Influence of Ulluco Starch Concentration on the Physicochemical Properties of Starch–Chitosan Biocomposite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Edible Films Preparation
2.3. Moisture Content, Swelling Power, and Solubility
2.4. Color and Transparency
2.5. Water Vapor Permeability
2.6. Mechanical Properties
2.7. Morphology of Edible Films
2.8. X-ray Diffraction of Edible Films
2.9. Fourier-Transform Infrared (ATR-FTIR) Spectroscopy of Starch and Edible Films
2.10. Contact Angle Measurement
2.11. Thermal Analysis
2.12. Statistical Analysis
3. Results
3.1. Moisture Content, Swelling Power, and Solubility
3.2. Water Vapor Permeability
3.3. Contact Angle
3.4. Optical Properties
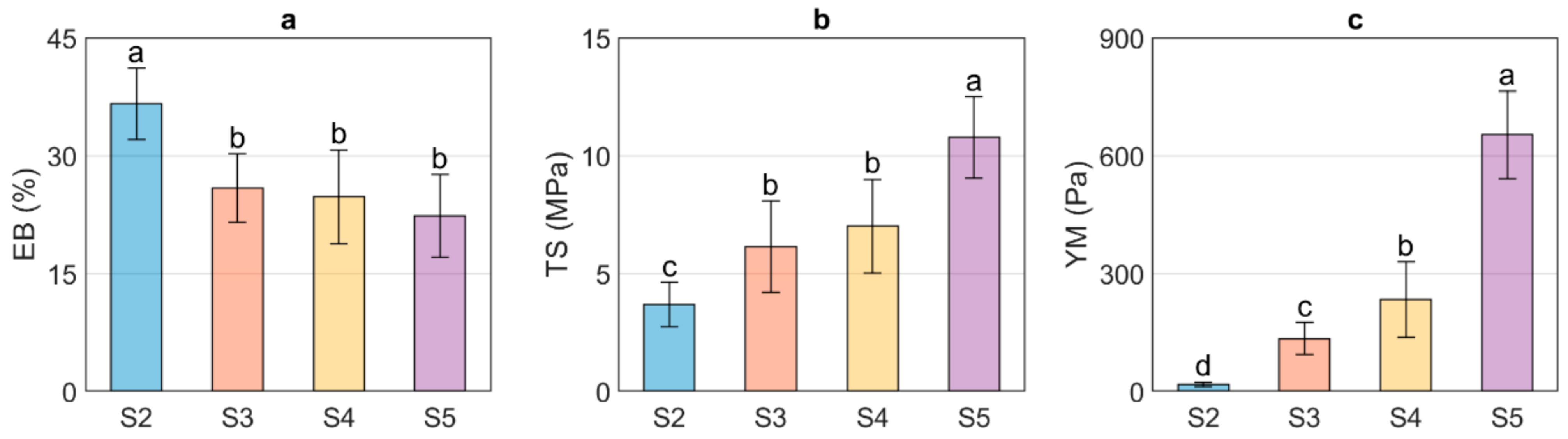
3.5. Mechanical Properties
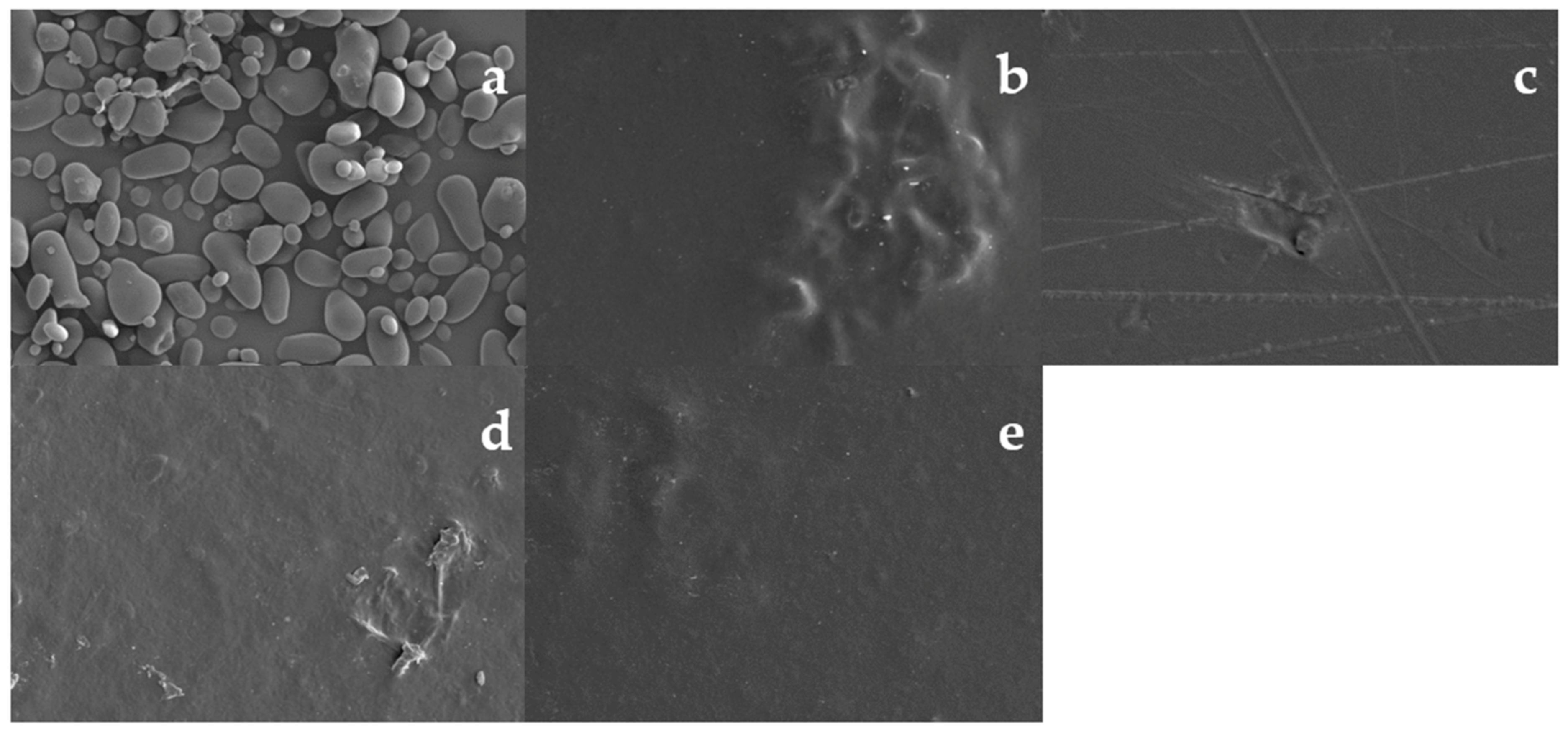
3.6. Morphology of Edible Films
3.7. X-ray Diffraction
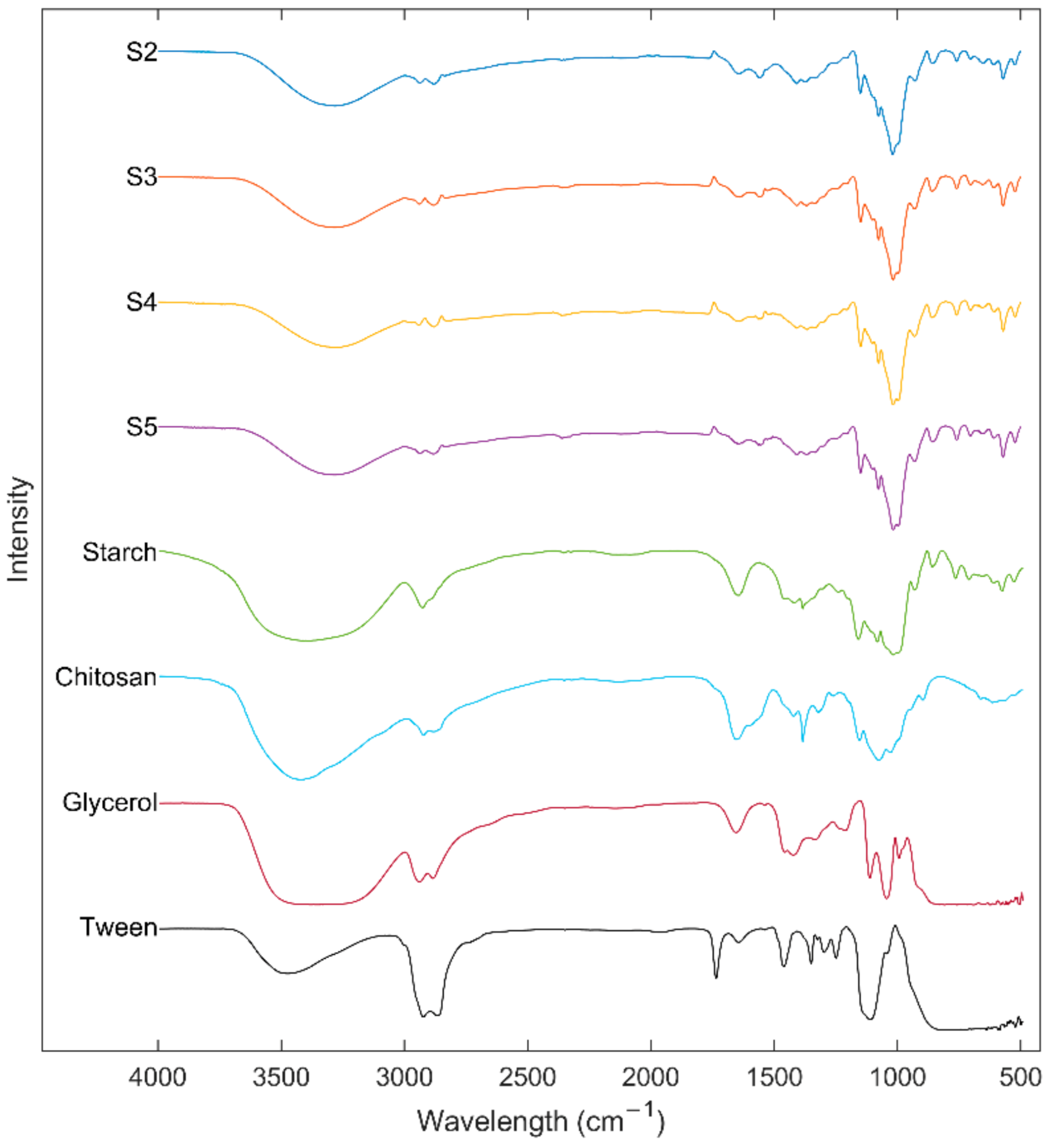
3.8. Fourier-Transform Infrared (ATR-FTIR) Spectroscopy of Starch and Edible Films
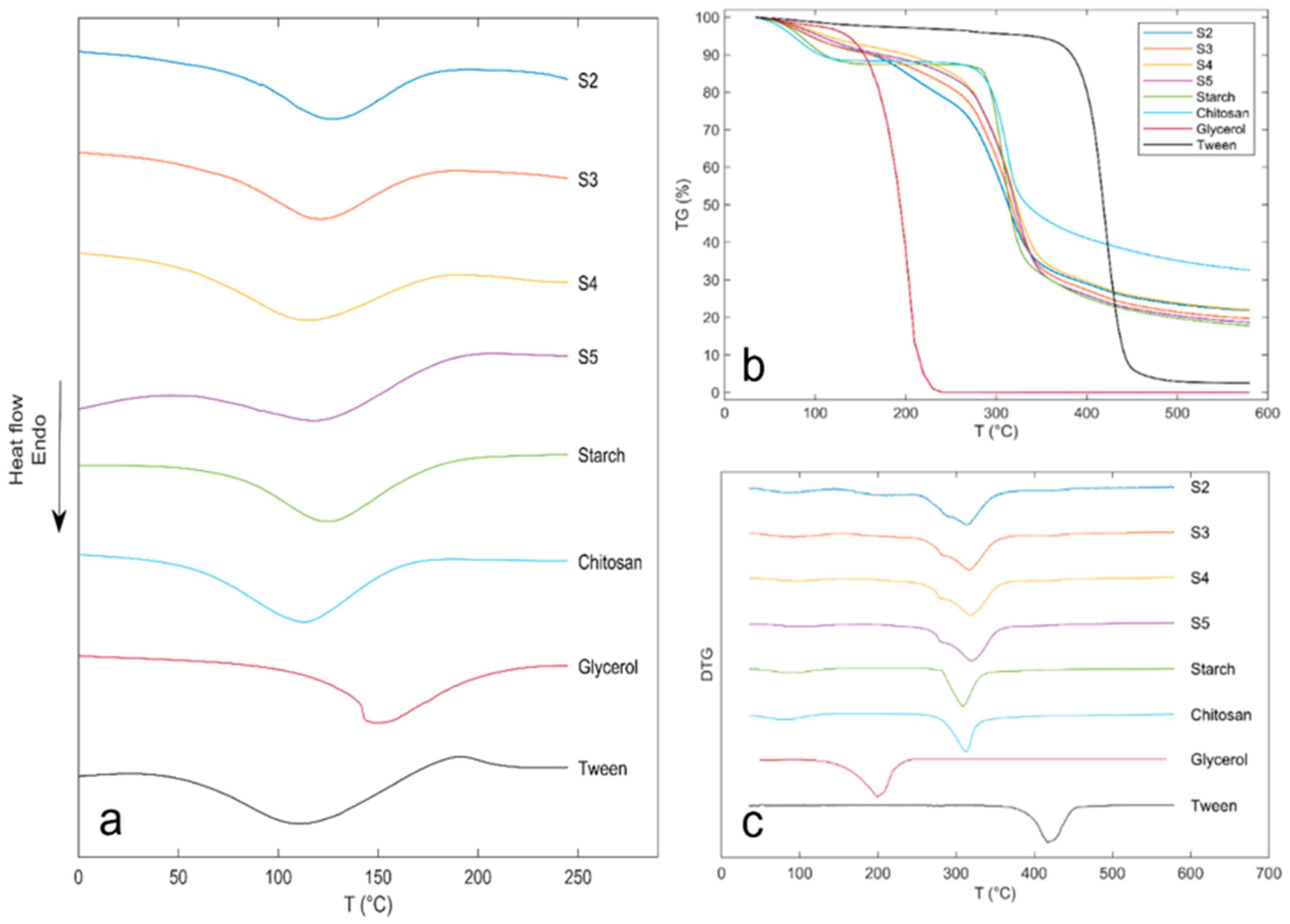
3.9. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. The Facts 2016: An Analysis of European Plastics Production, Demand and Waste Data 2016. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2016-Plastic-the-facts.pdf (accessed on 20 July 2020).
- Shapi’I, R.A.; Othman, S.H.; Nordin, N.; Basha, R.K.; Naim, M.N. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef] [PubMed]
- Spranz, R.; Schlüter, A.; Vollan, B. Morals, money or the master: The adoption of eco-friendly reusable bags. Mar. Policy 2018, 96, 270–277. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Holmberg, K.; Stripple, J. Need a bag? A review of public policies on plastic carrier bags–Where, how and to what effect? Waste Manag. 2019, 87, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.D.M.; Macedo, M.C.C.; Rodrigues, C.G.; dos Santos, A.N.; Loyola, A.C.D.F.E.; Fante, C.A. Biodegradable edible films of ripe banana peel and starch enriched with extract of Eriobotrya japonica leaves. Food Biosci. 2020, 38, 100750. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-acorn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef]
- Towle, M.A. The Ethnobotany of Pre-Columbian Peru, 1st ed.; Routledge: Chicago, IL, USA, 1961. [Google Scholar]
- Cruz, G.; Ribotta, P.; Ferrero, C.; Iturriaga, L. Physicochemical and rheological characterization of Andean tuber starches: Potato (Solanum tuberosumssp. Andigenum), Oca (Oxalis tuberosa Molina) and Papalisa (Ullucus tuberosus Caldas). Starch-Stärke 2016, 68, 1084–1094. [Google Scholar] [CrossRef]
- Valcárcel-Yamani, B.; Rondan-Sanabria, G.G.; Finardi-Filho, F. The physical, chemical and functional characterization of starches from Andean tubers: Oca (Oxalis tuberosa Molina), olluco (Ullucus tuberosus Caldas) and mashua (Tropaeolum tuberosum Ruiz & Pavón). Braz. J. Pharm. Sci. 2013, 49, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Galindez, A.; Daza, L.D.; Homez-Jara, A.; Eim, V.S.; Váquiro, H.A. Characterization of ulluco starch and its potential for use in edible films prepared at low drying temperature. Carbohydr. Polym. 2019, 215, 143–150. [Google Scholar] [CrossRef]
- Daza, L.D.; Homez, A.; Solanilla, J.F.; Váquiro, H.A. Effects of temperature, starch concentration, and plasticizer concentration on the physical properties of ulluco (Ullucus tuberosus Caldas)-based edible films. Int. J. Biol. Macromol. 2018, 120, 1834–1845. [Google Scholar] [CrossRef]
- Wang, B.; Sui, J.; Yu, B.; Yuan, C.; Guo, L.; El-Aty, A.A.; Cui, B. Physicochemical properties and antibacterial activity of corn starch-based films incorporated with Zanthoxylum bungeanum essential oil. Carbohydr. Polym. 2020, 254, 117314. [Google Scholar] [CrossRef]
- Podshivalov, A.; Zakharova, M.; Glazacheva, E.; Uspenskaya, M. Gelatin/potato starch edible biocomposite films: Correlation between morphology and physical properties. Carbohydr. Polym. 2017, 157, 1162–1172. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef]
- Pacheco, A.C.S.; Sousa, F.; Sarmento, B. Chitosan-based nanomedicine for brain delivery: Where are we heading? React. Funct. Polym. 2020, 146, 104430. [Google Scholar] [CrossRef]
- El-hack, M.E.A.; El-saadony, M.T.; Sha, M.E.; Zabermawi, N.M.; Arif, M.; Elsaber, G.; Khafaga, A.F.; El-hakim, Y.M.A.; Al-sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Luchese, C.L.; Pavoni, J.M.F.; Dos Santos, N.Z.; Quines, L.K.; Pollo, L.D.; Spada, J.C.; Tessaro, I.C. Effect of chitosan addition on the properties of films prepared with corn and cassava starches. J. Food Sci. Technol. 2018, 55, 2963–2973. [Google Scholar] [CrossRef]
- Homez-Jara, A.; Daza, L.D.; Aguirre, D.M.; Muñoz, J.A.; Solanilla, J.F.; Váquiro, H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Water Vapor Transmission of Materials; E96/E96M-16; ASTM International: West Conshohocken, PA, USA, 2016; pp. 719–725. [Google Scholar]
- ASTM. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; D882-18; ASTM International: West Conshohocken, PA, USA, 2018; Volume 8, pp. 182–190. [Google Scholar]
- Mohajer, S.; Rezaei, M.; Hosseini, S.F. Physico-chemical and microstructural properties of fish gelatin/agar bio-based blend films. Carbohydr. Polym. 2017, 157, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Khedri, S.; Sadeghi, E.; Rouhi, M.; Delshadian, Z.; Mortazavian, A.M.; Guimarães, J.D.T.; Fallah, M.; Mohammadi, R. Bioactive edible films: Development and characterization of gelatin edible films incorporated with casein phosphopeptides. LWT 2021, 138, 110649. [Google Scholar] [CrossRef]
- Jha, P. Effect of plasticizer and antimicrobial agents on functional properties of bionanocomposite films based on corn starch-chitosan for food packaging applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Saberi, B.; Pristijono, P.; Stathopoulus, C.E.; Golding, J.B.; Scarlett, C.J.; Bowyer, M.; Vuong, Q.V. Use of response surface methodology (RSM) to optimize pea starch-chitosan novel edible film formulation. J. Food Sci. Technol. 2017, 54, 2270–2278. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Pająk, P.; Przetaczek-Rożnowska, I.; Juszczak, L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Brahmakumar, M.; Abraham, T.E. Microstructural imaging and characterization of the mechanical, chemical, thermal, and swelling properties of starch–chitosan blend films. Biopolymers 2006, 82, 176–187. [Google Scholar] [CrossRef]
- Vásconez, M.B.; Flores, S.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Bof, M.J.; Bordagaray, V.C.; Locaso, D.E.; García, M.A. Chitosan molecular weight effect on starch-composite film properties. Food Hydrocoll. 2015, 51, 281–294. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Morphological characteristics and barrier properties of thermoplastic starch/chitosan blown film. Carbohydr. Polym. 2016, 150, 40–47. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Marvdashti, L.M.; Koocheki, A.; Yavarmanesh, M. Alyssum homolocarpum seed gum-polyvinyl alcohol biodegradable composite film: Physicochemical, mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 155, 280–293. [Google Scholar] [CrossRef]
- Jayasekara, R.; Harding, I.; Bowater, I.; Christie, G.; Lonergan, G. Preparation, surface modification and characterisation of solution cast starch PVA blended films. Polym. Test. 2004, 23, 17–27. [Google Scholar] [CrossRef]
- Filho, J.G.D.O.; Bezerra, C.C.D.O.N.; Albiero, B.R.; Oldoni, F.C.A.; Miranda, M.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. New approach in the development of edible films: The use of carnauba wax micro- or nanoemulsions in arrowroot starch-based films. Food Packag. Shelf Life 2020, 26, 100589. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, N.; Yokoyama, W.; Kim, Y. Effects of moisture content on mechanical properties, transparency, and thermal stability of yuba film. Food Chem. 2018, 243, 202–207. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Chillo, S.; Flores, S.; Mastromatteo, M.; Conte, A.; Gerschenson, L.; Del Nobile, M. Influence of glycerol and chitosan on tapioca starch-based edible film properties. J. Food Eng. 2008, 88, 159–168. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.; Gómez-Guillén, M. Polyphenol-rich extract from murta leaves on rheological properties of film-forming solutions based on different hydrocolloid blends. J. Food Eng. 2014, 140, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ding, J.; Fang, Y.; Pan, X.; Fan, F.; Li, P.; Hu, Q. Effect of ultrasonic power on properties of edible composite films based on rice protein hydrolysates and chitosan. Ultrason. Sonochem. 2020, 65, 105049. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Illias, H.A.; Kadir, M.F.Z. Conductivity and electrical properties of corn starch–chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys. Scr. 2014, 89, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2012, 116, 588–597. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Mostaghimi, M.; Ghiasi, F.; Tavakoli, S.; Naseri, M.; Hosseini, S.M.H. The effects of fatty acids chain length on the techno-functional properties of basil seed gum-based edible films. Int. J. Biol. Macromol. 2020, 160, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Chakravartula, S.S.N.; Lourenço, R.V.; Balestra, F.; Bittante, A.M.Q.B.; Sobral, P.J.D.A.; Rosa, M.D. Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Shi, J.; Zhang, X.; Lian, H.; Wang, Q.; Peng, Y. Effects of peanut shell and skin extracts on the antioxidant ability, physical and structure properties of starch-chitosan active packaging films. Int. J. Biol. Macromol. 2020, 152, 137–146. [Google Scholar] [CrossRef]

| Sample | Initial Concentration of Solutions | Final Concentration of Films | ||||
|---|---|---|---|---|---|---|
| US (%) | Ch (%) | US (%) | Ch (%) | Glycerol (%) | Tween 80® (%) | |
| S2 | 2.0 | 1.5 | 1.0 | 0.75 | 1 | 0.1 |
| S3 | 3.0 | 1.5 | 1.5 | 0.75 | 1 | 0.1 |
| S4 | 4.0 | 1.5 | 2.0 | 0.75 | 1 | 0.1 |
| S5 | 5.0 | 1.5 | 2.5 | 0.75 | 1 | 0.1 |
| Sample | MC (%) | Solubility (%) | SP (%) | WVP × 10−11 (g/m s Pa) | Opacity | YI |
|---|---|---|---|---|---|---|
| S2 | 33.4 ± 1.2 a | 17.5 ± 1.0 c | 39 ± 5 d | 5.6 ± 0.3 c | 0.40 ± 0.06 a | 5.7 ± 1.7 a |
| S3 | 21.1 ± 1.6 b | 19.6 ± 0.7 b | 116 ± 20 c | 6.9 ± 0.5 b | 0.32 ± 0.02 b | 5.3 ± 0.9 a |
| S4 | 17.9 ± 1.2 c | 19.7 ± 0.8 b | 177 ± 22 b | 6.4 ± 0.7 bc | 0.32 ± 0.03 b | 4.1 ± 0.4 b |
| S5 | 15.2 ± 0.8 d | 21.7 ± 1.1 a | 267 ± 18 a | 8.5 ± 0.9 a | 0.31 ± 0.01 b | 4.1 ± 0.4 b |
| Sample | Tm (°C) | Thermal Degradation Temperature (°C) | Total Loss (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | ||||||
| Tonset (°C) | Tmax (°C) | Tonset (°C) | Tmax. (°C) | Tonset (°C) | Tmax. (°C) | |||
| S2 | 119 ± 9.52 | 47.7 | 87.3 | 259.9 | 313.8 | 398.6 | 418.5 | 79.7 |
| S3 | 121 ± 0.98 | 47.7 | 91.9 | 263.9 | 316.5 | 391.1 | 419.2 | 80.3 |
| S4 | 114 ± 4.16 | 52.1 | 99.2 | 261.9 | 318.6 | 396.5 | 418.5 | 78.1 |
| S5 | 126 ± 5.60 | 52.1 | 98.4 | 263.9 | 319.8 | 393.5 | 418.4 | 81.5 |
| Glycerol | 145 ± 1.99 | 60.2 | 103.9 | 230.7 | 273.4 | - | - | 97.5 |
| Tween 80® | 115 ± 9.34 | 392.4 | 419.5 | - | - | - | - | 100 |
| Starch | 128 ± 5.26 | 50.0 | 89.3 | 281.9 | 308.5 | - | - | 78.3 |
| Chitosan | 113 ± 0.66 | 45.7 | 82.6 | 282.4 | 312.2 | - | - | 67.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daza, L.D.; Eim, V.S.; Váquiro, H.A. Influence of Ulluco Starch Concentration on the Physicochemical Properties of Starch–Chitosan Biocomposite Films. Polymers 2021, 13, 4232. https://doi.org/10.3390/polym13234232
Daza LD, Eim VS, Váquiro HA. Influence of Ulluco Starch Concentration on the Physicochemical Properties of Starch–Chitosan Biocomposite Films. Polymers. 2021; 13(23):4232. https://doi.org/10.3390/polym13234232
Chicago/Turabian StyleDaza, Luis Daniel, Valeria Soledad Eim, and Henry Alexander Váquiro. 2021. "Influence of Ulluco Starch Concentration on the Physicochemical Properties of Starch–Chitosan Biocomposite Films" Polymers 13, no. 23: 4232. https://doi.org/10.3390/polym13234232
APA StyleDaza, L. D., Eim, V. S., & Váquiro, H. A. (2021). Influence of Ulluco Starch Concentration on the Physicochemical Properties of Starch–Chitosan Biocomposite Films. Polymers, 13(23), 4232. https://doi.org/10.3390/polym13234232






