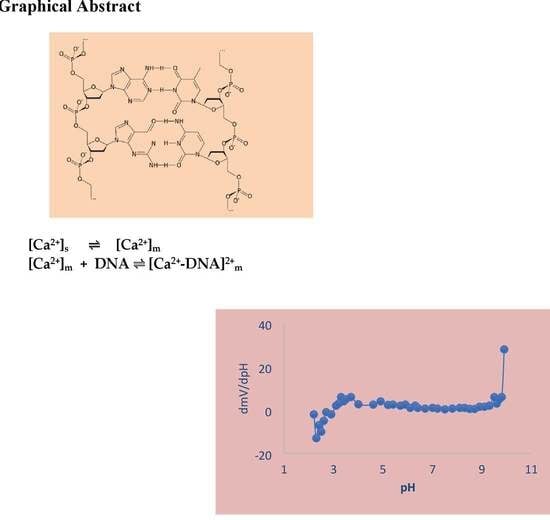
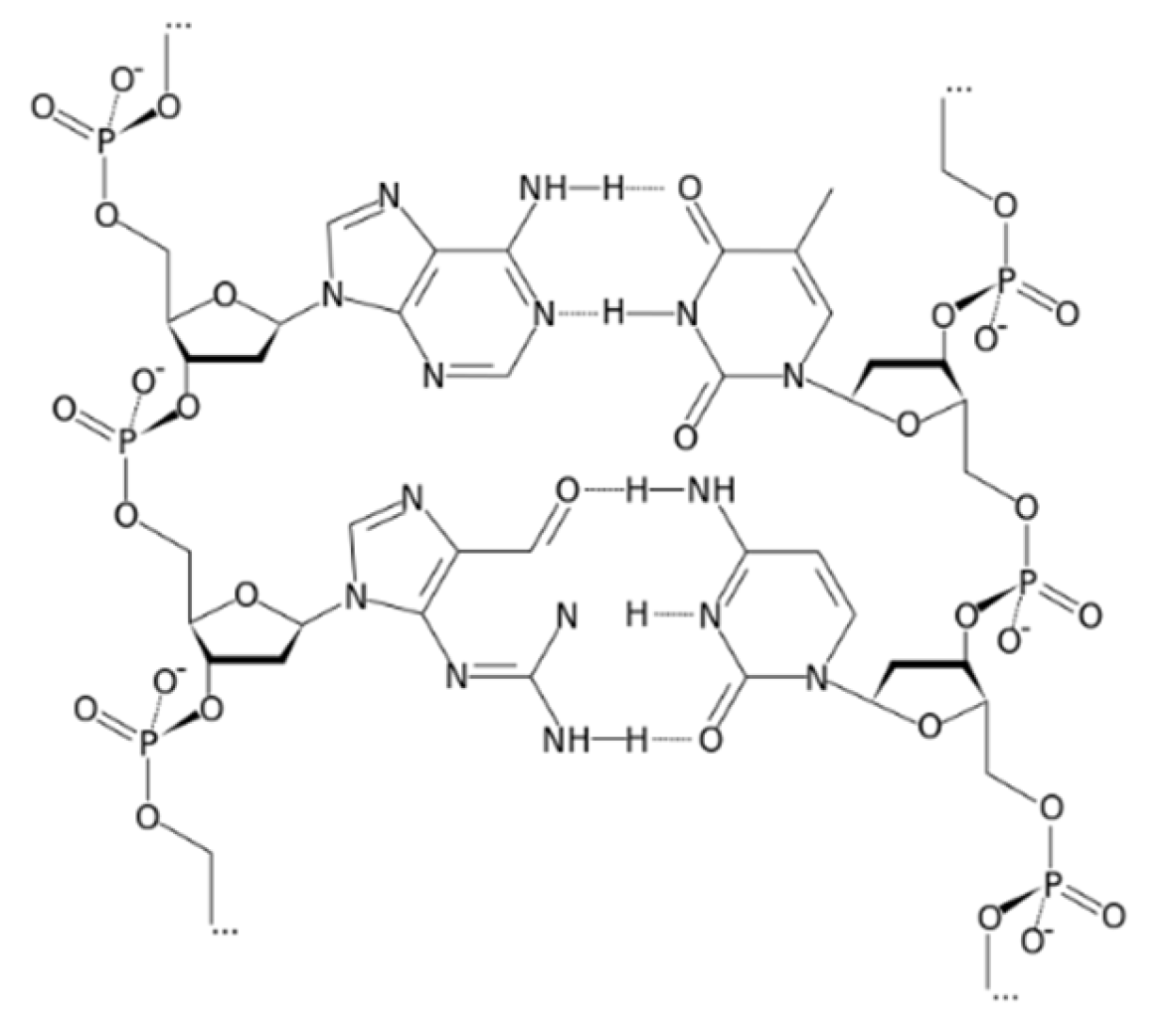
Polymeric Electrochemical Sensor for Calcium Based on DNA
Abstract
:1. Introduction
2. Experimental Methods
2.1. Reagents and Materials
2.2. Equipment
2.3. Electrode Preparation
2.4. Sensor Characterization Studies
| Silver–silver chloride (outer reference) | Test solution | Membrane | IF | Silver–silver chloride (inner reference) |
2.5. Determination of Ca in Real Samples
3. Results and Discussion
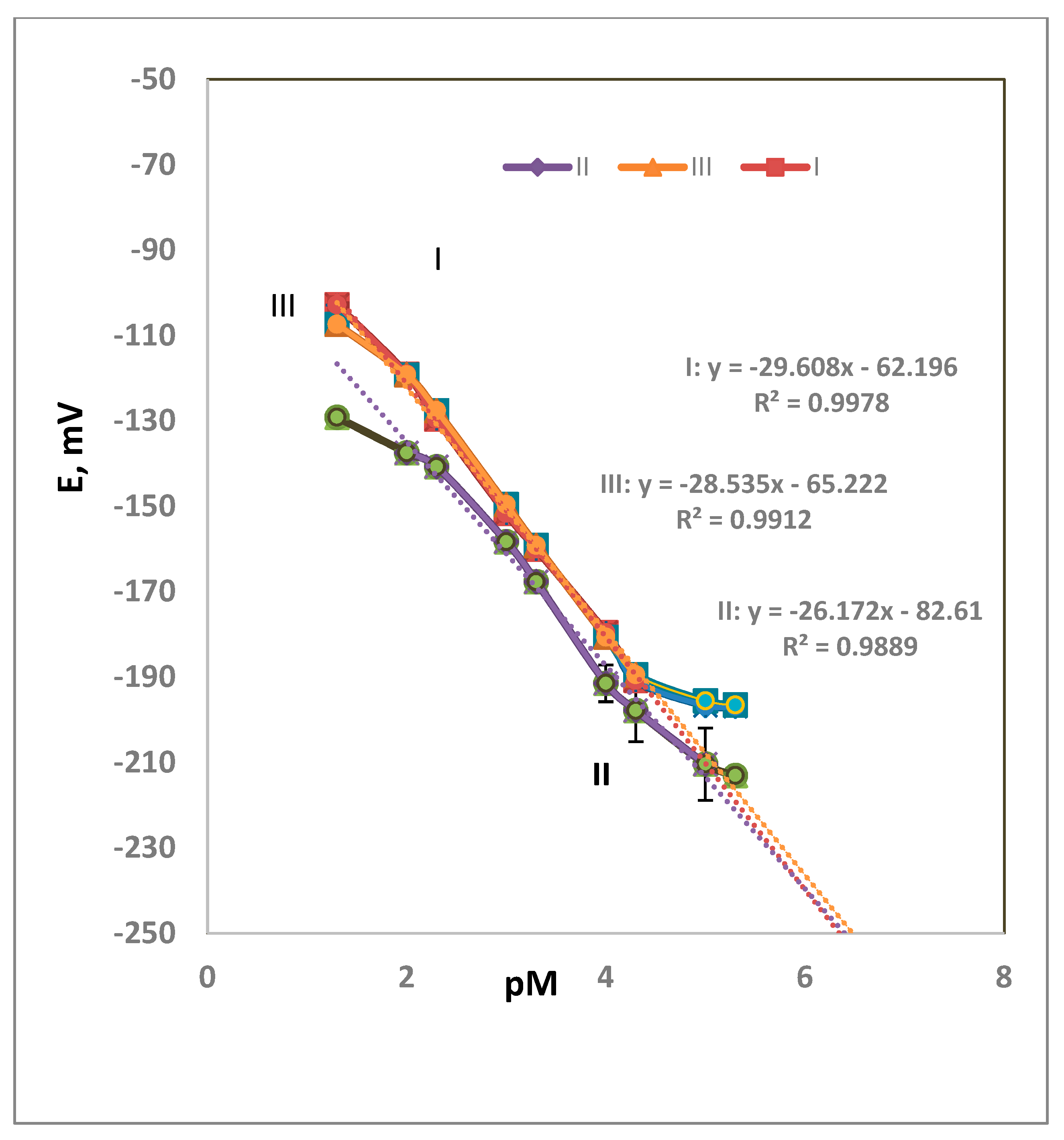
3.1. Composition Effect
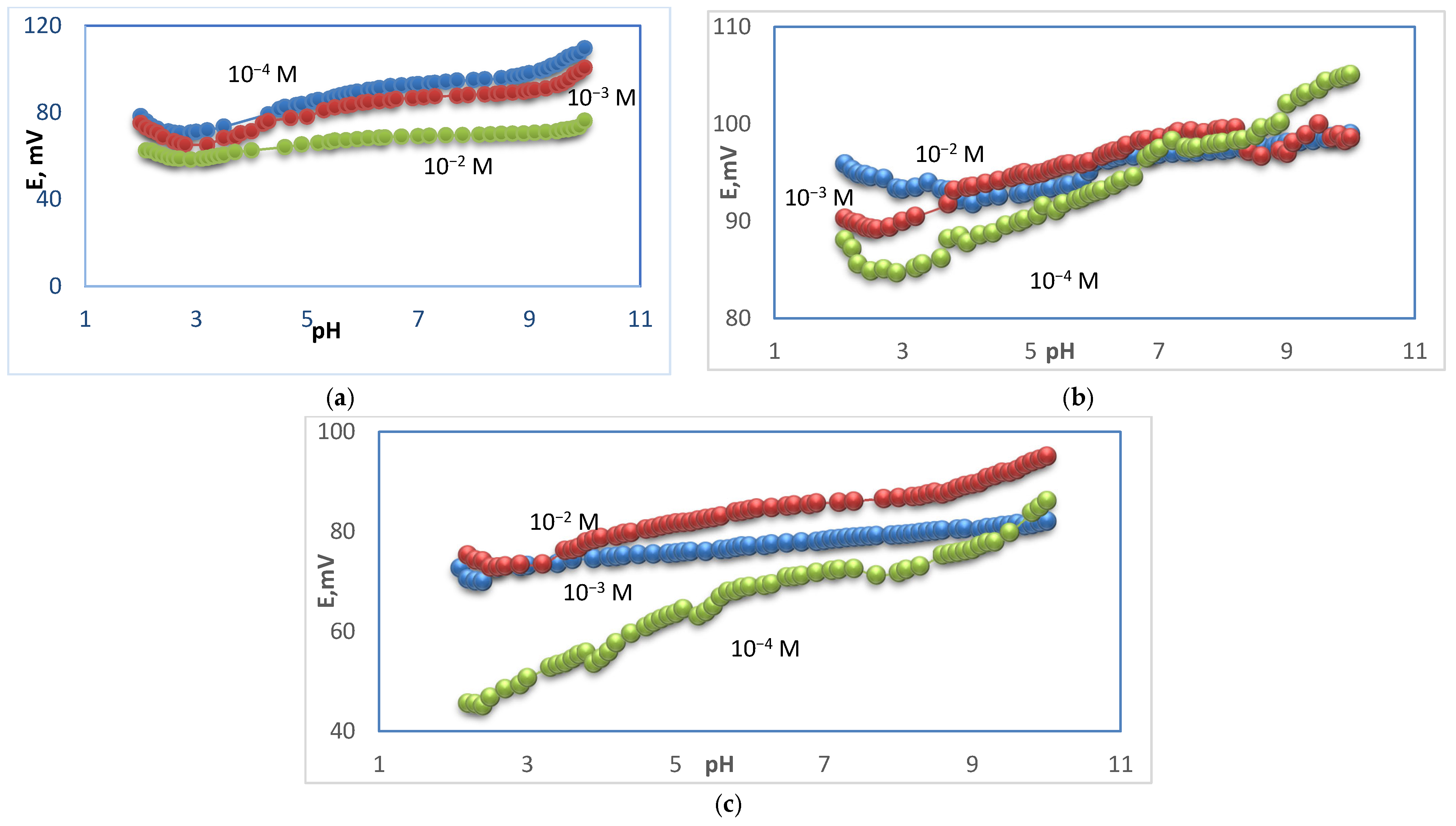
3.2. pH–Effect
3.3. Selective-Character Studies
3.4. Determination of Calcium in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.; Sun, C.; Ou, X.; Zhai, T.; Liu, B.; Lou, X.; Xia, F. Coordination-induced structural changes of DNA-based optical and electrochemical sensors for metal ions detection. Dalton Trans. 2019, 48, 5845–6292. [Google Scholar]
- Zhou, W.; Saran, R.; Jimmy Huang, P.-J.; Ding, J.; Liu, J. An Exceptionally Selective DNA Cooperatively Binding Two Ca2+Ions. ChemBioChem 2017, 18, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Shamkhalib, A.N.; Hanifehpourc, Y.; Jooc, S.W. A Ca2+ selective membrane electrode based on calcium-imprinted polymeric nanoparticles. N. J. Chem. 2016, 40, 8479–8487. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, W.; Liu, J. Screening of DNAzyme mutants for highly sensitive and selective detection of calcium in milk. Anal. Methods 2018, 10, 1740–1746. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Stelmach, E.; Szymczycha-Madeja, A.; Pohl, P. A Simplified Determination of Total Concentrations of Ca, Fe, Mg and Mn in Addition to Their Bioaccessible Fraction in Popular Instant Coffee Brews. Food Chem. 2016, 197, 388–394. [Google Scholar] [CrossRef]
- Lopez-Castilla, A.; Thomassin, J.L.; Bardiaux, B.; Zheng, W.L.; Nivaskumar, M.; Yu, X.; Nilges, M.; Egelman, E.H.; Izadi-Pruneyre, N.; Francetic, O. Structure of the Calcium-Dependent Type 2Secretion Pseudopilus. Nat. Microbiol. 2017, 2, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Ostad, M.A.; Hajinia, A.; Heidari, T. A Novel Direct and Cost-Effective Method for Fabricating Paper-Based Microfluidic Device by Commercial Eye Pencil and its Application for Determining Simultaneous Calcium and Magnesium. Microchem. J. 2017, 133, 545–550. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ma, X.; Dong, W.; Miao, P.; Tang, Y. Electrochemical Determination of Ca2+ Based On Recycling Formation of Highly Selective DNAzyme and Gold Nanoparticle-Mediated Amplification. Bioconjugate Chem. 2018, 29, 1021–1024. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wang, H.; Cheng, Z.; Liu, H.G. Hexametaphosphate-Capped Quantum Dots as Fluorescent Probes for Detection of Calcium Ion and Fluoride. Sens. Actuators 2016, 232, 306–312. [Google Scholar] [CrossRef]
- Umapathi, R.; Sonwal, S.; Lee, M.J.; Rani, G.M.; Lee, E.-S.; Jeon, T.-J.; Kang, S.-M.; Oh, M.-H.; Huh, Y.S. Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: New horizons, perspectives, and challenges. Coord. Chem. Rev. 2021, 446, 214061. [Google Scholar] [CrossRef]
- Vilian, A.E.; Umapathi, R.; Hwang, S.-K.; Huh, Y.S.; Han, Y.-K. Pd–Cu nanospheres supported on Mo2C for the electrochemical sensing of nitrites. J. Hazard. Mater. 2021, 408, 124914. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Thamaraiselvi, J. Calcium Ion Selective Electrode Based on Surface Modified Zeolite Based Ionophore and its Analytical Application. Orient J. Chem. 2017, 33, 396–401. [Google Scholar]
- Yang, Q.; Zhang, M.; Ming, C.; Liu, G.; Wang, M. All-solid-state Ca2+ Ion-selective Electrode with Black Phosphorus and Reduced Graphene Oxide as the Mediator Layer. Int. J. Electrochem. Sci. 2019, 14, 4933–4945. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Thamaraiselvi, J. Calcium Ion Selective Electrode Based on Schiff Base as Ionophore and Determination of Thermodynamic Functions and its Analytical Application. Orient. J. Chem. 2017, 33, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Van Dat, N.; Tuan, H.T.; Phong, H.Q.; Huong, H.L.; Dat, N.T.; Hien, O.T.M.; Purwadi, R. The preparation of a fine tip calcium ion selective electrode. Can Tho Univ. J. Sci. 2020, 12, 45–49. [Google Scholar]
- Zareh, M.; Zordek, W.; Abd-Alhady, A. Iron-Selective Electrode Based on Phosphorylated Calix-6-arene Derivative. J. Sens. Technol. 2014, 4, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ion selective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527. [Google Scholar] [CrossRef]
- Abu-Salah, K.M.; Zourob, M.M.; Mouffouk, F.; Alrokayan, S.A.; Alaamery, M.A.; Ansari, A.A. DNA-Based Nanobiosensors as an Emerging Platform for Detection of Disease. Sensors 2015, 15, 14539–14568. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Saran, R.; Liu, J. Metal Sensing by DNA. Am. Chem. Soc. 2017, 117, 8272–8325. [Google Scholar] [CrossRef] [Green Version]
- Saidura, M.R.; Abdul Aziza, A.R.; Basirun, W.J. Recent advances in DNA-based electrochemical biosensors for heavy metal ion detection: A Review. Biosens. Bioelectron. 2017, 90, 125–139. [Google Scholar] [CrossRef]
- Vlascici, D.; Plesu, N.; Fagadar-Cosma, G.; Lascu, A.; Petric, M.; Crisan, M.; Belean, A.; Fagadar-Cosma, E. Potentiometric Sensors for Iodide and Bromide Based on Pt(II)-Porphyrin. Sensors 2018, 18, 2297. [Google Scholar] [CrossRef] [Green Version]
- Zareh, M.M. Blank Membranes versus Ionophore-based Membranes for the Selective Determination of H+. Anal. Sci. 2009, 25, 1131. [Google Scholar] [CrossRef] [Green Version]
- Barreto, W.; Barreto, S.; Scarminio, I.; Ishikawa, D.; Soares, M.; de Proença, M. Determination of Ni(II) in metal alloys by spectrophotometry UV-Vis using dopasemiquinone. Quím. Nova 2010, 1, 33. [Google Scholar] [CrossRef]
- Buck Scientific. Manual Book, Buck Scientific 210/211 VGP Atomic Absorption Spectroscopy, Operator’s Manual February; VER 3.94 C; Buck Scientific: Norwalk, CT, USA, 2005. [Google Scholar]


| Plasticizer | Chemical Name | Molecular Formula | Molecular Weight | Physical Properties |
|---|---|---|---|---|
| DOP | Dioctyl phthalate | C24H38O4 | 390.57 | Colorless, transparent oily liquid, slight odor. Boiling Point 386°C. Viscosity 80 c.p. (20). Refractive index 1.4831.48620. Flash point 109 °F. Insoluble in water. |
| NPOE | 2-Nitrophenyloctyl ether | C14H21NO3 | 251.32 | Colorless liquid. Boiling Point 197–198 °C, 11 mm Hg. Flash Point > 230 °F. Refractive index n20/D 1.508 (lit.). Water Solubility, tetrahydrofuran: 0.1 g/mL. |
| DEP | Diethyl phthalate | C12H14O4 | 222.24 | Colorless liquid without significant odor. Insoluble in water.Boiling Point 295 °C. Flash Point 325 °F. Refractive index 1.5002 at 25 °C/D. |
| Composition, w/w% | PVC, mg | DNA, mg | DOP, mg | DEP, mg | NPOE, mg | Slope, mV/Decde | Detection Limit, M | Linear Range, M |
|---|---|---|---|---|---|---|---|---|
| I-membrane | 60 | 2 | 120 | _ | _ | 30 | 7.9 × 10−6 | 5.0 × 10−2–5.0 × 10−5 |
| II-membrane | 60 | 2 | _ | _ | 120 | 26 | 6.3 × 10−6 | 1.0 × 10−2–1.0 × 10−5 |
| III-membrane | 60 | 2 | _ | 120 | _ | 29 | 2.5 × 10−5 | 5.0 × 10−2–5.0 × 10−5 |
| No. | Inner Filling Solution | Slope, mV/Decade | Linear Range, M | R2 |
|---|---|---|---|---|
| A | (10−1 M CaCl2 + 10−1 M KCl) | 22 | 5.0 × 10−2–1.0 × 10−4 | 0.9917 |
| B | (10−2 M CaCl2 + 10−2 M KCl) | 25 | 5.0 × 10−2–5.0 × 10−5 | 0.9797 |
| C | (10−3 M CaCl2 + 10−3 M KCl) | 30 | 5.0 × 10−2–5.0 × 10−5 | 0.9978 |
| D | (10−4 M CaCl2 + 10−4 M KCl) | 23 | 5.0 × 10−2–1.0 × 10−4 | 0.9923 |
| Sensor/Ionophore | LDR, M | LOD, M | Slope, mV/Decade−1 | pH | Response Time, s | Age, Days | Ref. |
|---|---|---|---|---|---|---|---|
| Nano-sized Ca+2 imprinted polymers | 1.0 × 10−6–1.0 × 10−1 | 7.5 × 10−7 | 30.3 | 5.0–7.0 | 10 | – | [3] |
| Surface modified zeolite | 1.0 × 10−4–1.0 × 10−1 | – | 33.0 | 5.7–6.2 | – | – | [14] |
| Composite mediator layer of RGO-coated BP | 1.0 × 10−6–1.0 × 10−1 | 7.2 × 10−6 | 28.3 | – | 10 | 10 | [15] |
| Fine tip calcium ion selective electrode | 1.0 × 10−7–1.0 × 10−3 | 3.2 × 10−8 | 30.0 | – | 10 | – | [17] |
| Schiff base | – | – | – | – | – | 90 | [16] |
| DNA | 5.0 × 10−5–5.0 × 10−2 | 7.9 × 10−6 | 30.0 | 4.0–9.5 | 3 | 21 | Present work |
| Interference | KPotCa2+, jz+ | ||
|---|---|---|---|
| I-DOP | II-NPOE | III-DEP | |
| Ba2+ | 2.27 × 10−4 | 1.60 | 1.71 × 10−6 |
| Mg2+ | 4.21 × 10−4 | 1.05 | 2.91 × 10−6 |
| Cu2+ | 2.13 × 10−4 | 1.09 | 1.30 × 10−6 |
| Ni2+ | 3.60 × 10−4 | 0.49 | 4.29 × 10−6 |
| Zn2+ | 2.88 × 10−4 | 0.63 | 3.33 × 10−6 |
| Mn2+ | 3.51 × 10−4 | 0.59 | 3.47 × 10−6 |
| Pb2+ | 1.80 × 10−4 | 0.50 | 3.17 × 10−6 |
| NH4 + | 8.91 × 10−4 | 1.30 | 1.46 × 10−5 |
| K+ | 8.71 × 10−4 | 0.49 | 1.57 × 10−5 |
| Na+ | 9.33 × 10−4 | 0.52 | 9.75 × 10−6 |
| Fe3+ | 2.44 × 10−4 | 0.72 | 3.29 × 10−7 |
| Sr2+ | 2.40 × 10−4 | 0.99 | 1.77 × 10−6 |
| Hg2+ | 2.70 × 10−4 | 0.26 | 8.00 × 10−5 |
| Cr2+ | 5.37 × 10−4 | 0.60 | 3.28 × 10−6 |
| Cd2+ | 1.21 × 10−4 | 0.25 | 1.10 × 10−6 |
| No. | Sample | Ca2+, ppm | Recovery, % | RSD, % | |
|---|---|---|---|---|---|
| AAS Method | Ca-ISE Method | ||||
| A | Milk (Al-Marai) | 52.31 | 50.48 | 96.48 | 0.52 |
| B | Milk (Danone) | 101.98 | 100.71 | 88.19 | 0.18 |
| C | Powder milk (Nido) | 483.71 | 449.87 | 93.66 | 0.14 |
| D | Cheese (Domty) | 163.83 | 159.62 | 89.00 | 0.17 |
| E | Cheese (Président) | 479.56 | 449.87 | 83.47 | 0.24 |
| F | Yogurt (Danone) | 241.33 | 200.95 | 83.66 | 0.20 |
| G | Orange | 76.32 | 63.55 | 74.42 | 0.65 |
| H | Guava | 63.08 | 50.40 | 79.84 | 0.75 |
| I | Decal B12N (calcium syrup) | 67.86 | 56.63 | 93.63 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zareh, M.M.; Mohamed, S.F.; Elsheikh, A.M. Polymeric Electrochemical Sensor for Calcium Based on DNA. Polymers 2022, 14, 1896. https://doi.org/10.3390/polym14091896
Zareh MM, Mohamed SF, Elsheikh AM. Polymeric Electrochemical Sensor for Calcium Based on DNA. Polymers. 2022; 14(9):1896. https://doi.org/10.3390/polym14091896
Chicago/Turabian StyleZareh, Mohsen M., Soha F. Mohamed, and Anas M. Elsheikh. 2022. "Polymeric Electrochemical Sensor for Calcium Based on DNA" Polymers 14, no. 9: 1896. https://doi.org/10.3390/polym14091896