Molecular and Structure–Properties Comparison of an Anionically Synthesized Diblock Copolymer of the PS-b-PI Sequence and Its Hydrogenated or Sulfonated Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
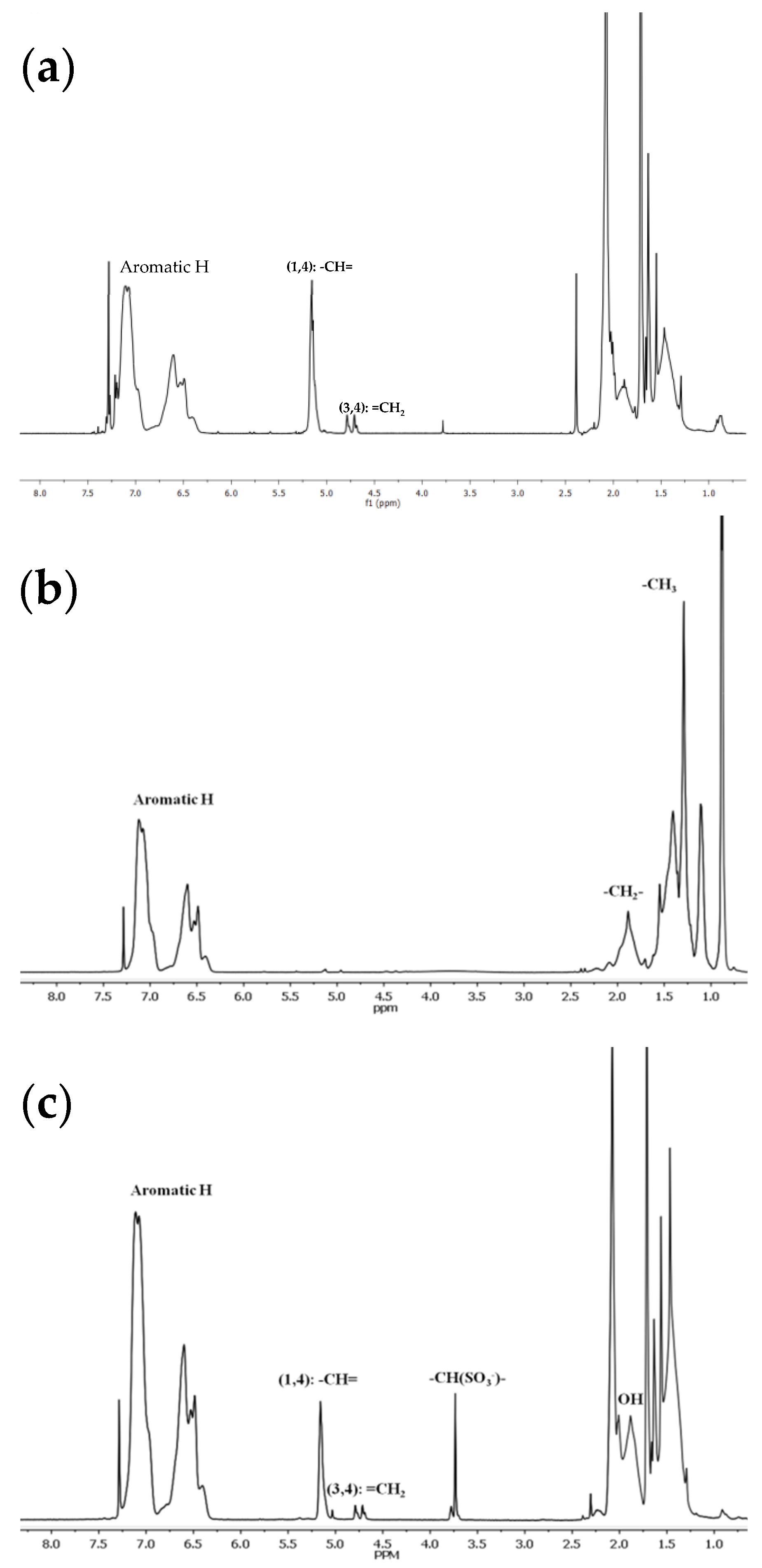
3.1. Molecular Characterization
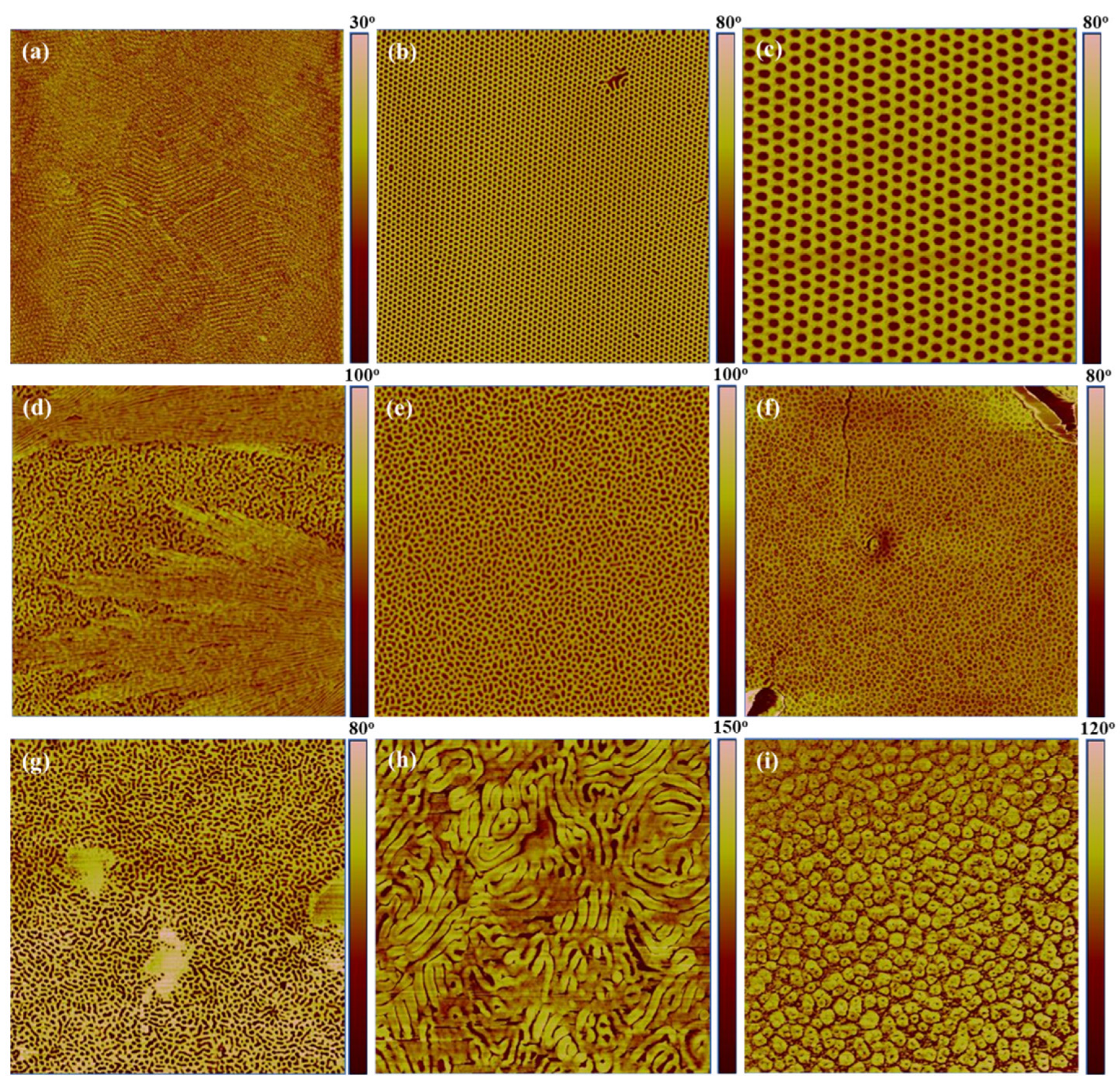
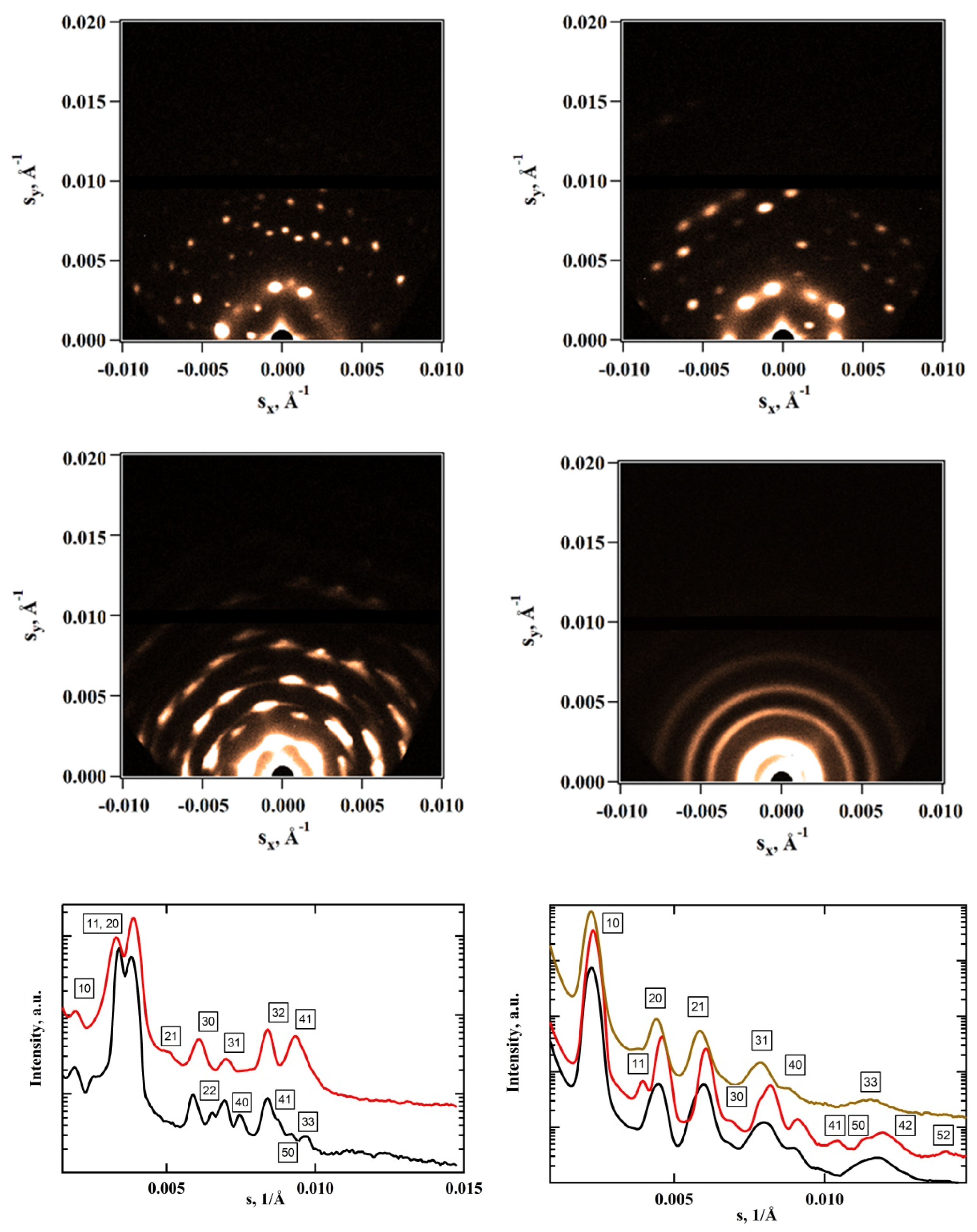
3.2. Morphological Characterization
3.3. Initial Diblock Copolymer (PS-b-PI or SI)
3.4. Hydrogenated Diblock Copolymer (SEP)
3.5. Partially Sulfonated Diblock Copolymer (SI/Sulf)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polymeropoulos, G.; Zapsas, G.; Ntetsikas, K.; Bilalis, P.; Gnanou, Y.; Hadjichristidis, N. 50th Anniversary Perspective: Polymers with Complex Architectures. Macromolecules 2017, 50, 1253–1290. [Google Scholar] [CrossRef]
- Leibler, L. Theory of Microphase Separation in Block Copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Lohse, D.J.; Hadjichristidis, N. Microphase separation in block copolymers. Curr. Opin. Colloid Interface Sci. 1997, 2, 171–176. [Google Scholar] [CrossRef]
- Romulus, J.; Henssler, J.T.; Weck, M. Postpolymerization Modification of Block Copolymers. Macromolecules 2014, 47, 5437–5449. [Google Scholar] [CrossRef]
- Politakos, N.; Kortaberria, G.; Zalakain, I.; Mondragon, I.; Avgeropoulos, A. Enhancing the hydrophobic properties of various commercial polymers through mixtures and coatings with a fluorinated diblock copolymer in low concentrations. Eur. Polym. J. 2013, 49, 1841–1851. [Google Scholar] [CrossRef]
- Politakos, N.; Kortaberria, G.; Zalakain, I.; Avgeropoulos, A.; Mondragon, I. Modified diblock copolymer bearing fluoro groups and evaluation of its hydrophobic properties. Macromol. Symp. 2012, 321–322, 53–58. [Google Scholar] [CrossRef]
- Politakos, N.; Weinman, C.J.; Paik, M.Y.; Sundaram, H.S.; Ober, C.K.; Avgeropoulos, A. Synthesis, molecular, and morphological characterization of initial and modified diblock copolymers with organic acid chloride derivatives. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4292–4305. [Google Scholar] [CrossRef]
- Kimishima, K.; Jinnai, H.; Hashimoto, T. Control of Self-Assembled Structures in Binary Mixtures of A-B Diblock Copolymer and A-C Diblock Copolymer by Changing the Interaction between B and C Block Chains. Macromolecules 1999, 32, 2585–2596. [Google Scholar] [CrossRef]
- Tureau, M.S.; Epps, T.H. Effect of partial hydrogenation on the phase behavior of poly(isoprene-b-styrene-b-methyl methacrylate) triblock copolymers. Macromolecules 2012, 45, 8347–8355. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Bates, F.S. Synthesis and characterization of model polyalkane-poly(ethylene oxide) block copolymers. Macromolecules 1996, 29, 6994–7002. [Google Scholar] [CrossRef]
- Laurer, J.H.; Khan, S.A.; Spontak, R.J.; Satkowski, M.M.; Grothaus, J.T.; Smith, S.D.; Lin, J.S. Morphology and rheology of SIS and SEPS triblock copolymers in the presence of a midblock-selective solvent. Langmuir 1999, 15, 7947–7955. [Google Scholar] [CrossRef]
- Hu, H.; Gopinadhan, M.; Osuji, C.O. Directed self-Assembly of block copolymers: A tutorial review of strategies for enabling nanotechnology with soft matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef]
- Park, C.; Yoon, J.; Thomas, E.L. Enabling nanotechnology with self assembled block copolymer patterns. Polymer 2003, 44, 6725–6760. [Google Scholar] [CrossRef] [Green Version]
- Davidock, D.A.; Hillmyer, M.A.; Lodge, T.P. Mapping large regions of diblock copolymer phase space by selective chemical modification. Macromolecules 2004, 37, 397–407. [Google Scholar] [CrossRef]
- Allgaier, J.; Poppe, A.; Willner, L.; Richter, D. Synthesis and Characterization of Poly[1,4-isoprene-b-(ethylene oxide)] and Poly[ethylene-co-propylene-b-(ethylene oxide)] Block Copolymers. Macromolecules 1997, 30, 1582–1586. [Google Scholar] [CrossRef]
- Mani, S.; Weiss, R.A.; Hahn, S.F.; Williams, C.E.; Cantino, M.E.; Khairallah, L.H. Microstructure of block copolymers of polystyrene and poly(ethylene-alt-propylene). Polymer 1998, 39, 2023–2033. [Google Scholar] [CrossRef]
- Charmondusit, K.; Prasassarakich, P.; Mcmanus, N.T.; Rempel, G.L. Hydrogenation of cis-1,4-Poly(isoprene) Catalyzed by OsHCl(CO)(O2)(PCy3)2. J. Appl. Polym. Sci. 2002, 89, 142–152. [Google Scholar] [CrossRef]
- Kongparakul, S.; Prasassarakich, P.; Rempel, G.L. Catalytic hydrogenation of styrene-g-natural rubber (ST-g-NR) in the presence of OsHCl(CO)(O2)(PCy3)2. Eur. Polym. J. 2009, 45, 2358–2373. [Google Scholar] [CrossRef]
- Barrios, V.A.E.; Nájera, R.H.; Petit, A.; Pla, F. Selective hydrogenation of butadiene-styrene copolymers using a Ziegler-Natta type catalyst 1. Kinetic study. Eur. Polym. J. 2000, 36, 1817–1834. [Google Scholar] [CrossRef]
- Alfonzo, C.G.; Fleury, G.; Chaffin, K.A.; Bates, F.S. Synthesis and characterization of elastomeric heptablock terpolymers structured by crystallization. Macromolecules 2010, 43, 5295–5305. [Google Scholar] [CrossRef]
- Schmalz, H.; Müller, A.J.; Abetz, V. Crystallization in ABC triblock copolymers with two different crystalline end blocks: Influence of confinement on self-nucleation behavior. Macromol. Chem. Phys. 2003, 204, 111–124. [Google Scholar] [CrossRef]
- Kaewsaiha, P.; Matsumoto, K.; Matsuoka, H. Synthesis and nanostructure of strong polyelectrolyte brushes in amphiphilic diblock copolymer monolayers on a water surface. Langmuir 2004, 20, 6754–6761. [Google Scholar] [CrossRef]
- Tamada, Y.; Murata, M.; Makino, K.; Yoshida, Y.; Yoshida, T.; Hayashi, T. Anticoagulant effects of sulphonated polpisoprenes. Biomaterials 1998, 19, 745–750. [Google Scholar] [CrossRef]
- Tamada, Y.; Murata, M.; Hayashi, T.; Goto, K. Anticoagulant mechanism of sulfonated polyisoprenes. Biomaterials 2002, 23, 1375–1382. [Google Scholar] [CrossRef]
- Yakubchik, A.I.; Tichomirov, B.I.; Sulimov, V.S. Hydrogenation of Natural and Synthetic cis-1,4-Polyisoprene. Rubber Chem. Technol. 1962, 35, 1063–1065. [Google Scholar] [CrossRef]
- Alley, W.M.; Hamdemir, I.K.; Johnson, K.A.; Finke, R.G. Ziegler-type hydrogenation catalysts made from group 8-10 transition metal precatalysts and AlR3 cocatalysts: A critical review of the literature. J. Mol. Catal. A Chem. 2010, 315, 1–27. [Google Scholar] [CrossRef]
- Von Tiedemann, P.; Yan, J.; Barent, R.D.; Spontak, R.J.; Floudas, G.; Frey, H.; Register, R.A. Tapered Multiblock Star Copolymers: Synthesis, Selective Hydrogenation, and Properties. Macromolecules 2020, 53, 4422–4434. [Google Scholar] [CrossRef]
- Samran, J.; Phinyocheep, P.; Daniel, P.; Kittipoom, S. Hydrogenation of unsaturated rubbers using diimide as a reducing agent. J. Appl. Polym. Sci. 2005, 95, 16–27. [Google Scholar] [CrossRef]
- Ricci, G.; Boccia, A.C.; Leone, G.; Pierro, I.; Zanchin, G.; Scoti, M.; Auriemma, F.; De Rosa, C. Isotactic and syndiotactic alternating ethylene/propylene copolymers obtained through non-catalytic hydrogenation of highly stereoregular cis-1,4 poly(1,3-diene)s. Molecules 2017, 22, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, S.F. An improved method for the diimide hydrogenation of butadiene and isoprene containing polymers. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 397–408. [Google Scholar] [CrossRef]
- Gacal, B.N.; Filiz, V.; Shishatskiy, S.; Rangou, S.; Neumann, S.; Abetz, V. Modification of polyisoprene-block-poly(vinyl trimethylsilane) block copolymers via hydrosilylation and hydrogenation, and their gas transport properties. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1252–1261. [Google Scholar] [CrossRef]
- Kaditi, E.; Mountrichas, G.; Pispas, S. Amphiphilic block copolymers by a combination of anionic polymerization and selective post-polymerization functionalization. Eur. Polym. J. 2011, 47, 415–434. [Google Scholar] [CrossRef] [Green Version]
- Szczubiałka, K.; Ishikawa, K.; Morishima, Y. Micelle Formation of Diblock Copolymers of Styrene and Sulfonated Isoprene in Aqueous Solution. Langmuir 1999, 15, 454–462. [Google Scholar] [CrossRef]
- Szczubiałka, K.; Ishikawa, K.; Morishima, Y. Associating behavior of sulfonated polyisoprene block copolymers with short polystyrene blocks at both chain ends. Langmuir 2000, 16, 2083–2092. [Google Scholar] [CrossRef]
- Burns, A.B.; Register, R.A. Strategies for the Synthesis of Well-Defined Star Polymers by Anionic Polymerization with Chlorosilane Coupling and Preservation of the Star Architecture during Catalytic Hydrogenation. Macromolecules 2016, 49, 2063–2070. [Google Scholar] [CrossRef]
- Tsai, B.-H.; Lin, T.-A.; Cheng, C.-H.; Lin, J.-C. Studies of the Sulfonated Hydrogenated Styrene–Isoprene–Styrene Block Copolymer and Its Surface Properties, Cytotoxicity, and Platelet-Contacting Characteristics. Polymers 2021, 13, 235. [Google Scholar] [CrossRef]
- Yeo, J.; Kim, S.Y.; Kim, S.; Ryu, D.Y.; Kim, T.H.; Park, M.J. Mechanically and structurally robust sulfonated block copolymer membranes for water purification applications. Nanotechnology 2012, 23, 245703. [Google Scholar] [CrossRef] [PubMed]
- Isaacs Sodeye, A.I.; Huang, T.; Gido, S.P.; Mays, J.W. Polymer electrolyte membranes from fluorinated polyisoprene-block- sulfonated polystyrene: Membrane structure and transport properties. Polymer 2011, 52, 1963–1970. [Google Scholar] [CrossRef]
- Isaacs Sodeye, A.I.; Huang, T.; Gido, S.P.; Mays, J.W. Polymer electrolyte membranes from fluorinated polyisoprene-block- sulfonated polystyrene: Structural evolution with hydration and heating. Polymer 2011, 52, 3201–3208. [Google Scholar] [CrossRef]
- Isaacs Sodeye, A.I.; Huang, T.; Gido, S.P.; Mays, J.W. Polymer electrolyte membranes from fluorinated polyisoprene-block- sulfonated polystyrene: Microdomain orientation by external field. Polymer 2011, 52, 5393–5396. [Google Scholar] [CrossRef]
- Van Der Velden, P.M.; Smolders, C.A. New cation-exchange membranes for hyperfiltration processes. J. Appl. Polym. Sci. 1977, 21, 1445–1457. [Google Scholar] [CrossRef] [Green Version]
- Jonquières, A.; Clément, R.; Lochon, P. Permeability of block copolymers to vapors and liquids. Prog. Polym. Sci. 2002, 27, 1803–1877. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, F.; Sun, M.; Xie, F.; Ying, S.; Yang, Z.; Zhou, C.; Xia, J.; Li, A. Thermally Stable and Well-Defined Pentadiene-Derived Copolymers Prepared by Anionic Alternating Copolymerization and Subsequent Controlled Postmodification. Macromol. Chem. Phys. 2020, 221, 1–9. [Google Scholar] [CrossRef]
- Ashraf, A.R.; Ryan, J.J.; Satkowski, M.M.; Smith, S.D.; Spontak, R.J. Effect of Systematic Hydrogenation on the Phase Behavior and Nanostructural Dimensions of Block Copolymers. ACS Appl. Mater. Interfaces 2018, 10, 3186–3190. [Google Scholar] [CrossRef] [PubMed]
- Taksapattanakul, K.; Tulyapitak, T.; Phinyocheep, P.; Ruamcharoen, P.; Ruamcharoen, J.; Lagarde, F.; Daniel, P. The effect of percent hydrogenation and vulcanization system on ozone stability of hydrogenated natural rubber vulcanizates using Raman spectroscopy. Polym. Degrad. Stab. 2017, 141, 58–68. [Google Scholar] [CrossRef]
- Shiraki, Y.; Yokoyama, H. Novel hydrophobic and oleophobic surfaces using polyurethane with hydrogenated polyisoprene soft segment. Mater. Today Commun. 2020, 24, 101243. [Google Scholar] [CrossRef]
- Politakos, N.; Kortaberria, G. Exploring the self-assembly capabilities of ABA-type SBS, SIS, and their analogous hydrogenated copolymers onto different nanostructures using atomic force microscopy. Materials 2018, 11, 1529. [Google Scholar] [CrossRef] [Green Version]
- Goswami, M.; Sumpter, B.G.; Huang, T.; Messman, J.M.; Gido, S.P.; Isaacs-Sodeye, A.I.; Mays, J.W. Tunable morphologies from charged block copolymers. Soft Matter 2010, 6, 6146–6154. [Google Scholar] [CrossRef]
- Apolinar, Y.; Ramos, L.F.; Saade, H.; Díaz De Leon, R.; López, R.G. Polyisoprene nanoparticles prepared by polymerization in microemulsion. J. Nanomater. 2010, 2010, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Han, C.D. Order-disorder transition induced by the hydroxylation of homogeneous polystyrene-block-polyisoprene copolymer. Macromolecules 2002, 35, 760–769. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Chang, J.; Chen, L. Enhanced thermoelectric properties of CNT/PANI composite nanofibers by highly orienting the arrangement of polymer chains. J. Mater. Chem. 2012, 22, 17612–17618. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kataoka, H.; Ishizone, T.; Kato, T.; Ono, T.; Kobukata, S.; Ogi, H. Synthesis and properties of new thermoplastic elastomers containing poly[4-(1-adamantyl)styrene] hard segments. Macromolecules 2008, 41, 5502–5508. [Google Scholar] [CrossRef]
- Kakiage, M.; Sekiya, M.; Yamanobe, T.; Uehara, H. Structural change with blending of crystalline/amorphous block copolymers having different types of microphase separations. Polymer 2011, 52, 6146–6153. [Google Scholar] [CrossRef]
- Khandpur, A.K.; Förster, S.; Bates, F.S.; Hamley, I.W.; Ryan, A.J.; Bras, W.; Almdal, K.; Mortensen, K. Polyisoprene-Polystyrene Diblock Copolymer Phase Diagram near the Order-Disorder Transition. Macromolecules 1995, 28, 8796–8806. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- Lindvig, T.; Michelsen, M.L.; Kontogeorgis, G.M. A Flory-Huggins model based on the Hansen solubility parameters. Fluid Phase Equilib. 2002, 203, 247–260. [Google Scholar] [CrossRef]
- van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contribution, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 201–257. [Google Scholar]
- Stühn, B. The relation between the microphase separation transition and the glass transition in diblock copolymers. J. Polym. Sci. Part B Polym. Phys. 1992, 30, 1013–1019. [Google Scholar] [CrossRef]
- Ren, Y.; Lodge, T.P.; Hillmyer, M.A. Synthesis, characterization, and interaction strengths of difluorocarbene-modified polystyrene-polyisoprene block copolymers. Macromolecules 2000, 33, 866–876. [Google Scholar] [CrossRef]
- Davidock, D.A.; Hillmyer, M.A.; Lodge, T.P. Persistence of the gyroid morphology at strong segregation in diblock copolymers. Macromolecules 2003, 36, 4682–4685. [Google Scholar] [CrossRef]
- Lai, C.; Russel, W.B.; Register, R.A.; Marchand, G.R.; Adamson, D.H. Phase behavior of styrene-isoprene diblock derivatives with varying conformational asymmetry. Macromolecules 2000, 33, 3461–3466. [Google Scholar] [CrossRef]



| χAB → T(K) | 300 K | 353 K | 373 K | 393 K |
|---|---|---|---|---|
| χPS/PI | 0.051 | 0.043 | 0.040 | 0.039 |
| χPS/PEP | 0.102 | 0.087 | 0.082 | 0.079 |
| χPS/PIsulf | 1.350 | 1.180 | 1.080 | 1.046 |
| χPI/PIsulf | 1.927 | 1.638 | 1.541 | 1.493 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politakos, N.; Moutsios, I.; Manesi, G.-M.; Artopoiadis, K.; Tsitoni, K.; Moschovas, D.; Piryazev, A.A.; Kotlyarskiy, D.S.; Kortaberria, G.; Ivanov, D.A.; et al. Molecular and Structure–Properties Comparison of an Anionically Synthesized Diblock Copolymer of the PS-b-PI Sequence and Its Hydrogenated or Sulfonated Derivatives. Polymers 2021, 13, 4167. https://doi.org/10.3390/polym13234167
Politakos N, Moutsios I, Manesi G-M, Artopoiadis K, Tsitoni K, Moschovas D, Piryazev AA, Kotlyarskiy DS, Kortaberria G, Ivanov DA, et al. Molecular and Structure–Properties Comparison of an Anionically Synthesized Diblock Copolymer of the PS-b-PI Sequence and Its Hydrogenated or Sulfonated Derivatives. Polymers. 2021; 13(23):4167. https://doi.org/10.3390/polym13234167
Chicago/Turabian StylePolitakos, Nikolaos, Ioannis Moutsios, Gkreti-Maria Manesi, Konstantinos Artopoiadis, Konstantina Tsitoni, Dimitrios Moschovas, Alexey A. Piryazev, Denis S. Kotlyarskiy, Galder Kortaberria, Dimitri A. Ivanov, and et al. 2021. "Molecular and Structure–Properties Comparison of an Anionically Synthesized Diblock Copolymer of the PS-b-PI Sequence and Its Hydrogenated or Sulfonated Derivatives" Polymers 13, no. 23: 4167. https://doi.org/10.3390/polym13234167








