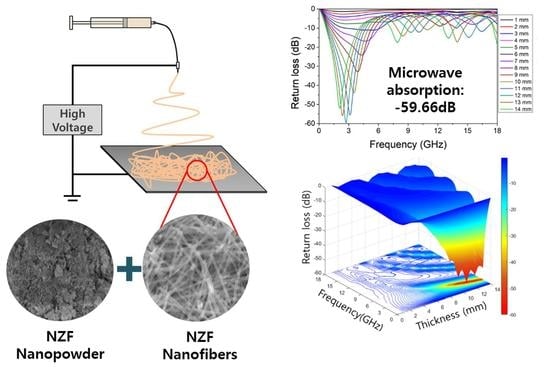
Fabrication of Electrospun Ni0.5Zn0.5Fe2O4 Nanofibers Using Polyvinyl Pyrrolidone Precursors and Electromagnetic Wave Absorption Performance Improvement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
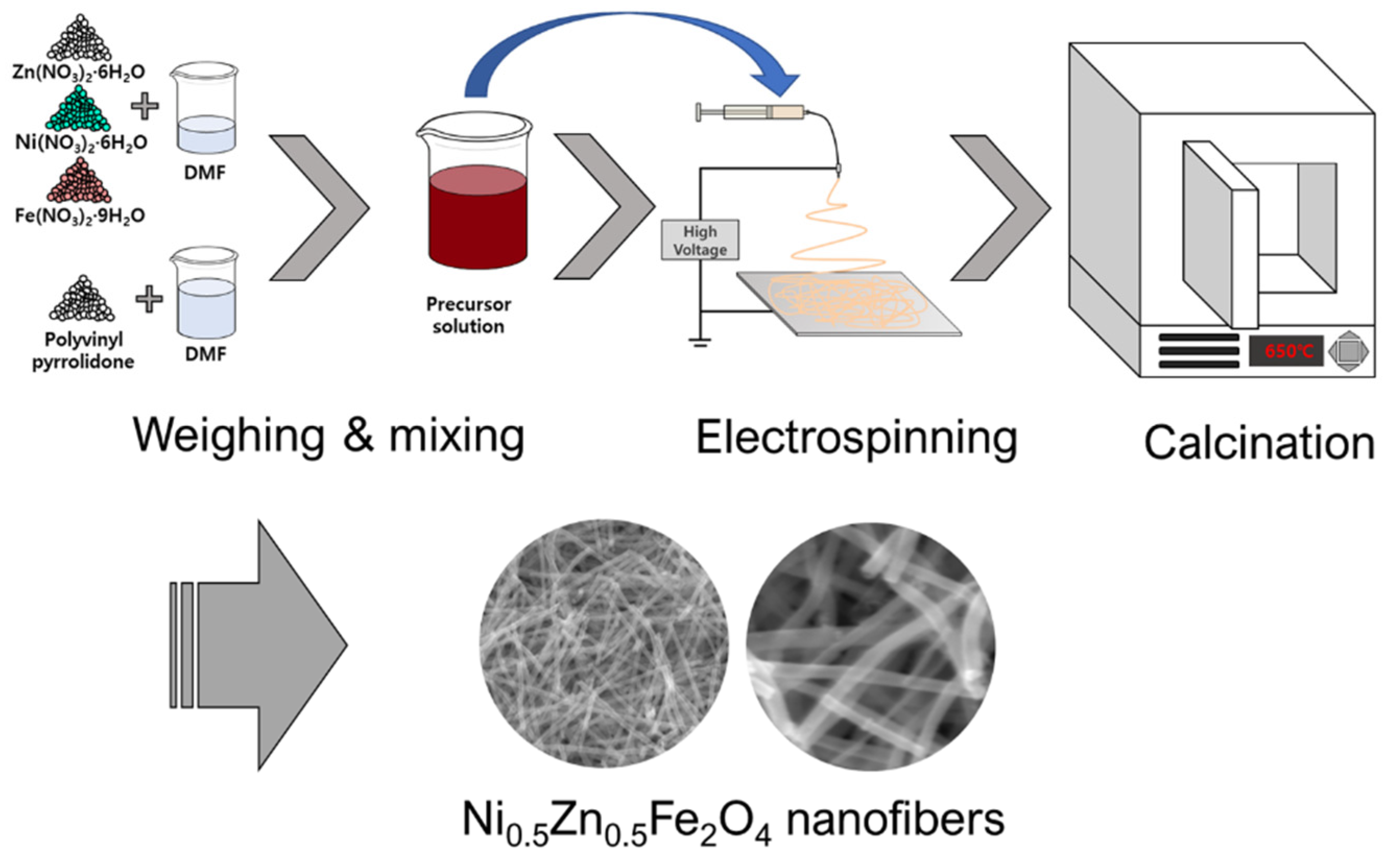
2.2. Electrospinning Process
2.3. Fabrication of RL Measuring Sample
2.4. Characterization
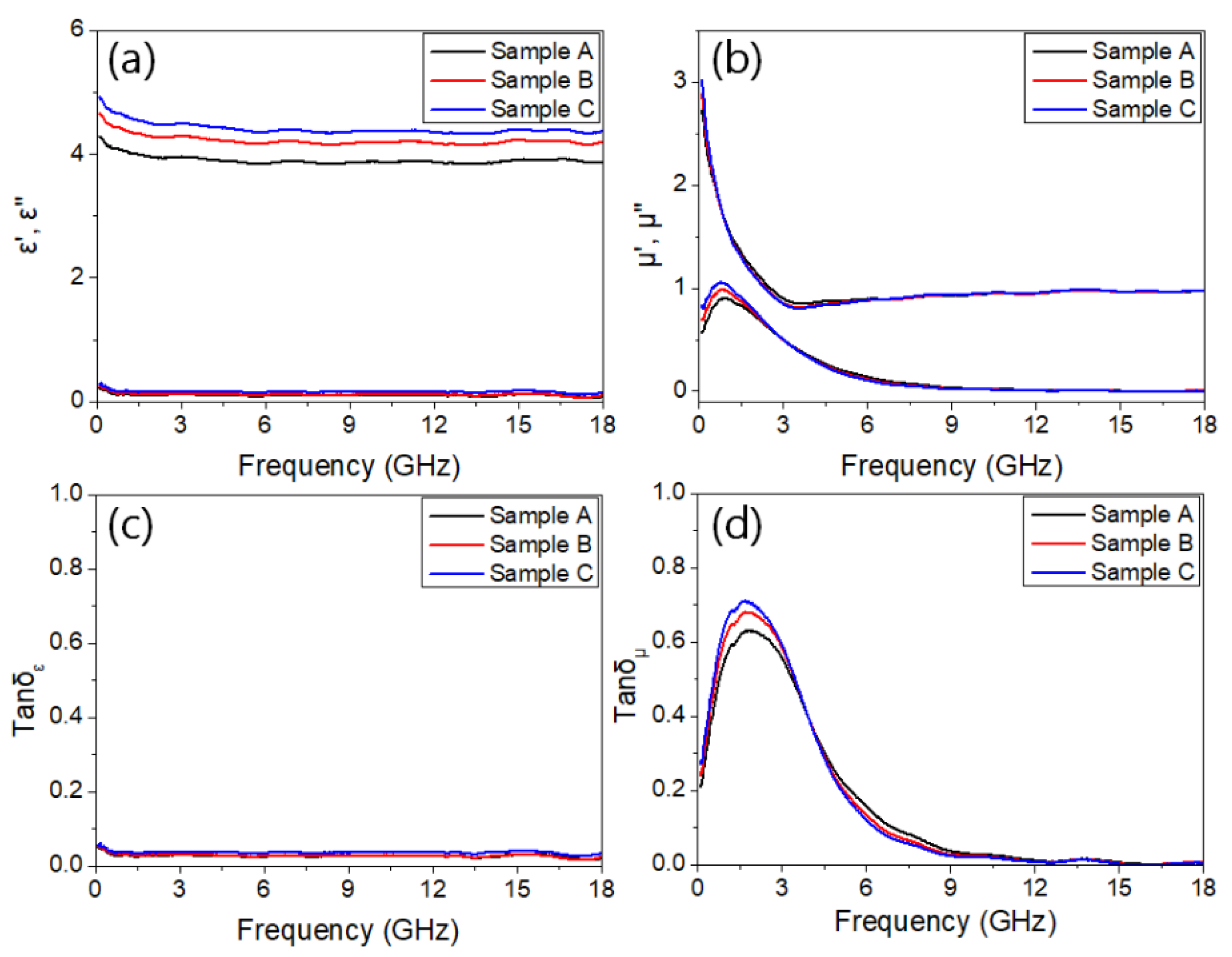
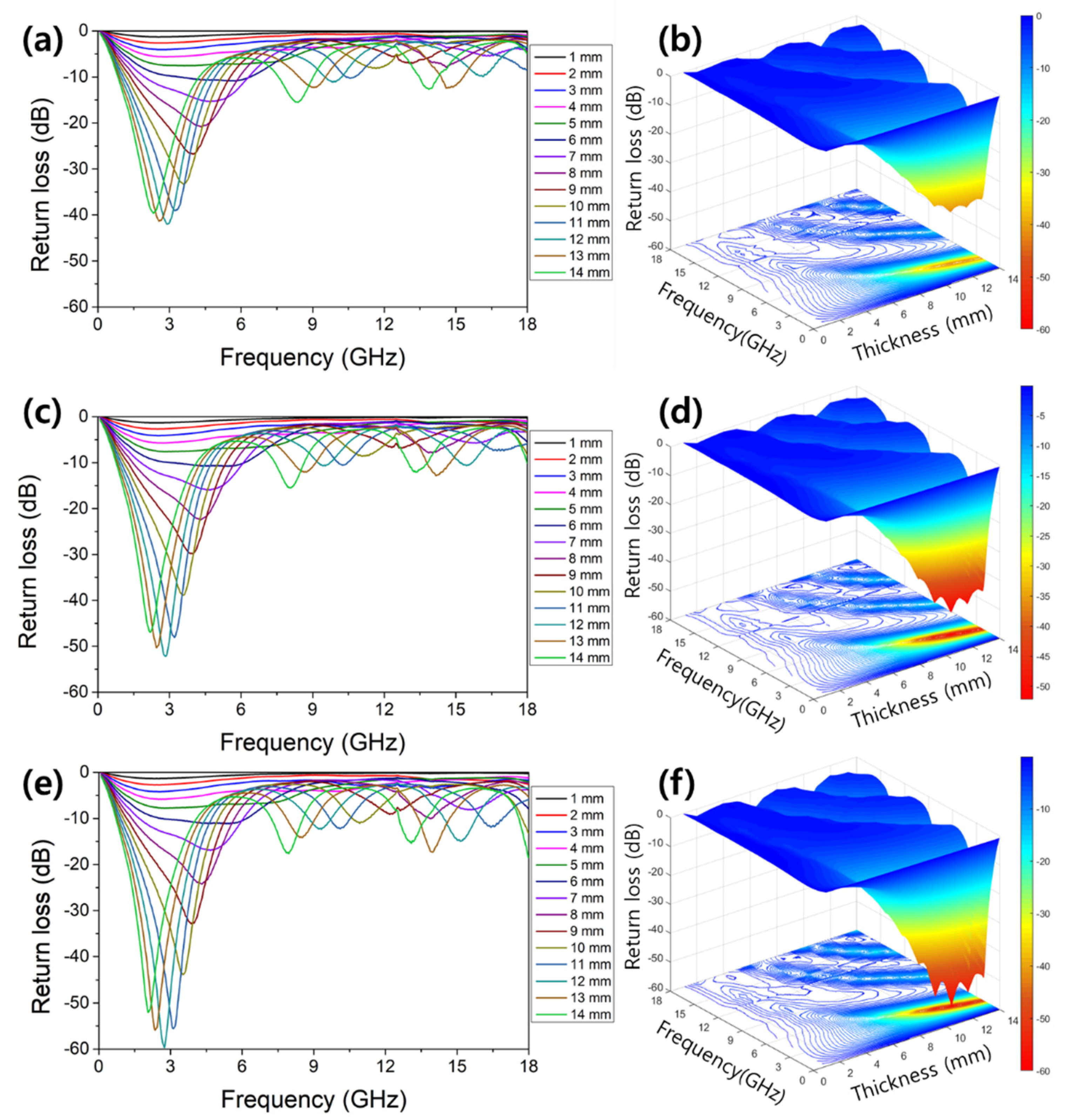
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anyaka, B.O.; Akuru, U.B. Electromagnetic wave effect on human health: Challenges for developing countries. In Proceedings of the 2012 International Conference on Cyber-Enabled Distributed Computing and Knowledge Discovery, Sanya, China, 10–12 October 2012; pp. 447–452. [Google Scholar]
- Shen, J.-E. Analysis of the harm of electromagnetic wave pollution and its protection strategies. In Proceedings of the 2020 Cross Strait Radio Science & Wireless Technology Conference (CSRSWTC), Fuzhou, China, 13–16 December 2020; pp. 1–2. [Google Scholar]
- Xing, J.Y. Electromagnetic radiation on human health hazards and protective measures in modern society. In Advanced Materials Research; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2012; Volume 518, pp. 1022–1026. [Google Scholar]
- Singh, S.K.; Akhtar, M.J.; Kar, K.K. Hierarchical carbon nanotube-coated carbon fiber: Ultra lightweight, thin, and highly efficient microwave absorber. ACS Appl. Mater. Interfaces 2018, 10, 24816–24828. [Google Scholar] [CrossRef]
- Mahanta, U.J.; Gogoi, J.P.; Borah, D.; Bhattacharyya, N.S. Dielectric characterization and microwave absorption of expanded graphite integrated polyaniline multiphase nanocomposites in X-band. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 194–201. [Google Scholar] [CrossRef]
- Chen, H.; Lu, W.-B.; Liu, Z.-G.; Zhang, J.; Zhang, A.-Q.; Wu, B. Experimental demonstration of microwave absorber using large-area multilayer graphene-based frequency selective surface. IEEE Trans. Microw. Theory Tech. 2018, 66, 3807–3816. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Y.; Li, F.; Tang, L.; Wang, T.; Qiao, L. Submicron carbonyl iron particles as an efficient microwave absorber in the low frequency band. J. Phys. D Appl. Phys. 2017, 50, 475001. [Google Scholar] [CrossRef] [Green Version]
- Min, D. Thin-thickness and wide-bandwidth microwave absorber with highly aligned carbonyl iron flakes and graphene nanosheets. J. Mater. Sci. Mater. Electron. 2019, 30, 17119–17127. [Google Scholar] [CrossRef]
- He, J.; Luo, H.; He, L.; Yan, S.; Shan, D.; Huang, S.; Deng, L. Investigation on microwave dielectric behavior of flaky carbonyl iron composites. J. Mater. Sci. Mater. Electron. 2018, 29, 15112–15118. [Google Scholar] [CrossRef]
- Singh, J.; Singh, C.; Kaur, D.; Narang, S.B.; Jotania, R.B.; Ateia, E.; Kagdi, A.; Joshi, R.; Sombra, A.S.B.; Zhou, D. Development of doped Ba–Sr hexagonal ferrites for microwave absorber applications: Structural characterization, tunable thickness, absorption peaks and electromagnetic parameters. J. Alloys Compd. 2021, 855, 157242. [Google Scholar] [CrossRef]
- Dhruv, P.N.; Pullar, R.C.; Singh, C.; Carvalho, F.E.; Jotania, R.B.; Meena, S.S.; Singh, J. Design and development of Ga-substituted Z-type hexaferrites for microwave absorber applications: Mössbauer, static and dynamic properties. Ceram. Int. 2021, 47, 1145–1162. [Google Scholar] [CrossRef]
- Narang, S.B.; Pubby, K. Electromagnetic characterization of Co-Ti-Doped Ba-M ferrite-based frequency-tunable microwave absorber in 12.4–40 GHz. J. Supercond. Nov. Magn. 2017, 30, 511–520. [Google Scholar] [CrossRef]
- Zhang, G.; Shu, R.; Xie, Y.; Xia, H.; Gan, Y.; Shi, J.; He, J. Cubic MnFe2O4 particles decorated reduced graphene oxide with excellent microwave absorption properties. Mater. Lett. 2018, 231, 209–212. [Google Scholar] [CrossRef]
- Mandal, D.; Mandal, K. Enhancement of electromagnetic wave absorption in MnFe2O4 nano-hollow spheres. J. Appl. Phys. 2021, 129, 74902. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhang, W.; Huang, S. One-pot synthesis of MnFe2O4 nanoparticles-decorated reduced graphene oxide for enhanced microwave absorption properties. Mater. Technol. 2017, 32, 32–37. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Zhang, Y.; Feng, R.; Chen, X.; Xiong, C.; Dong, L. Broadband and lightweight microwave absorber constructed by in situ growth of hierarchical CoFe2O4/reduced graphene oxide porous nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 13860–13868. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, G.; Wu, H.; Liu, B.; Fang, X.; Zhang, D.; Zhu, J. Double-layer microwave absorber based on nanocrystalline CoFe2O4 and CoFe2O4/PANI multi-core/shell composites. Mater. Sci. Pol. 2017, 35, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, Y.; Xue, H.; Xie, J.; Feng, C.; Li, H.; Shi, D.; Muhammad, S.; Jiao, Q. Preparation of flower-like CoFe2O4@graphene composites and their microwave absorbing properties. Mater. Lett. 2018, 223, 186–189. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Wu, X.; Zhang, W.; Luo, C.; Liu, P. Facile design of 3D hierarchical NiFe2O4/N-GN/ZnO composite as a high performance electromagnetic wave absorber. Chem. Eng. J. 2019, 375, 121942. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilčáková, J.; Machovský, M.; Škoda, D.; Urbánek, P.; Masař, M.; Gořalik, M.; Urbánek, M.; Kalina, L. Polypropylene nanocomposite filled with spinel ferrite NiFe2O4 nanoparticles and in-situ thermally-reduced graphene oxide for electromagnetic interference shielding application. Nanomaterials 2019, 9, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; He, Y.; Chen, D.; Du, B.; Cao, W.; Lv, Y.; Ding, Z. Hydrothermal Synthesis and Microwave Absorption Properties of Nickel Ferrite/Multiwalled Carbon Nanotubes Composites. Coatings 2021, 11, 534. [Google Scholar] [CrossRef]
- Ali, K.; Iqbal, J.; Jana, T.; Ahmad, N.; Ahmad, I.; Wan, D. Enhancement of microwaves absorption properties of CuFe2O4 magnetic nanoparticles embedded in MgO matrix. J. Alloys Compd. 2017, 696, 711–717. [Google Scholar] [CrossRef]
- Elayakumar, K.; Manikandan, A.; Dinesh, A.; Thanrasu, K.; Raja, K.K.; Kumar, R.T.; Slimani, Y.; Jaganathan, S.K.; Baykal, A. Enhanced magnetic property and antibacterial biomedical activity of Ce3+ doped CuFe2O4 spinel nanoparticles synthesized by sol-gel method. J. Magn. Magn. Mater. 2019, 478, 140–147. [Google Scholar] [CrossRef]
- Ali, K.; Iqbal, J.; Jan, T.; Ahmad, I.; Wan, D.; Bahadur, A.; Iqbal, S. Synthesis of CuFe2O4-ZnO nanocomposites with enhanced electromagnetic wave absorption properties. J. Alloys Compd. 2017, 705, 559–565. [Google Scholar] [CrossRef]
- Heidari, P.; Masoudpanah, S.M. A facial synthesis of MgFe2O4/RGO nanocomposite powders as a high performance microwave absorber. J. Alloys Compd. 2020, 834, 155166. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Q.; Zhu, X.; Fu, M. One-step in situ growth of magnesium ferrite nanorods on graphene and their microwave-absorbing properties. Appl. Organomet. Chem. 2018, 32, e4017. [Google Scholar] [CrossRef]
- He, G.; Duan, Y.; Pang, H.; Zhang, X. Rational design of mesoporous MnO2 microwave absorber with tunable microwave frequency response. Appl. Surf. Sci. 2019, 490, 372–382. [Google Scholar] [CrossRef]
- Zhan, J.; Yao, Y.; Zhang, C.; Li, C. Synthesis and microwave absorbing properties of quasione-dimensional mesoporous NiCo2O4 nanostructure. J. Alloys Compd. 2014, 585, 240–244. [Google Scholar] [CrossRef]
- Liu, H.; Cui, G.; Li, L.; Zhang, Z.; Lv, X.; Wang, X. Polypyrrole Chains Decorated on CoS Spheres: A Core-Shell Like Heterostructure for High-Performance Microwave Absorption. Nanomaterials 2020, 10, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Deng, Y.; Han, C.; Zhu, H.; Yan, C.; Zhang, H. Enhanced Microwave Absorption Bandwidth in Graphene-Encapsulated Iron Nanoparticles with Core–Shell Structure. Nanomaterials 2020, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, R.; Huang, Z.; Kang, F.; Gu, J. Synthesis and microwave absorbing properties of FeCo alloy particles/graphite nanoflake composites. J. Alloys Compd. 2011, 509, 494–498. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, N.; Muhammad, J.; Wang, D.; Duan, Y.; Zhang, X.; Dong, X.; Zhang, Z. Graphene nanoflakes with optimized nitrogen doping fabricated by arc discharge as highly efficient absorbers toward microwave absorption. Carbon 2019, 148, 204–213. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Xiao, S.; Chen, G. The cobalt zinc spinel ferrite nanofiber: Lightweight and efficient microwave absorber. J. Am. Ceram. Soc. 2014, 97, 1363–1366. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Lai, M.; Sang, T. Preparation and microwave absorption mechanisms of the NiZn ferrite nanofibers. J. Alloys Compd. 2015, 627, 367–373. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; Cheng, J.; Cao, Q.; Zheng, G.; Liang, S.; Wang, H.; Cao, M.-S. Lightweight and high-performance microwave absorber based on 2D WS2–RGO heterostructures. Nano-Micro Lett. 2019, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, D.; Hou, L.; Wang, S.; Luo, H.; Deng, L.; He, J.; Song, M. Facile synthesis of Fe/Fe3C-C core-shell nanoparticles as a high-efficiency microwave absorber. Appl. Surf. Sci. 2019, 493, 1083–1089. [Google Scholar] [CrossRef]
- Liu, P.; Peng, J.; Chen, Y.; Liu, M.; Tang, W.; Guo, Z.-H.; Yue, K. A general and robust strategy for in-situ templated synthesis of patterned inorganic nanoparticle assemblies. Giant 2021, 8, 100076. [Google Scholar] [CrossRef]
- Ghasemi, A.; Liu, X.; Morisako, A. Magnetic and microwave absorption properties of BaFe12−x(Mn0.5Cu0.5Zr)x/2O19 synthesized by sol–gel processing. J. Magn. Magn. Mater. 2007, 316, e105–e108. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, J.; Wu, J.; Du, J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2021, 14, 1–18. [Google Scholar] [CrossRef]
- Bayan, M.A.H.; Taromi, F.A.; Lanzi, M.; Pierini, F. Enhanced efficiency in hollow core electrospun nanofiber-based organic solar cells. Sci. Rep. 2021, 11, 21144. [Google Scholar]
- Massaro, L.M.; Gentilini, S.; Portone, A.; Camposeo, A.; Pisignano, D.; Conti, C.; Ghofraniha, N. Heterogeneous random laser with switching activity visualized by replica symmetry breaking maps. ACS Photonics 2021, 8, 376–383. [Google Scholar] [CrossRef]
- Derakhshani, M.; Taheri-Nassaj, E.; Jazirehpour, M.; Masoudpanah, S.M. Structural, magnetic, and gigahertz-range electromagnetic wave absorption properties of bulk Ni–Zn ferrite. Sci. Rep. 2021, 11, 9468. [Google Scholar]
- Wolska, E.; Wolski, W.; Piszora, P.; Pietrusik, M.; Šubrt, J.; Grygar, T.; Nejezchleba, M. X-ray powder diffraction and Mössbauer studies on the formation of Cd0.5Ni0.5Fe2O4/Zn0.5Ni0.5Fe2O4 spinel solid solutions. Int. J. Inorg. Mater. 1999, 1, 187–192. [Google Scholar] [CrossRef]
- Zhu, T.; Chang, S.; Song, Y.-F.; Lahoubi, M.; Wang, W. PVP-encapsulated CoFe2O4/rGO composites with controllable electromagnetic wave absorption performance. Chem. Eng. J. 2019, 373, 755–766. [Google Scholar] [CrossRef]
- Wen, F.; Yi, H.; Qiao, L.; Zheng, H.; Zhou, D.; Li, F. Analyses on double resonance behavior in microwave magnetic permeability of multiwalled carbon nanotube composites containing Ni catalyst. Appl. Phys. Lett. 2008, 92, 42507. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Z.; Ng, V.M.H.; Zhou, J.; Kong, L.B.; Yue, K. Facile synthesis of ultrasmall Fe3O4 nanoparticles on MXenes for high microwave absorption performance. Compos. Part A Appl. Sci. Manuf. 2018, 115, 371–382. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Z.; Zhou, J.; Yang, Z.; Kong, L.B. Small magnetic Co-doped NiZn ferrite/graphene nanocomposites and their dual-region microwave absorption performance. J. Mater. Chem. C 2016, 4, 9738–9749. [Google Scholar] [CrossRef]


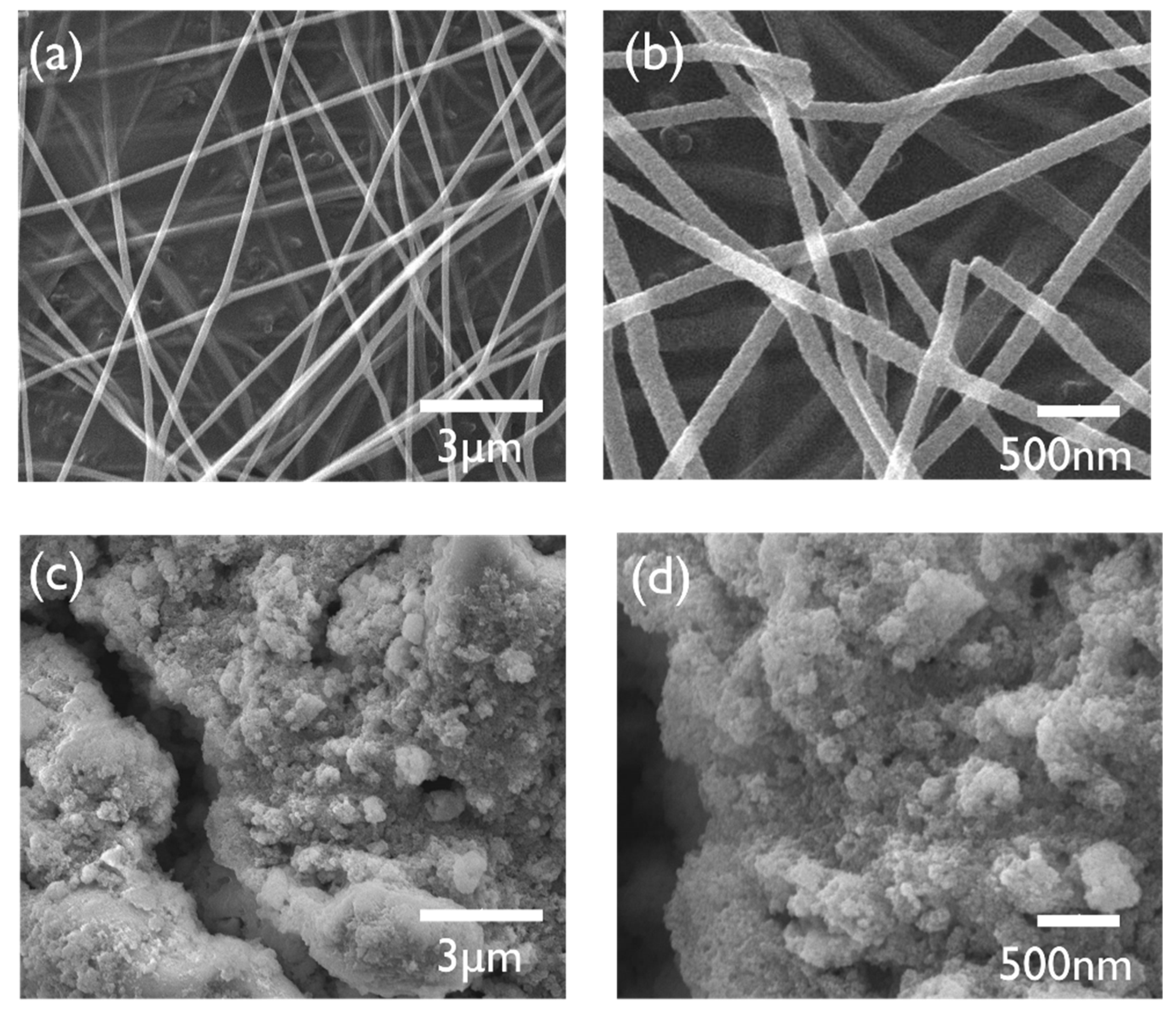
| Sample | Plane | 2θ (°) | FWHM (°) | Calculated Average Grain Size (Diameter, nm) |
|---|---|---|---|---|
| Commercial | (022) | 41.5 | 1.11 | 9.22 |
| (131) | 35.22 | 1.12 | ||
| (004) | 67.4 | 1.24 | ||
| 450 °C | (022) | 41.46 | 1.22 | 8.44 |
| (131) | 35.14 | 1.27 | ||
| (004) | 67.3 | 1.3 | ||
| 550 °C | (022) | 41.48 | 1.15 | 8.79 |
| (131) | 35.18 | 1.23 | ||
| (004) | 67.38 | 1.26 | ||
| 650 °C | (022) | 41.48 | 1.01 | 10.17 |
| (131) | 35.2 | 0.97 | ||
| (004) | 67.36 | 1.17 | ||
| 750 °C | (022) | 41.46 | 0.66 | 14.51 |
| (131) | 35.14 | 0.93 | ||
| (004) | 67.34 | 0.68 |
| Element | Weight Percent (%) | Atomic Percent (%) | Net Int. | Error (%) |
|---|---|---|---|---|
| Fe | 46.58 | 29.71 | 1082.70 | 2.56 |
| Ni | 13.23 | 8.03 | 221.19 | 5.08 |
| Zn | 16.19 | 8.82 | 176.36 | 5.41 |
| O | 24.00 | 53.44 | 916.52 | 7.08 |
| Sample | NZF Nanoparticles (g) | NZF Nanofibers (g) | Epoxy Binder (g) |
|---|---|---|---|
| A | 0.450 | - | 0.050 |
| B | 0.405 | 0.045 | 0.050 |
| C | 0.360 | 0.09 | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, K.-H.; Jang, K.-P.; Kim, S.-W.; Choi, W.-Y. Fabrication of Electrospun Ni0.5Zn0.5Fe2O4 Nanofibers Using Polyvinyl Pyrrolidone Precursors and Electromagnetic Wave Absorption Performance Improvement. Polymers 2021, 13, 4247. https://doi.org/10.3390/polym13234247
Na K-H, Jang K-P, Kim S-W, Choi W-Y. Fabrication of Electrospun Ni0.5Zn0.5Fe2O4 Nanofibers Using Polyvinyl Pyrrolidone Precursors and Electromagnetic Wave Absorption Performance Improvement. Polymers. 2021; 13(23):4247. https://doi.org/10.3390/polym13234247
Chicago/Turabian StyleNa, Kyeong-Han, Kyong-Pil Jang, Sung-Wook Kim, and Won-Youl Choi. 2021. "Fabrication of Electrospun Ni0.5Zn0.5Fe2O4 Nanofibers Using Polyvinyl Pyrrolidone Precursors and Electromagnetic Wave Absorption Performance Improvement" Polymers 13, no. 23: 4247. https://doi.org/10.3390/polym13234247
APA StyleNa, K.-H., Jang, K.-P., Kim, S.-W., & Choi, W.-Y. (2021). Fabrication of Electrospun Ni0.5Zn0.5Fe2O4 Nanofibers Using Polyvinyl Pyrrolidone Precursors and Electromagnetic Wave Absorption Performance Improvement. Polymers, 13(23), 4247. https://doi.org/10.3390/polym13234247