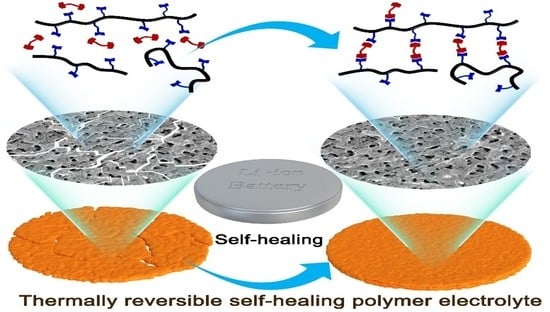
Self-Healing of a Covalently Cross-Linked Polymer Electrolyte Membrane by Diels-Alder Cycloaddition and Electrolyte Embedding for Lithium Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
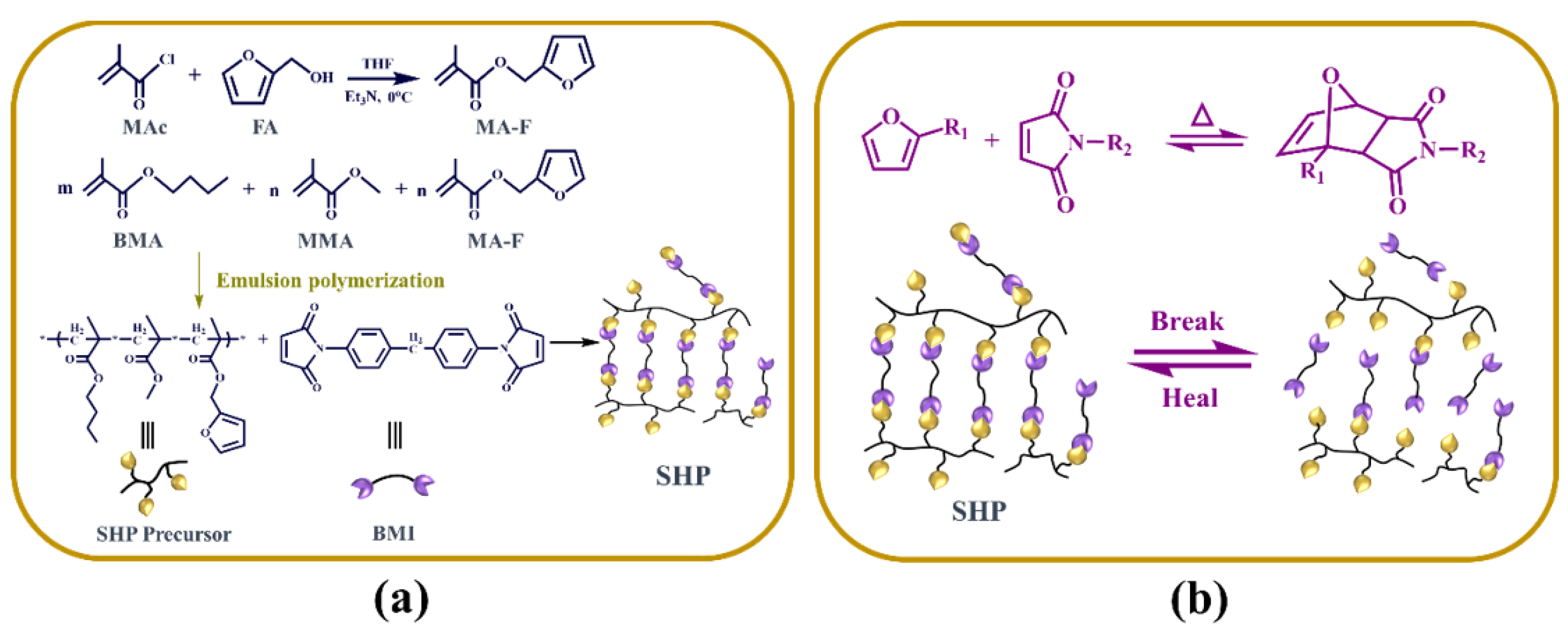
2.2. Preparation of the Functional Monomer Furfuryl Methacrylate
2.3. Preparation of the Self-Healing Polymer Precursor
2.4. Preparation of the Self-Healing Polymer Electrolyte Membrane
2.5. Fabrication of the Self-Healing Polymer Lithium Ion Battery
2.6. Characterizations and Electrochemical Tests
3. Results
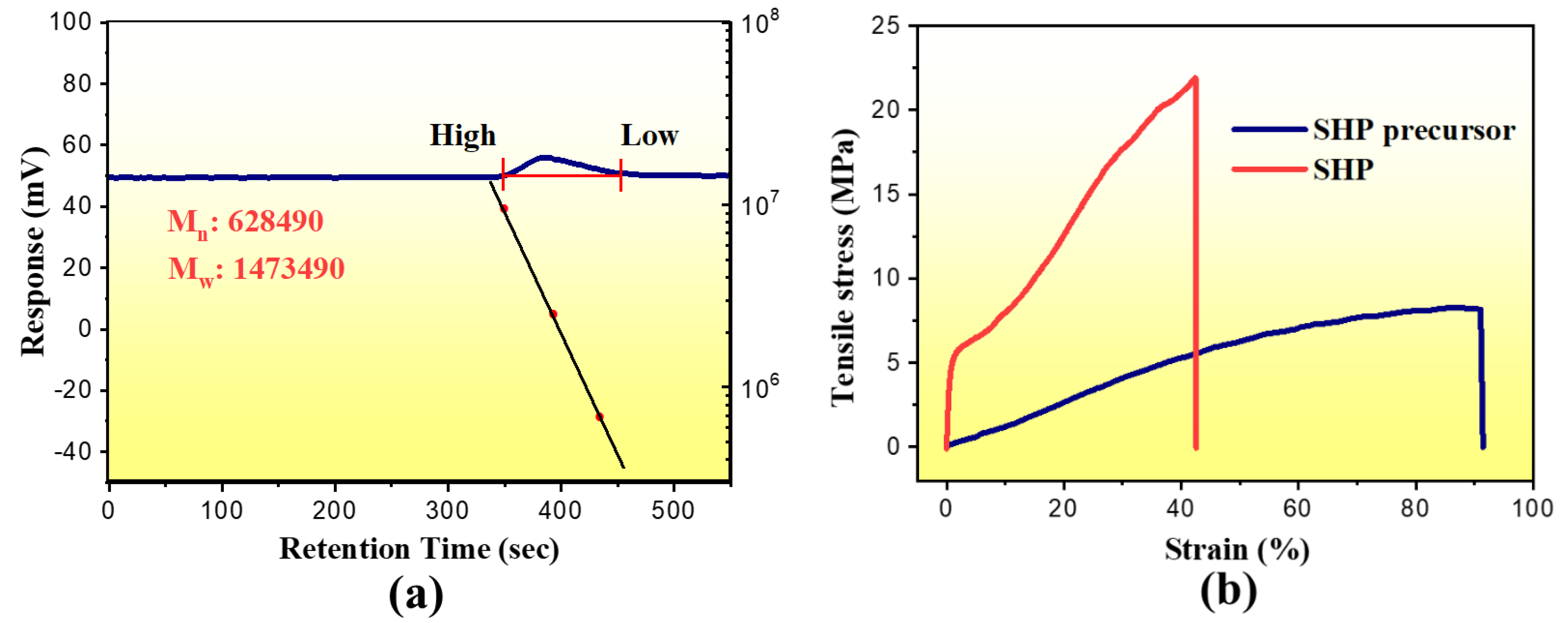
3.1. Synthesis and Characterization of SHP
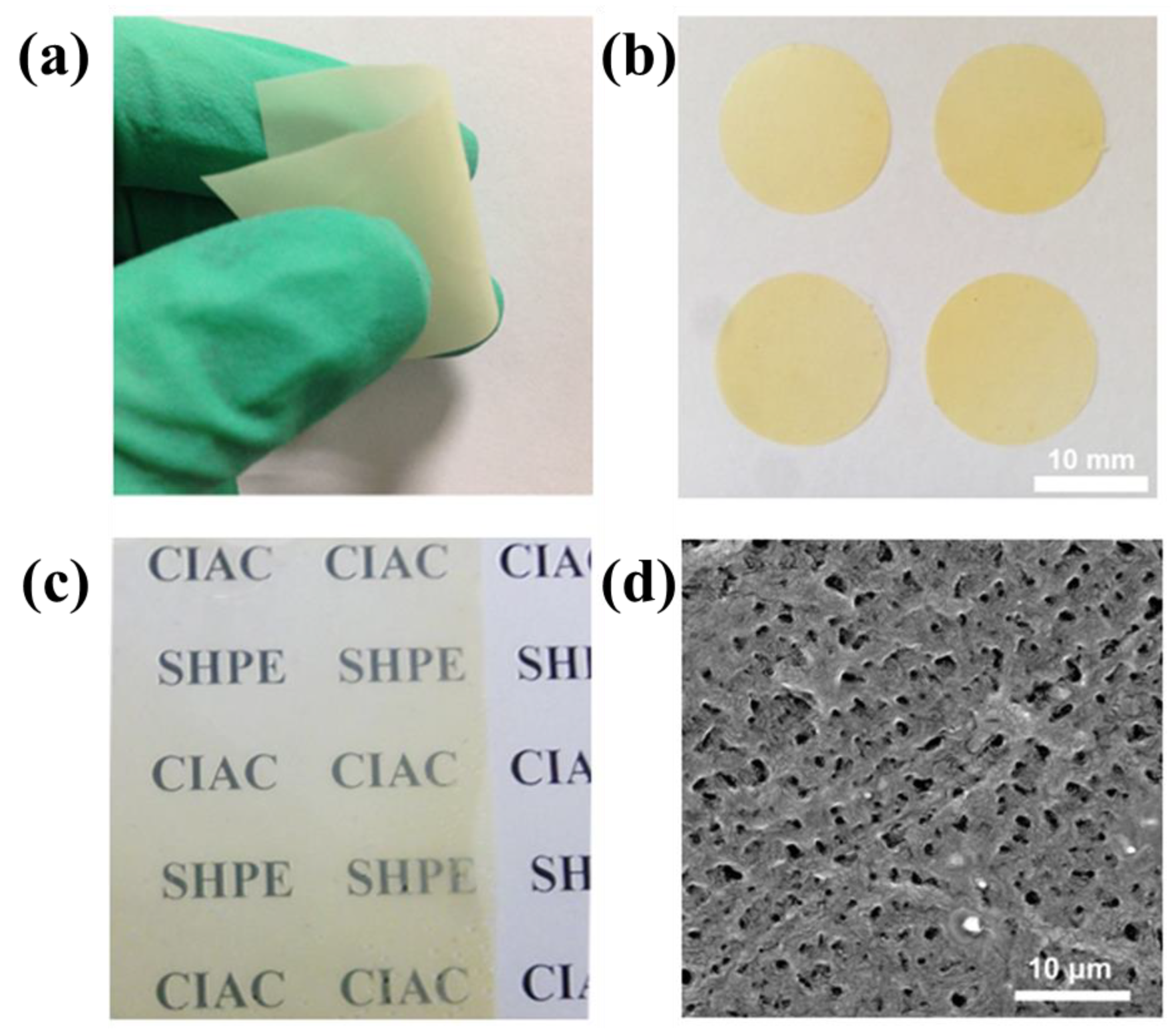
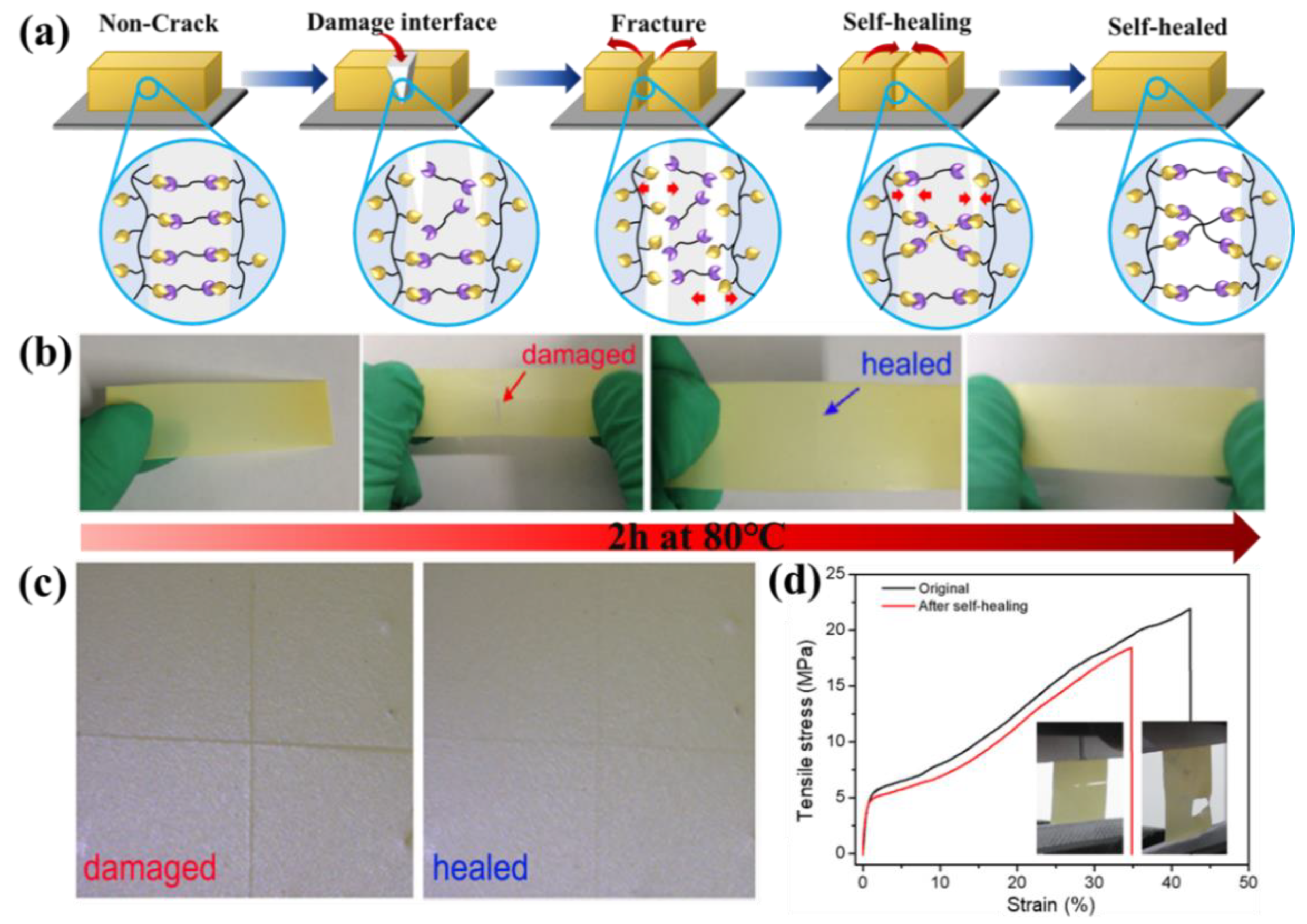
3.2. Self-Healing and Mechanical Property of the SHP Electrolyte Membranes
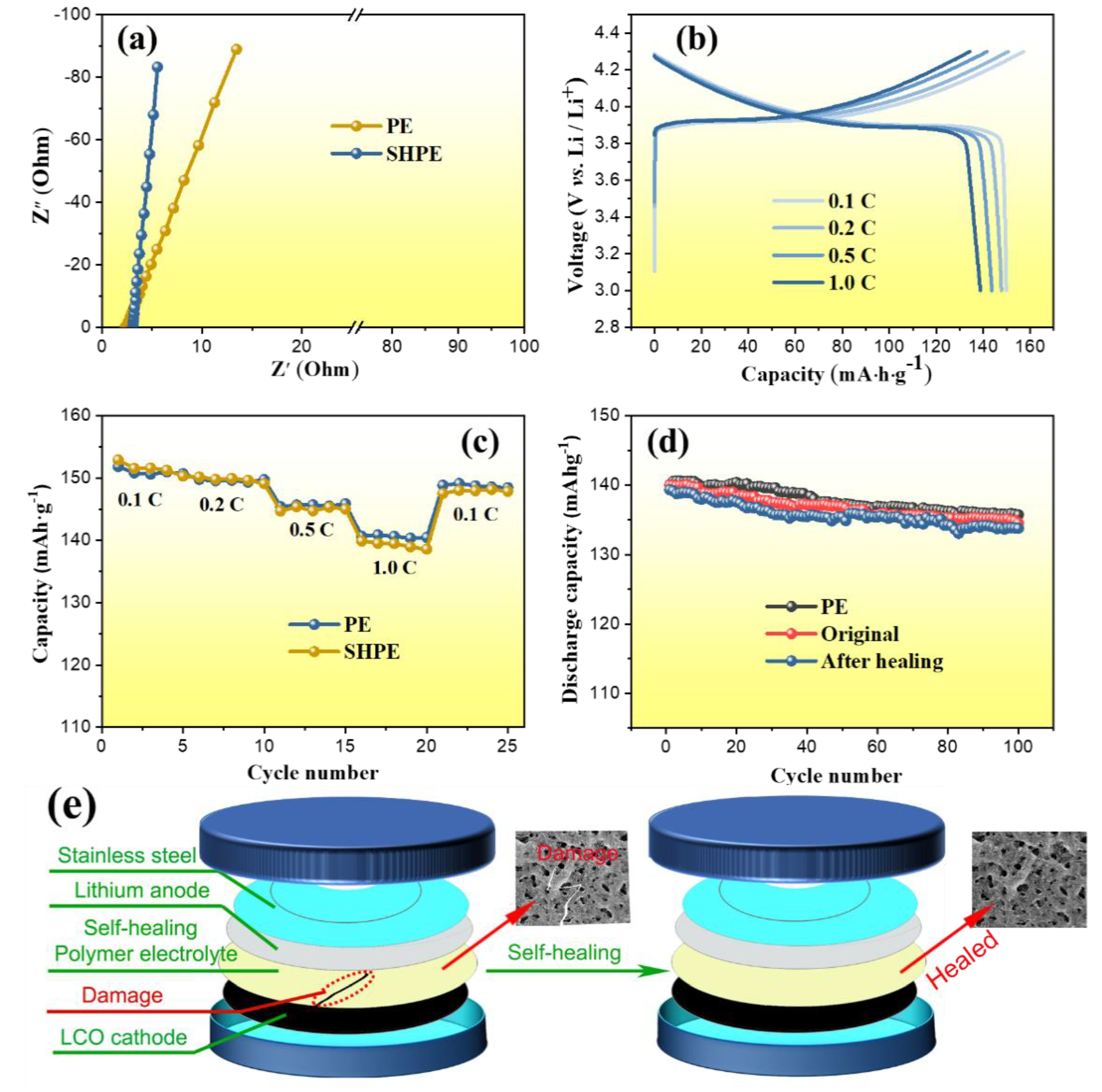
3.3. Electrochemical Performances of SHP Electrolyte Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.L.; Kushner, A.M.; Williams, G.A.; Guan, Z.B. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418. [Google Scholar] [CrossRef]
- Bergman, S.D.; Wudl, F. Mendable polymers. J. Mater. Chem. 2008, 18, 41–62. [Google Scholar] [CrossRef]
- Hager, M.D.; Greil, P.; Leyens, C.; Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Chappell, D.; Colquhoun, H.M.; Hayes, W. Healable polymeric materials: A tutorial review. Chem. Soc. Rev. 2010, 39, 1973–1985. [Google Scholar] [CrossRef] [Green Version]
- South, A.B.; Lyon, L.A. Autonomic self-healing of hydrogel thin films. Angew. Chem. Int. Ed. 2010, 49, 767–771. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Hansen, C.J.; Wu, W.; Toohey, K.S.; Sottos, N.R.; White, S.R.; Lewis, J.A. Self-healing materials with interpenetrating microvascular networks. Adv. Mater. 2009, 21, 4143–4147. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Dong, L.; Manjunatha, R.; Tan, M.; Yan, W.; Zhang, J. A review of self-healing electrode and electrolyte materials and their mitigating degradation of lithium batteries. Nano Energy 2021, 84, 105907. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Song, S.; Yang, H.; Cui, Y.; Tang, Y.; Chen, Y.; Yang, B.; Yuan, J.; Huang, J. Mussel inspired, self-healing polymer blends. Polymer 2020, 198, 122528. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Kramer, S.L.B.; Grady, M.E.; Mcllroy, D.A.; Moore, J.S.; Sottos, N.R.; White, S.R. Autonomic restoration of electrical conductivity. Adv. Mater. 2012, 24, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.; Wu, M.; Sun, J. Polyelectrolyte multilayers impart healability to highly electrically conductive films. Adv. Mater. 2012, 24, 4578–4582. [Google Scholar] [CrossRef] [PubMed]
- Palleau, E.; Reece, S.; Desai, S.C.; Smith, M.E.; Dickey, M.D. Self-healing stretchable wires for reconfigurable circuit wiring and 3D microfluidics. Adv. Mater. 2013, 25, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Haick, H. Self-healing, fully functional, and multiparametric flexible sensing platform. Adv. Mater. 2016, 28, 138–143. [Google Scholar] [CrossRef]
- Han, S.T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An overview of the development of flexible sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef]
- Huynh, T.P.; Haick, H. Autonomous flexible sensors for health monitoring. Adv. Mater. 2018, 30, 1802337. [Google Scholar] [CrossRef]
- Tee, B.C.K.; Wang, C.; Allen, R.; Bao, Z.N. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotech. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.J.; Li, S.; Lee, W.W.; Guo, H.; Cai, Y.; Wang, C.; Tee, B.C.K. Self-healing electronic skins for aquatic environments. Nat. Electron. 2019, 2, 75–82. [Google Scholar] [CrossRef]
- Wu, H.; Su, Z.; Shi, M.; Miao, L.; Song, Y.; Chen, H.; Han, M.; Zhang, H. Self-powered noncontact electronic skin for motion sensing. Adv. Funct. Mater. 2018, 28, 1704641. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Chen, Z.; McDowell, M.T.; Cui, Y.; Bao, Z.N. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 2013, 5, 1042–1048. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, S.; Oh, P.; Kim, Y.; Cho, J. High performance LiMn2O4 cathode materials grown with epitaxial layered nanostructure for Li-ion batteries. Nano Lett. 2014, 14, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K.; Chen, Z.H.; Noh, H.J.; Lee, D.J.; Jung, H.G.; Ren, Y.; Wang, S.; Yoon, C.S.; Myung, S.T.; Amine, K. Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 2012, 11, 942–947. [Google Scholar] [CrossRef]
- Zhu, Z.; Hong, M.; Guo, D.; Shi, J.; Tao, Z.; Chen, J. All-solid-state lithium organic battery with composite polymer electrolyte and pillar[5]quinone cathode. J. Am. Chem. Soc. 2014, 136, 16461–16464. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hackenberg, K.; Fu, Q.; Ajayan, P.M.; Ardebili, H. High ion conducting polymer nanocomposite electrolytes using hybrid nanofillers. Nano Lett. 2012, 12, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xie, Z.; Wang, J.; Yu, T.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Lithium-salt-containing ionic liquid-incorporated Li-Al-layered double hydroxide-based solid electrolyte with high-performance and safety in solid-state lithium batteries. ACS Sustain. Chem. Eng. 2020, 8, 12378–12387. [Google Scholar] [CrossRef]
- Nohara, T.; Koseki, K.; Tabata, K.; Shimada, R.; Suzuki, Y.; Umemoto, K.; Takeda, M.; Sato, R.; Rodbuntum, S.; Arita, T.; et al. Core size-dependent proton conductivity of silica filler-functionalized polymer electrolyte membrane. ACS Sustain. Chem. Eng. 2020, 8, 14674–14678. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Chen, Z.; Xu, K.; Lu, J. New concepts in electrolytes. Chem. Rev. 2020, 120, 6783–6819. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Rojaee, R.; Shahbazian-Yassar, R. Two-dimensional materials to address the lithium battery challenges. ACS Nano 2020, 14, 2628–2658. [Google Scholar] [CrossRef] [PubMed]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Natarajan, S.; Subramanyam, K.; Dhanalakshmi, R.B.; Stephan, A.M.; Aravindan, V. Regeneration of polyolefin separators from spent Li-ion batteryfor second life. Batter. Supercaps 2020, 3, 581–586. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Wang, H.; Wang, Q. Self-recovering tough gel electrolyte with adjustable supercapacitor performance. Adv. Mater. 2014, 26, 4370–4375. [Google Scholar] [CrossRef]
- Whiteley, J.M.; Taynton, P.; Zhang, W.; Lee, S.H. Ultra-thin solid-state Li-ion electrolyte membrane facilitated by a self-healing polymer matrix. Adv. Mater. 2015, 27, 6922–6927. [Google Scholar] [CrossRef]
- Liu, Y.; Chuo, T.W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Gan, H.; Zhang, Y.; Li, S.; Yu, L.; Wang, J.; Xue, Z. Self-healing single-ion conducting polymer electrolyte formed via supramolecular networks for lithium metal batteries. ACS Appl. Energy Mater. 2021, 4, 482–491. [Google Scholar] [CrossRef]
- Wu, N.; Shi, Y.; Lang, S.; Zhou, J.; Liang, J.; Wang, W.; Tan, S.; Yin, Y.; Wen, R.; Guo, Y. Self-healable solid polymeric electrolytes for stable and flexible lithium metal batteries. Angew. Chem. Int. Edit. 2019, 58, 18146–18149. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Cai, X.; Sun, Z.; Zhang, B.; Bao, Y.; Liu, Z.; Han, D.; Niu, L. Self-Healing of a Covalently Cross-Linked Polymer Electrolyte Membrane by Diels-Alder Cycloaddition and Electrolyte Embedding for Lithium Ion Batteries. Polymers 2021, 13, 4155. https://doi.org/10.3390/polym13234155
Chen L, Cai X, Sun Z, Zhang B, Bao Y, Liu Z, Han D, Niu L. Self-Healing of a Covalently Cross-Linked Polymer Electrolyte Membrane by Diels-Alder Cycloaddition and Electrolyte Embedding for Lithium Ion Batteries. Polymers. 2021; 13(23):4155. https://doi.org/10.3390/polym13234155
Chicago/Turabian StyleChen, Lijuan, Xisen Cai, Zhonghui Sun, Baohua Zhang, Yu Bao, Zhenbang Liu, Dongxue Han, and Li Niu. 2021. "Self-Healing of a Covalently Cross-Linked Polymer Electrolyte Membrane by Diels-Alder Cycloaddition and Electrolyte Embedding for Lithium Ion Batteries" Polymers 13, no. 23: 4155. https://doi.org/10.3390/polym13234155
APA StyleChen, L., Cai, X., Sun, Z., Zhang, B., Bao, Y., Liu, Z., Han, D., & Niu, L. (2021). Self-Healing of a Covalently Cross-Linked Polymer Electrolyte Membrane by Diels-Alder Cycloaddition and Electrolyte Embedding for Lithium Ion Batteries. Polymers, 13(23), 4155. https://doi.org/10.3390/polym13234155