Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis
Abstract
:1. Introduction
2. Materials and Methods
3. Formulation of the Problem
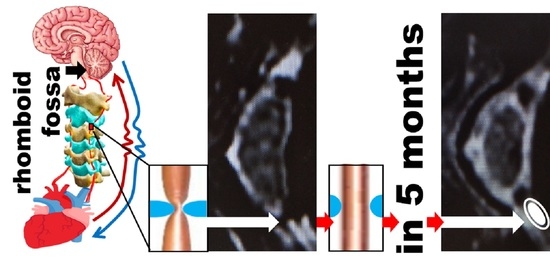
4. The Explanation of the Hypothesis
- Increment of arterial linear blood flow velocity(VA) through cervical arteries, since namely they have access to the oxygen detector.
- Decrement of BP.
- Restoration of measurable body parameters like pulse, pH, [Fe], etc., to the normal values.
- Restoration of cartilage, starting from its biopolymeric part, easily visible on MRI.
5. Discussion on the Hypothesis Verification
6. Conclusions
- The restoration of the arterial BP;
- The restoration of the organism’s homeostatic ability to self-repair the cartilage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Liu, T.H.; Liu, Y.Q.; Peng, B.G. Cervical intervertebral disc degeneration and dizziness. World J. Clin. Cases 2021, 9, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Armbrecht, G.; European Vertebral Osteoporosis Study; European Prospective Osteoporosis Study Groups. Degenerative inter-vertebral disc disease osteochondrosis intervertebralis in Europe: Prevalence, geographic variation and radiological correlates in men and women aged 50 and over. Rheumatology 2017, 56, 1189–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, M. The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review. Cells 2020, 9, 2208. [Google Scholar] [CrossRef]
- Choy, W.J. Annular closure device for disc herniation: Meta-analysis of clinical outcome and complications. BMC Musculoskelet. Disord. 2018, 19, 290. [Google Scholar] [CrossRef]
- Kerr, D.; Zhao, W.; Lurie, J.D. What Are Long-term Predictors of Outcomes for Lumbar Disc Herniation? A Randomized and Observational Study. Clin. Orthop. Relat. Res. 2015, 473, 1920–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, T. Rehabilitation after lumbar disc surgery. Cochrane Database Syst. Rev. 2014, 3, CD003007. [Google Scholar] [CrossRef] [Green Version]
- Spoto, M.M.; Dixon, G. An integrated approach to the examination and treatment of a patient with chronic low back pain. Physiother. Theory Pract. 2015, 31, 67–75. [Google Scholar] [CrossRef]
- Azevedo, D.C. Movement System Impairment-Based Classification Versus General Exercise for Chronic Low Back Pain: Protocol of a Randomized Controlled Trial. Phys. Ther 2015, 95, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M. Clinical diagnosis and treatment of a patient with low back pain using the patient response model: A case report. Physiother. Theory Pract. 2016, 32, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Diwan, A.D.; Tipper, J.L. Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus. Int. J. Mol. Sci. 2020, 21, 4889. [Google Scholar] [CrossRef]
- Choi, Y.; Park, M.H.; Lee, K. Tissue Engineering Strategies for Intervertebral Disc Treatment Using Functional Polymers. Polymers 2019, 11, 872. [Google Scholar] [CrossRef] [Green Version]
- Chu, G. Biomechanics in Annulus Fibrosus Degeneration and Regeneration. Adv. Exp. Med. Biol. 2018, 1078, 409–420. [Google Scholar] [PubMed]
- Moriguchi, Y. In vivo annular repair using high-density collagen gel seeded with annulus fibrosus cells. Acta Biomater. 2018, 79, 230–238. [Google Scholar] [CrossRef]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef]
- Benneker, L.M. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur. Spine J. 2005, 14, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laor, T.; Clarke, J.; Yin, H. Development of the long bones in the hands and feet of children: Radiographic and MR imaging correlation. Pediatr. Radiol. 2016, 46, 551–561. [Google Scholar] [CrossRef]
- Karsdal, M.A. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res. Ther. 2008, 10, R63. [Google Scholar] [CrossRef] [Green Version]
- Billman, G.E. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front. Physiol. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Tlidi, M. Observation and modelling of vegetation spirals and arcs in isotropic environmental conditions: Dissipative structures in arid landscapes. Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 2135–2146. [Google Scholar] [CrossRef] [Green Version]
- Tlidi, M.; Clerc, M.G.; Panajotov, K. Dissipative structures in matter out of equilibrium: From chemistry, photonics and biology, the legacy of Ilya Prigogine (part 2). Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 2147–2155. [Google Scholar]
- Tlidi, M.; Clerc, M.G.; Panajotov, K. Dissipative structures in matter out of equilibrium: From chemistry, photonics and biology, the legacy of Ilya Prigogine (part 1). Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 2124–2134. [Google Scholar]
- Vetcher, A.A. The cervical blood flow parameters with the best correlation from arterial blood pressure in hypertension cases. Int. J. Rec. Sci. Res. 2021, 12, 42957–42958. [Google Scholar]
- Shishonin, A. Method for Treating Cervical Osteochondrosis. Patent of RF RU 2 243 758C2, 2003. [Google Scholar]
- Belousov, B.P. A periodic reaction and its mechanism. In Collection of Short Papers on Radiation Medicine Conference for 1958; Medgiz: Moscow, Russia, 1959. [Google Scholar]
- Sel’kov, E.E. Self-Oscillations in Glycolysis.1. A Simple Kinetic Model. Eur. J. Biochem. 1968, 4, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Frederick, D.W. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab. 2016, 24, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, E.; Kim, M.S. Brain Regulation of Energy Metabolism. Endocrinol. Metab. 2016, 31, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; W.H.Freeman: New York, NY, USA, 2008. [Google Scholar]
- Dobroborsky, B.S. Thermodynamics of Biological Systems; North-Western State Medical University Press: Saint-Petersburg, Russia, 2006. [Google Scholar]
- Curtelin, D. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J. Cereb. Blood Flow Metab. 2018, 38, 136–150. [Google Scholar] [CrossRef] [Green Version]
- He, Z.B. Atlantoaxial Misalignment Causes High Blood Pressure in Rats: A Novel Hypertension Model. Biomed. Res. Int. 2017, 2017, 5986957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvani, A. Brain-heart interactions: Physiology and clinical implications. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 2067. [Google Scholar] [CrossRef]
- Elek, G.; Muller, M. The living matter according to Ervin Bauer (1890–1938), (on the 75th anniversary of his tragic death) (History). Acta Physiol. Hung. 2013, 100, 124–132. [Google Scholar] [CrossRef]
- Levit, S. Type 2 diabetes therapeutic strategies: Why don’t we see the ELEPHANT in the room? Diabetes Mellitus 2016, 19, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Beltrame, R.T. Automatic and manual Doppler velocimetry measurements of the uterine artery in pregnant ewes. Anim. Reprod. Sci. 2017, 181, 103–107. [Google Scholar] [CrossRef]
- Schoning, M.; Walter, J.; Scheel, P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke 1994, 25, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Eckstein, F. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging--from image to data, from data to theory. Anat. Embryol. 2001, 203, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Gwadry-Sridhar, F.H. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: A systematic review by the ISPOR medication adherence and persistence special interest group. Value Health 2013, 16, 863–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, E. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension 2012, 59, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, T.O.; Anderson, A.; Bertram, D. Effect of indomethacin on blood pressure in elderly people with essential hypertension well controlled on amlodipine or enalapril. Am. J. Hypertens. 2000, 13, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, J. European headache federation guideline on idiopathic intracranial hypertension. J. Headache Pain 2018, 19, 93. [Google Scholar] [CrossRef]
- Kroll, L.S. Level of physical activity, well-being, stress and self-rated health in persons with migraine and co-existing tension-type headache and neck pain. J. Headache Pain 2017, 18, 46. [Google Scholar] [CrossRef] [Green Version]
- De Backer, J. A reliable set of reference genes to normalize oxygen-dependent cytoglobin gene expression levels in melanoma. Sci. Rep. 2021, 11, 10879. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, B. Beta-blockers in COPD: Time for reappraisal. Eur. Respir. J. 2016, 48, 880–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, M. Beta-blocker therapy in heart failure in the elderly. Int. J. Cardiol. 2008, 125, 149–153. [Google Scholar] [CrossRef]
- Couffignal, C. Timing of beta-Blocker Reintroduction and the Occurrence of Postoperative Atrial Fibrillation after Cardiac Surgery: A Prospective Cohort Study. Anesthesiology 2020, 132, 267–279. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Hyperchloremic normal gap metabolic acidosis. Minerva. Endocrinol. 2019, 44, 363–377. [Google Scholar] [CrossRef]
- Regolisti, G. Metabolic acidosis. G. Ital. Nefrol. 2016, 33–72. [Google Scholar]
- Sajan, A. Recurrent Anion Gap Metabolic Acidosis. Am. J. Med. Case Rep. 2019, 7, 200–202. [Google Scholar] [CrossRef] [Green Version]
- Gloy, V. Uncertainties about the need for ethics approval in Swtzerland: A mixed method study. Swiss. Med. Wkly. 2020, 150, w20318. [Google Scholar]
- Talantov, P. Unapproved clinical trials in Russia: Exception or norm? BMC Medical. Ethics 2021, 22, 46. [Google Scholar] [CrossRef]




| Parameter | Patients Number | % of Total |
|---|---|---|
| Lowering BP on 10–20 torr | 740 * | 28 |
| Lowering BP on 20–40 torr | 1604 * | 61 |
| Lowering BP for ≥40 torr | 278 * | 11 |
| Pulse normalization | 2342 | 89 |
| Increment of VA ≥ 25% | 2622 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukov, K.V.; Vetcher, A.A.; Gasparuan, B.A.; Shishonin, A.Y. Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis. Polymers 2021, 13, 4248. https://doi.org/10.3390/polym13234248
Zhukov KV, Vetcher AA, Gasparuan BA, Shishonin AY. Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis. Polymers. 2021; 13(23):4248. https://doi.org/10.3390/polym13234248
Chicago/Turabian StyleZhukov, Kirill V., Alexandre A. Vetcher, Bagrat A. Gasparuan, and Alexander Y. Shishonin. 2021. "Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis" Polymers 13, no. 23: 4248. https://doi.org/10.3390/polym13234248
APA StyleZhukov, K. V., Vetcher, A. A., Gasparuan, B. A., & Shishonin, A. Y. (2021). Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis. Polymers, 13(23), 4248. https://doi.org/10.3390/polym13234248