In Situ-Forming Cellulose/Albumin-Based Injectable Hydrogels for Localized Antitumor Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Animals
2.3. Synthesis of Hydroxypropylcellulose-graft-Abietic Acid (HPC-g-AA)
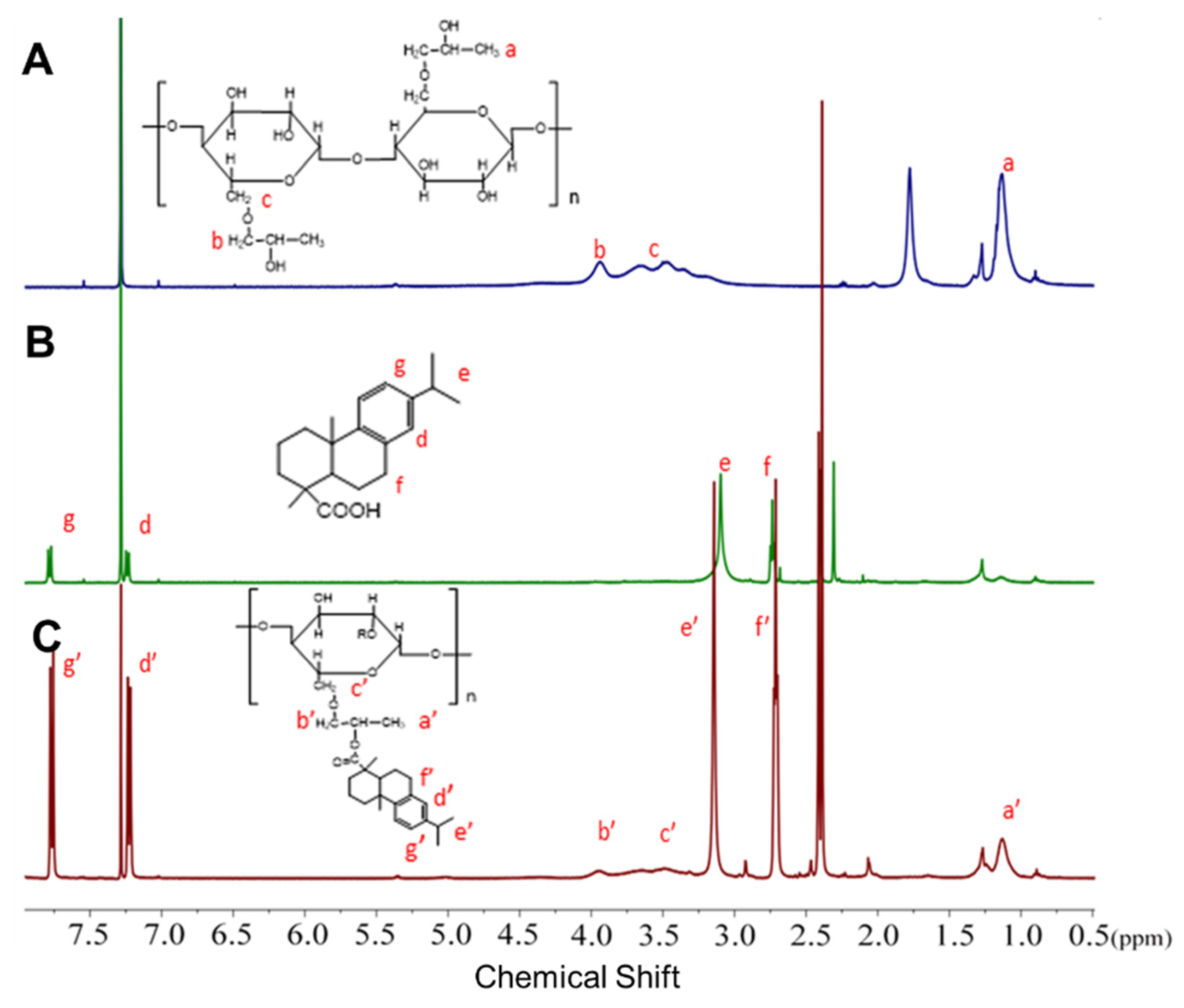
2.4. 1H NMR Characterization
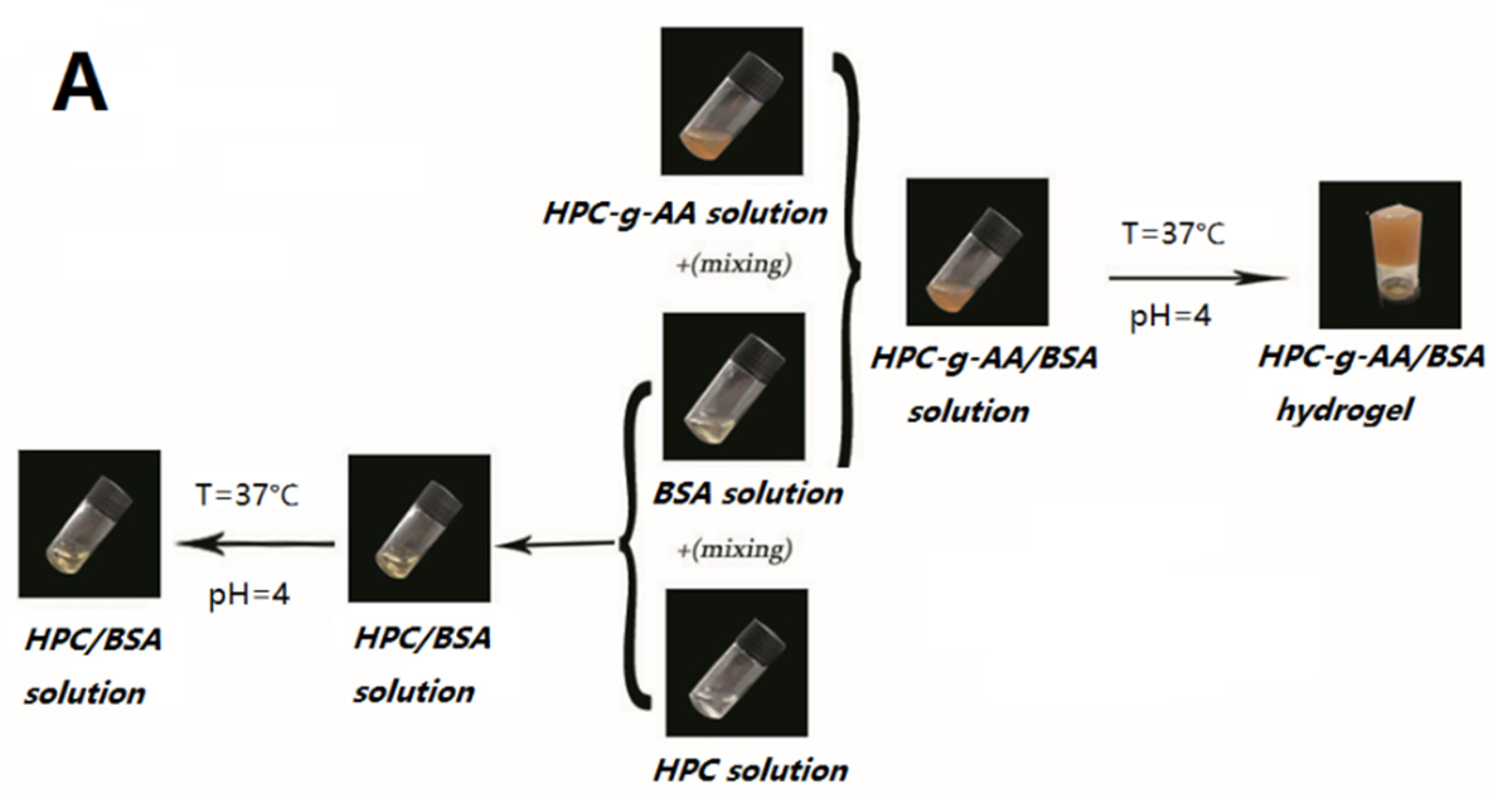
2.5. Preparation of HPC-g-AA/BSA Hydrogels
2.6. Rheological Analysis
2.7. Preparation of DOX@HPC-g-AA/BSA Hydrogels
2.8. Biocompatibility Studies of HPC-g-AA/BSA Hydrogels
2.9. Cytotoxicity of DOX@HPC-g-AA/BSA Hydrogels
2.10. In Vivo Antitumor Efficacy
2.11. Histological Studies
3. Results and Discussion
3.1. Synthesis and Characterization on HPC-graft-AA (HPC-g-AA)
3.2. In Situ Gelatin and Thermoresponsive Behavior
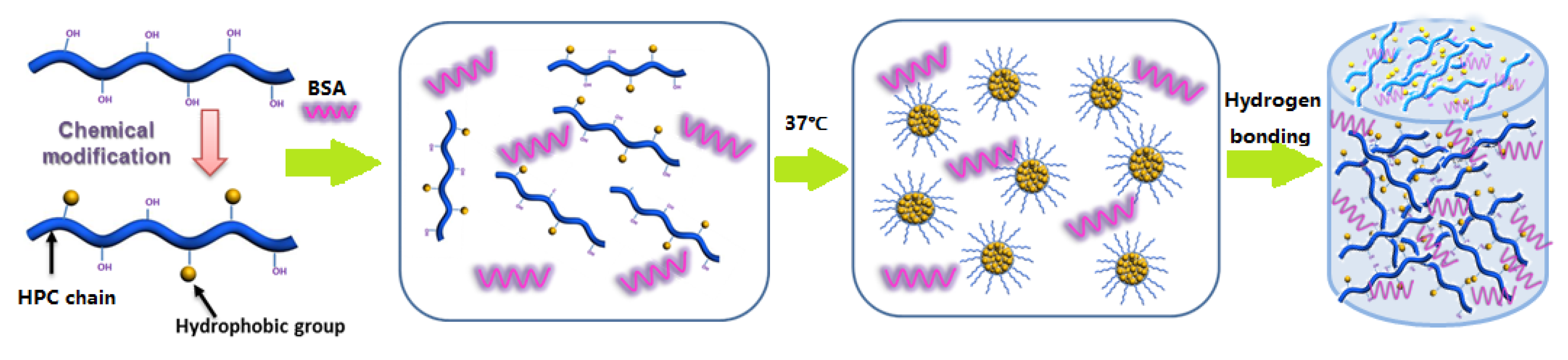
3.3. Formation Mechanism of HPC-g-AA/BSA Hydrogels
3.4. Cytotoxicity of DOX Delivered by Hydrogels
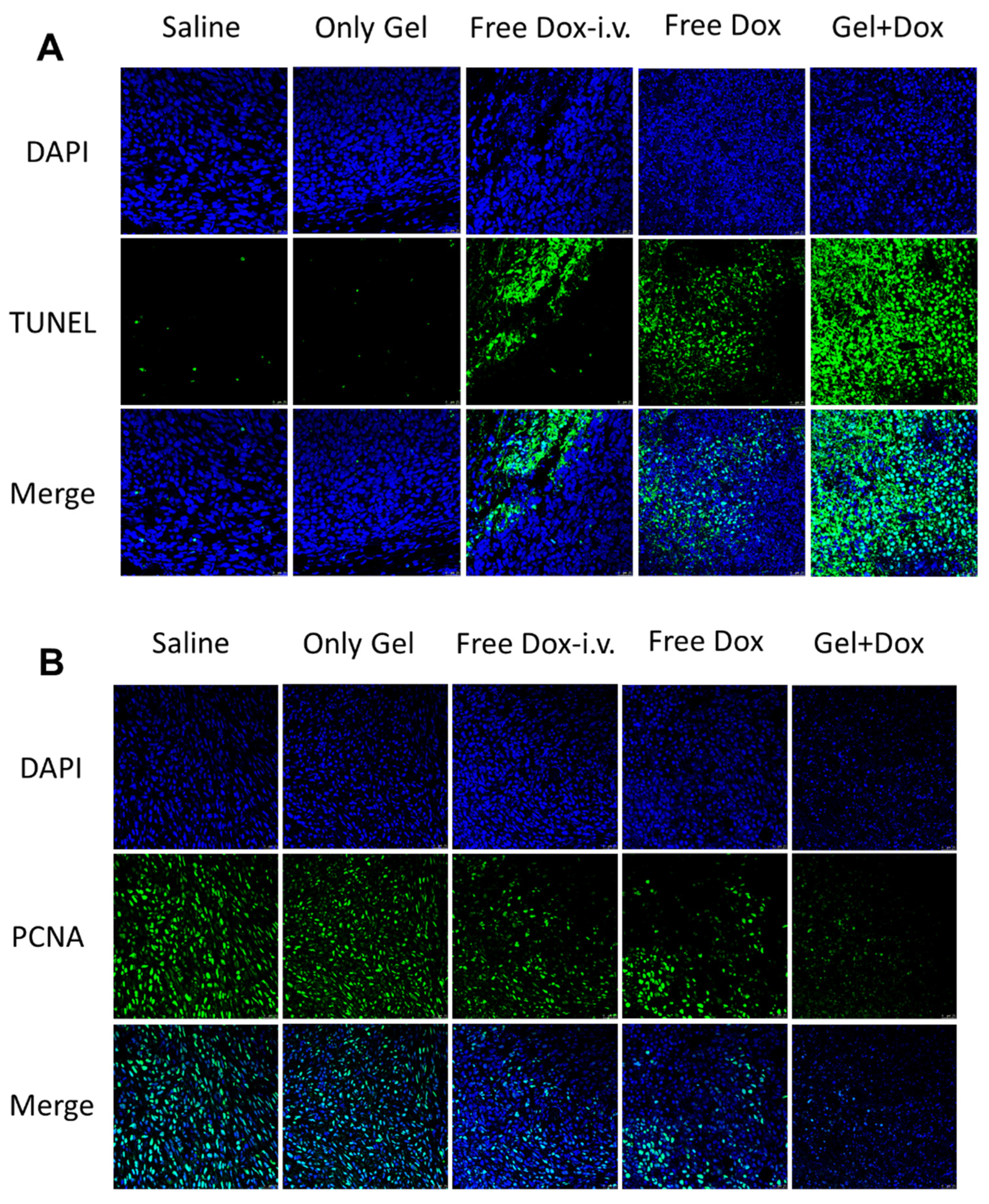
3.5. Subcutaneous Injectability and Antitumor Efficacy In Vivo
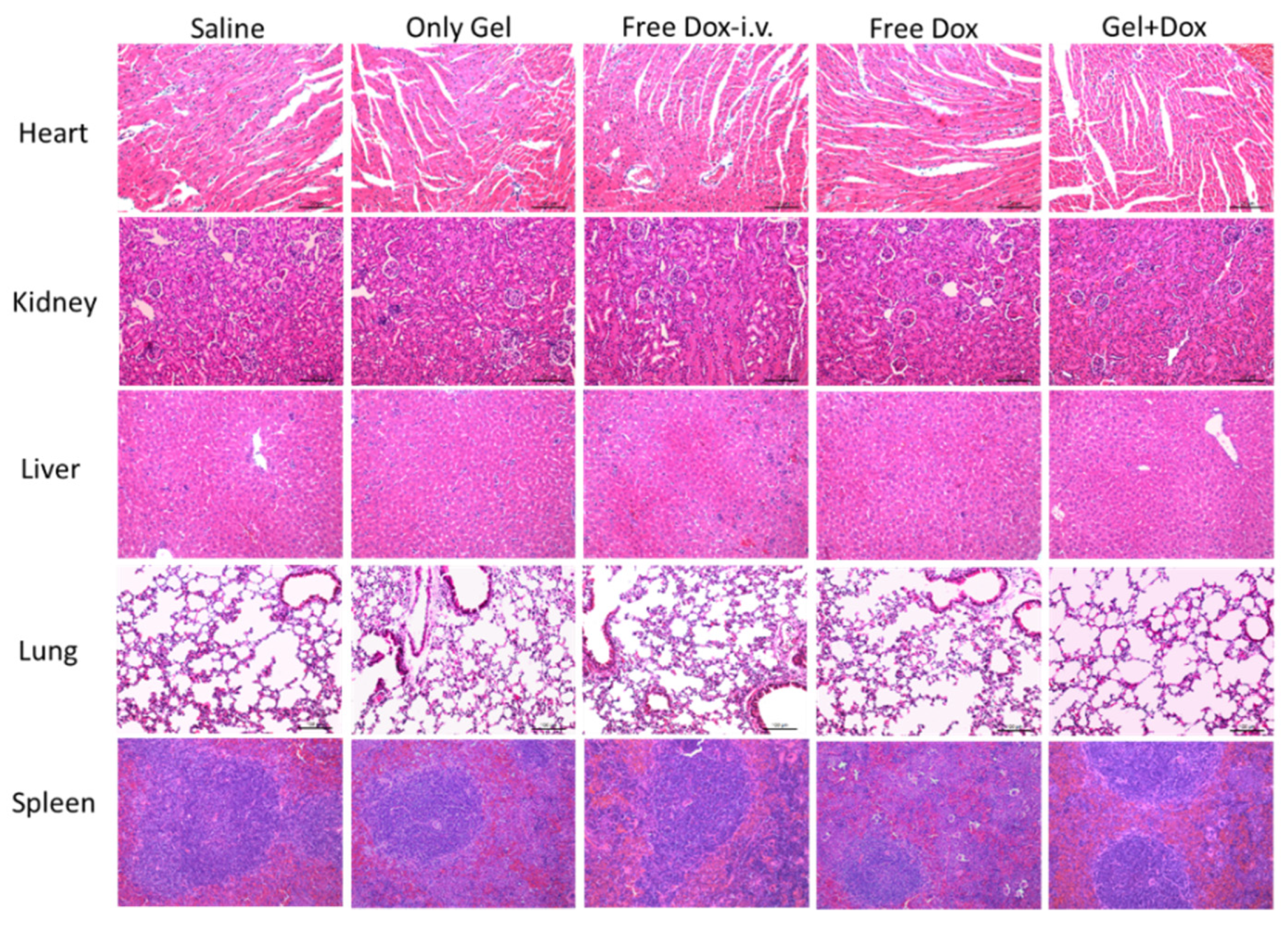
3.6. Histological Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chouhan, D.; Lohe, T.U. In Situ Forming Injectable Silk Fibroin Hydrogel Promotes Skin Regeneration in Full Thickness Burn Wounds. Adv. Healthc. Mater. 2018, 7, 1801092. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug. Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Geng, S.; Zhao, H.; Zhan, G.; Zhao, Y.; Yang, X. Injectable in Situ Forming Hydrogels of Thermosensitive Polypyrrole Nanoplatforms for Precisely Synergistic Photothermo-Chemotherapy. ACS Appl. Mater. Inter. 2020, 12, 7995–8005. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Manigandan, A.; Chinnaswamy, P.; Subramanian, A.; Sethuraman, S. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials 2018, 162, 82–98. [Google Scholar] [CrossRef]
- Pupkaite, J.; Rosenquist, J.; Hilborn, J.N.; Samanta, A. Injectable Shape-Holding Collagen Hydrogel for Cell Encapsulation and Delivery Cross-linked Using Thiol-Michael Addition Click Reaction. Biomacromolecules 2019, 20, 3475–3484. [Google Scholar] [CrossRef]
- Cheng, B.; Yan, Y.; Qi, J.; Deng, L.; Shao, Z.W.; Zhang, K.Q.; Li, B.; Sun, Z.; Li, X. Cooperative Assembly of a Peptide Gelator and Silk Fibroin Afford an Injectable Hydrogel for Tissue Engineering. ACS Appl. Mater. Inter. 2018, 10, 12474–12484. [Google Scholar] [CrossRef] [PubMed]
- Turabee, M.H.; Thambi, T.; Duong, H.T.T.; Jeong, J.H.; Lee, D.S. A pH- and temperature-responsive bioresorbable injectable hydrogel based on polypeptide block copolymers for the sustained delivery of proteins in vivo. Biomater. Sci. 2018, 6, 661–671. [Google Scholar] [CrossRef]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohyd. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Zhang, C.; Yang, C.; Tian, R.; Sun, T.; Zhang, W.; Chang, J.; Wang, H. An injectable hydrogel co-loading with cyanobacteria and up conversion nanoparticles for enhanced photodynamic tumor therapy. Colloid. Surface B 2021, 201, 111640. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Yang, H.; Peng, K.; Zhang, X. Injectable self-healing hydrogels formed via thiol/disulfide exchange of thiol functionalized F127 and dithiolane modified PEG. J. Mater. Chem. B 2017, 5, 4121–4127. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.B.; Koh, J.T.; Song, S.C. Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials 2017, 122, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Sheng, N.; Zhang, X.; Luan, Z.; Qi, P.; Lin, M.; Tan, Y.; Xia, Y.; Li, Y.; Sui, K. Design of injectable agar/NaCl/polyacrylamide ionic hydrogels for high performance strain sensors. Carbohyd. Polym. 2019, 211, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, Q.; Thundat, T.; Zeng, H. Stretchable, Injectable, and Self-Healing Conductive Hydrogel Enabled by Multiple Hydrogen Bonding toward Wearable Electronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Zou, L.; Braegelman, A.S.; Webber, M.J. Spatially Defined Drug Targeting by in Situ Host–Guest Chemistry in a Living Animal. ACS Cent. Sci. 2019, 5, 1035–1043. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Cai, Z.; Ye, T.; Yu, X.; Chen, Z.; Yan, Y.; Qi, J.; Wang, L.; Liu, Z.; Cui, W. Injectable Polypeptide-Protein Hydrogels for Promoting Infected Wound Healing. Adv. Funct. Mater. 2020, 30, 2001196. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.C.; Lai, J.Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Injectable and 3D Bioprinted Polysaccharide Hydrogels: From Cartilage to Osteochondral Tissue Engineering. Biomacromolecules 2017, 18, 1–26. [Google Scholar] [CrossRef]
- Ren, B.; Chen, X.; Du, S.; Ma, Y.; Chen, H.; Yuan, G.; Li, J.; Xiong, D.; Tan, H.; Ling, Z. Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Int. J. Biol. Macromol. 2018, 118, 1257–1266. [Google Scholar] [CrossRef]
- Jang, H.; Zhi, K.; Wang, J.; Zhao, H.; Li, B.; Yang, X. Enhanced therapeutic effect of paclitaxel with a natural polysaccharide carrier for local injection in breast cancer. Int. J. Biol. Macromol. 2020, 148, 163–172. [Google Scholar] [CrossRef]
- Flynn, J.; Durack, E.; Collins, M.N.; Hudson, S.P. Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J. Mater. Chem. B. 2020, 8, 4029–4038. [Google Scholar] [CrossRef]
- Resmi, R.; Parvathy, J.; John, A.; Joseph, R. Injectable self-crosslinking hydrogels for meniscal repair: A study with oxidized alginate and gelatin Injectable self-crosslinking hydrogels for meniscal repair: A study with oxidized alginate and gelatin. Carbohyd Polym. 2020, 234, 115902. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Tsintou, M.; Dalamagkas, K.; Seifalian, A. Injectable Hydrogel versus Plastically Compressed Collagen Scaffold for Central Nervous System Applications. Int. J. Biol. Macromol. 2018, 2018, 3514019. [Google Scholar] [CrossRef]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef]
- Pereira, I.; Fraga, S.; Maltez, L.; Requicha, J.; Guardão, L.; Oliveira, J.; Prada, J.; Alves, H.; Santos, J.D.; Teixeira, J.P. In vivo systemic toxicity assessment of an oxidized dextrin-based hydrogel and its effectiveness as a carrier and stabilizer of granular synthetic bone substitutes. J. Biomed. Mater. Res. A 2019, 107, 1678–1689. [Google Scholar]
- Zhao, N.; Suzuki, A.; Zhang, X.; Shi, P.; Abune, L.; Coyne, J.; Jia, H.; Xiong, N.; Zhang, G.; Wang, Y. Dual Aptamer-Functionalized in Situ Injectable Fibrin Hydrogel for Promotion of Angiogenesis via Codelivery of Vascular Endothelial Growth Factor and Platelet-Derived Growth Factor-BB. ACS Appl. Mater. Iinter. 2019, 11, 18123–18132. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Qian, K.; Cai, J.; Yang, Y.; Zhu, L.; Liu, B. Therapy for Gastric Cancer with Peritoneal Metastasis Using Injectable Albumin Hydrogel Hybridized with Paclitaxel-Loaded Red Blood Cell Membrane Nanoparticles. ACS Biomater. Sci. Eng. 2019, 5, 1100–1112. [Google Scholar] [CrossRef]
- Bubpamala, T.; Pasuwat, K.V.; Pholpabu, P. Injectable Poly(ethylene glycol) Hydrogels Cross-Linked by Metal–Phenolic Complex and Albumin for Controlled Drug Release. ACS Omega 2020, 5, 19437–19445. [Google Scholar] [CrossRef]
- Chruszcz, M.; Mikolajczak, K.; Mank, N. Serum albumins—unusual allergens. Biochim. Biophys. Acta 2013, 1830, 5375–5381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voltolini, S.; Spigno, F.; Al, A. Bovine Serum Albumin: A double allergy risk. Eur. Ann. Allergy Clin. Immunol. 2013, 45, 144–147. [Google Scholar] [PubMed]

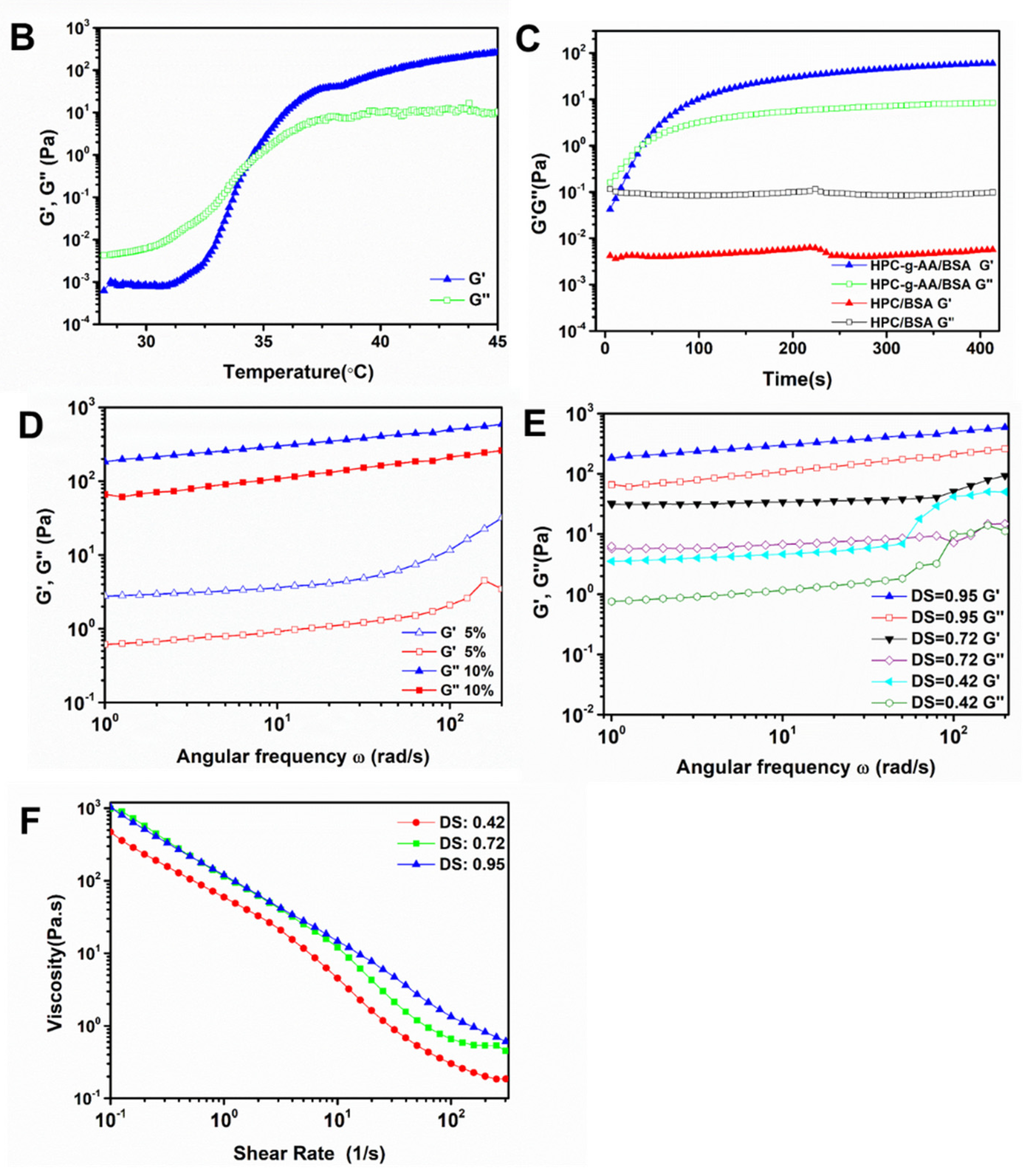


| Experiment | Measured Parameters | Temperature (°C) | Strain (%) | Frequency (Hz) |
|---|---|---|---|---|
| Time sweep | G′, G″, gelling time | 37 | 0.1 | 0.16 |
| Frequency sweep | G′, G″ | 37 | 0.1 | 0.16–31.83 |
| Temperature sweep | G′, G″, Tgel | 25–45 | 0.1 | 0.16 |
| Molar Feed Ratio (HPCunit/AA/Tos-Cl) | DS | Yield (%) | LCST (°C) | |
|---|---|---|---|---|
| HPC-g-AA-1 | 1:0.6:0.6 | 0.42 | 76.2 | 39 |
| HPC-g-AA-2 | 1:0.8:0.8 | 0.72 | 62.1 | 37 |
| HPC-g-AA-3 | 1:1:1 | 0.95 | 81.7 | 35 |
| HPC-g-AA | BSA | HPC-g-AA/BSA Hydrogel | |||
|---|---|---|---|---|---|
| DS | Concentration (wt%) | Concentration (wt%) | Tgel (°C) | Gelling Time (s) | G′ (Pa) |
| 0.95 | 3.0 | 7.0 | 36 | 410 | 264 |
| 0.95 | 5.0 | 5.0 | 35 | 40 | 632 |
| 0.95 | 7.0 | 3.0 | 34 | 180 | 97 |
| 0.72 | 3.0 | 7.0 | 37 | 312 | 276 |
| 0.72 | 5.0 | 5.0 | 37 | 212 | 233 |
| 0.72 | 7.0 | 3.0 | 36 | 198 | 115 |
| 0.42 | 3.0 | 7.0 | 37 | 804 | 168 |
| 0.42 | 5.0 | 5.0 | 37 | 412 | 210 |
| 0.42 | 7.0 | 3.0 | 37 | 500 | 65 |
| 0 | 5.0 | 5.0 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, X.; Huang, Y.; Kuang, P.; Wang, Y.; Liu, Y.; Yin, W.; Zan, J.; Liu, Y.; Yin, C.; et al. In Situ-Forming Cellulose/Albumin-Based Injectable Hydrogels for Localized Antitumor Therapy. Polymers 2021, 13, 4221. https://doi.org/10.3390/polym13234221
Chen Y, Wang X, Huang Y, Kuang P, Wang Y, Liu Y, Yin W, Zan J, Liu Y, Yin C, et al. In Situ-Forming Cellulose/Albumin-Based Injectable Hydrogels for Localized Antitumor Therapy. Polymers. 2021; 13(23):4221. https://doi.org/10.3390/polym13234221
Chicago/Turabian StyleChen, Ying, Xiaomin Wang, Yudong Huang, Peipei Kuang, Yushu Wang, Yong Liu, Weihan Yin, Jiahui Zan, Yupeng Liu, Chao Yin, and et al. 2021. "In Situ-Forming Cellulose/Albumin-Based Injectable Hydrogels for Localized Antitumor Therapy" Polymers 13, no. 23: 4221. https://doi.org/10.3390/polym13234221
APA StyleChen, Y., Wang, X., Huang, Y., Kuang, P., Wang, Y., Liu, Y., Yin, W., Zan, J., Liu, Y., Yin, C., & Fan, Q. (2021). In Situ-Forming Cellulose/Albumin-Based Injectable Hydrogels for Localized Antitumor Therapy. Polymers, 13(23), 4221. https://doi.org/10.3390/polym13234221







