Effect of Engineered Biomaterials and Magnetite on Wastewater Treatment: Biogas and Kinetic Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Engineered Biomaterials and Characterisation Techniques
2.2. Synthesis and Characteristics of the Magnetite (Fe3O4)
2.3. Wastewater and Inoculum Samples
2.4. Biochemical Methane Potential (BMP) Test
2.5. Kinetic Study of BMP System
3. Results and Discussion
3.1. Surface Morphology
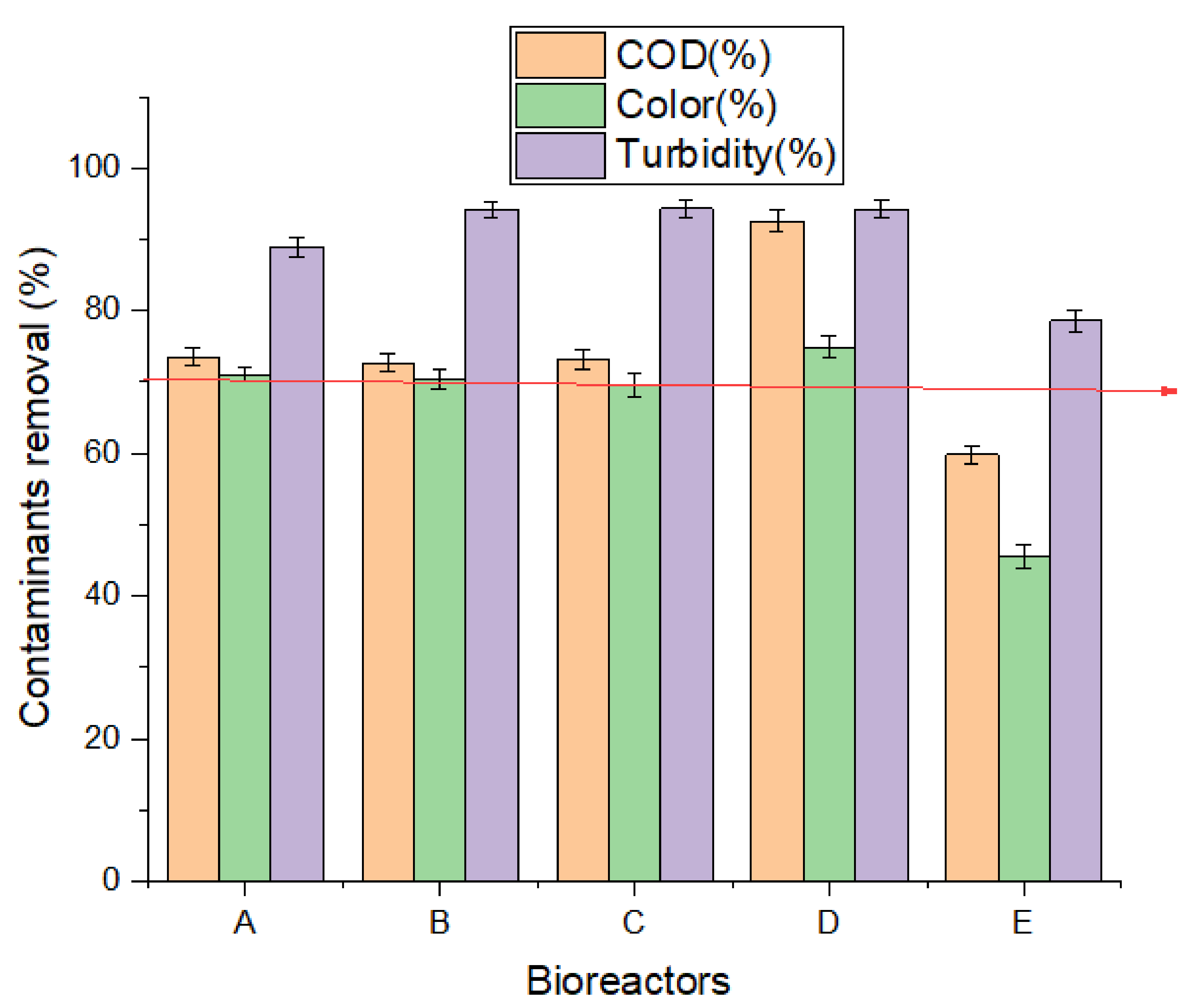
3.2. Biodegradation Efficiency
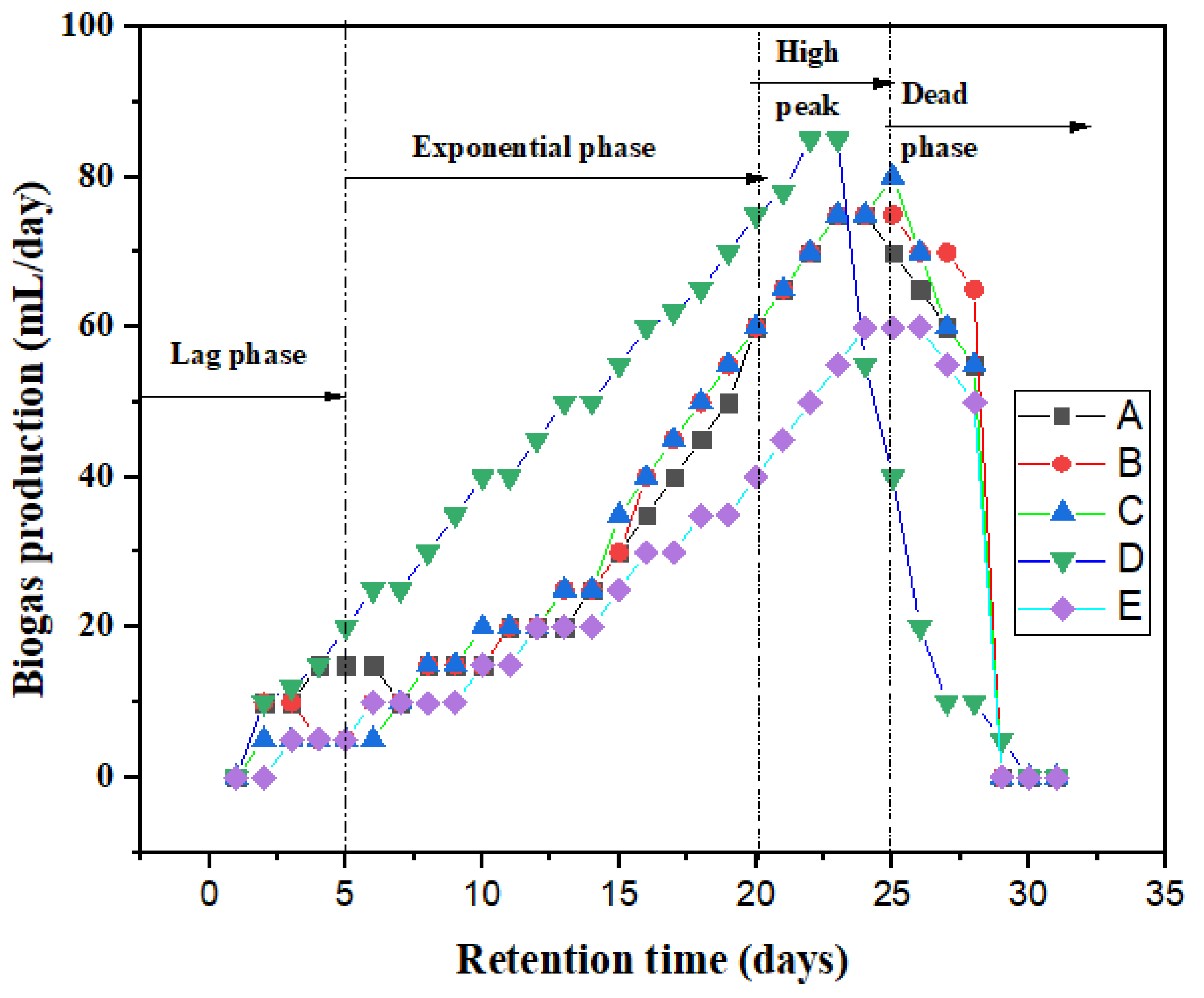
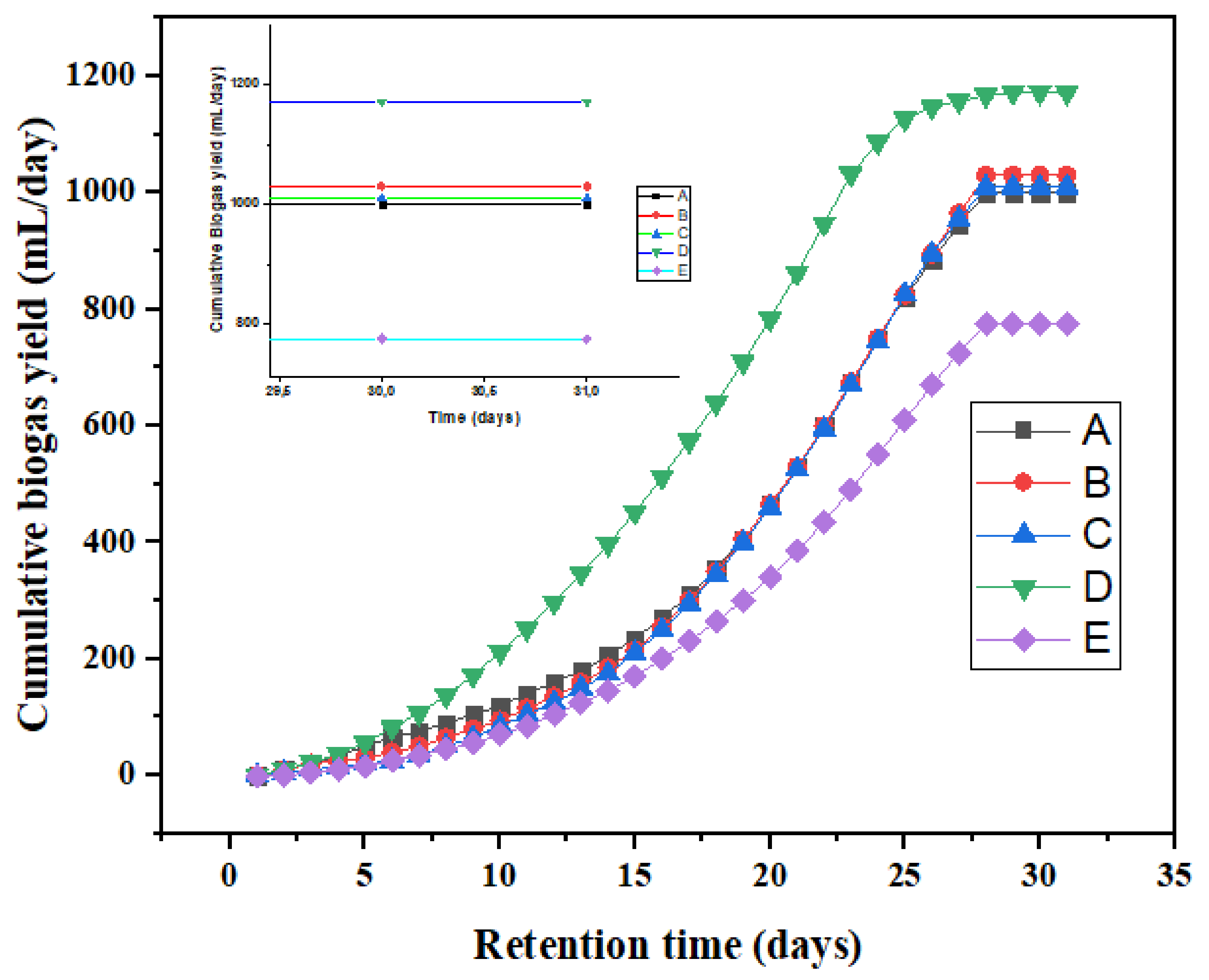
3.3. Effect of Biosorbent on Biogas Yield
3.4. Kinetic Model of the Biogas Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelwahab, T.A.M.; Mohanty, M.K.; Sahoo, P.K.; Behera, D. Application of nanoparticles for biogas production: Current status and perspectives. Energy Sources Part A Recover. Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Zaidi, S.A.A.; RuiZhe, F.; Shi, Y.; Khan, S.Z.; Mushtaq, K. Nanoparticles augmentation on biogas yield from microalgal biomass anaerobic digestion. Int. J. Hydrogen Energy 2018, 43, 14202–14213. [Google Scholar] [CrossRef]
- Msibi, S.S.; Kornelius, G. Potential for domestic biogas as household energy supply in South Africa. J. Energy S. Afr. 2017, 28, 1–13. [Google Scholar] [CrossRef]
- Omara, A.E.-D.; Elsakhawy, T.; Alshaal, T.; El-Ramady, H.; Kovács, Z.; Fári, M. Nanoparticles: A Novel Approach for Sustainable Agro-productivity. Environ. Biodivers. Soil Secur. 2019, 3, 30–40. [Google Scholar] [CrossRef]
- Chollom, M.; Rathilal, S.; Swalaha, F.; Bakare, B.; Tetteh, E. Removal of Antibiotics During the Anaerobic Digestion of Slaughterhouse Wastewater. Int. J. Sustain. Dev. Plan. 2020, 15, 335–342. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total. Environ. 2020, 711, 134847. [Google Scholar] [CrossRef] [PubMed]
- Fermoso, F.G.; van Hullebusch, E.; Collins, G.; Roussel, J.; Mucha, A.P.; Esposito, G. Trace Elements in Anaerobic Biotechnologies; IWA Publishing: London, UK, 2019. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Kinetics and Nanoparticle Catalytic Enhancement of Biogas Production from Wastewater Using a Magnetized Biochemical Methane Potential (MBMP) System. Catalysts 2020, 10, 1200. [Google Scholar] [CrossRef]
- Ángeles, R.; Vega-Quiel, M.J.; Batista, A.; Fernández-Ramos, O.; Lebrero, R.; Muñoz, R. Influence of biogas supply regime on photosynthetic biogas upgrading performance in an enclosed algal-bacterial photobioreactor. Algal Res. 2021, 57, 102350. [Google Scholar] [CrossRef]
- Nethengwe, N.S.; Uhunamure, S.E.; Tinarwo, D. Potentials of biogas as a source of renewable energy: A case study of South Africa. Int. J. Renew. Energy Res. 2018, 8, 1112–1123. [Google Scholar]
- Budzianowski, W.M.; Postawa, K. Renewable energy from biogas with reduced carbon dioxide footprint: Implications of applying different plant configurations and operating pressures. Renew. Sustain. Energy Rev. 2017, 68, 852–868. [Google Scholar] [CrossRef]
- Michailos, S.; Walker, M.; Moody, A.; Poggio, D.; Pourkashanian, M. Biomethane production using an integrated anaerobic digestion, gasification and CO2 biomethanation process in a real waste water treatment plant: A techno-economic assessment. Energy Convers. Manag. 2020, 209, 112663. [Google Scholar] [CrossRef]
- Hülsemann, B.; Zhou, L.; Merkle, W.; Hassa, J.; Müller, J.; Oechsner, H. Biomethane Potential Test: Influence of Inoculum and the Digestion System. Appl. Sci. 2020, 10, 2589. [Google Scholar] [CrossRef] [Green Version]
- Lohani, S.P.; Havukainen, J. Anaerobic Digestion: Factors Affecting Anaerobic Digestion Process; Springer: Singapore, 2018; pp. 343–359. [Google Scholar]
- Rehman, M.L.U.; Iqbal, A.; Chang, C.; Li, W.; Ju, M. Anaerobic digestion. Water Environ. Res. 2019, 91, 1253–1271. [Google Scholar] [CrossRef] [Green Version]
- Sarto, S.; Hildayati, R.; Syaichurrozi, I. Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew. Energy 2019, 132, 335–350. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H.; Lee, D.-J.; Tung, K.-L.; Jin, P.; Wang, J.; Wu, Y. Challenges in biogas production from anaerobic membrane bioreactors. Renew. Energy 2016, 98, 120–134. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Yoshida, K.; Shimizu, N. Biogas production management systems with model predictive control of anaerobic digestion processes. Bioprocess Biosyst. Eng. 2020, 43, 2189–2200. [Google Scholar] [CrossRef]
- Xu, D.; Ji, H.; Ren, H.; Geng, J.; Li, K.; Xu, K. Inhibition effect of magnetic field on nitrous oxide emission from sequencing batch reactor treating domestic wastewater at low temperature. J. Environ. Sci. 2020, 87, 205–212. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef]
- Luna-Delrisco, M.; Orupõld, K.; Dubourguier, H.-C. Particle-size effect of CuO and ZnO on biogas and methane production during anaerobic digestion. J. Hazard. Mater. 2011, 189, 603–608. [Google Scholar] [CrossRef]
- Goli, J.; Sahu, O. Development of heterogeneous alkali catalyst from waste chicken eggshell for biodiesel production. Renew. Energy 2018, 128, 142–154. [Google Scholar] [CrossRef]
- Sridhar, N.; Senthilkumar, J.S.; Subburayan, M.R. Removal of Toxic Metals (Lead & Copper) From Automotive Industry Waste Water By Using Fruit Peels. Int. J. Adv. Inf. Commun. Technol. 2014, 1, 188–191. [Google Scholar]
- Amo-Duodu, G.; Rathilal, S.; Chollom, M.N.; Tetteh, E.K. Application of metallic nanoparticles for biogas enhancement using the biomethane potential test. Sci. Afr. 2021, 12, e00728. [Google Scholar] [CrossRef]
- Hassanein, A.; Keller, E.; Lansing, S. Effect of metal nanoparticles in anaerobic digestion production and plant uptake from effluent fertilizer. Bioresour. Technol. 2021, 321, 124455. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, W.; Fan, J.; Zhang, K.; Gu, Z. Removal of silver nanoparticles in aqueous solution by activated sludge: Mechanism and characteristics. Sci. Total Environ. 2020, 711, 135155. [Google Scholar] [CrossRef] [PubMed]
- Toor, R.; Mohseni, M. UV-H2O2 based AOP and its integration with biological activated carbon treatment for DBP reduction in drinking water. Chemosphere 2007, 66, 2087–2095. [Google Scholar] [CrossRef]
- Pirilä, M. Adsorption and Photocatalysis in Water Treatment: Active, Abundant and Inexpensive Materials and Methods; Acta Universitatis Ouluensis, University of Oulu: Oulu, Finland, 2015; Available online: http://jultika.oulu.fi/files/isbn9789526207629.pdf (accessed on 20 May 2021).
- Lakshmanan, R. Application of Magnetic Nanoparticles and Reactive Filter Materials for Wastewater Treatment Ramnath Lakshmanan. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2013. [Google Scholar]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-enabled water and wastewater treatment. NanoImpact 2016, 3–4, 22–39. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Zhu, Y.; Long, C.; Su, L.; Liu, S.; Tang, Z. Applications of Nanomaterials in Asymmetric Photocatalysis: Recent Progress, Challenges, and Opportunities. Adv. Mater. 2021, 33, e2001731. [Google Scholar] [CrossRef]
- Maksoud, M.I.A.A.; Elgarahy, A.; Farrell, C.; Al-Muhtaseb, A.H.; Rooney, D.W.; Osman, A. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord. Chem. Rev. 2020, 403, 213096. [Google Scholar] [CrossRef]
- Peeters, K.; Lespes, G.; Zuliani, T.; Ščančar, J.; Milačič, R. The fate of iron nanoparticles in environmental waters treated with nanoscale zero-valent iron, FeONPs and Fe3O4NPs. Water Res. 2016, 94, 315–327. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Amankwa, M.O.; Armah, E.K.; Rathilal, S. Fate of COVID-19 Occurrences in Wastewater Systems: Emerging Detection and Treatment Technologies—A Review. Water 2020, 12, 2680. [Google Scholar] [CrossRef]
- Lombi, E.; Donner, E.; Tavakkoli, E.; Turney, T.; Naidu, R.; Miller, B.W.; Scheckel, K. Fate of Zinc Oxide Nanoparticles during Anaerobic Digestion of Wastewater and Post-Treatment Processing of Sewage Sludge. Environ. Sci. Technol. 2012, 46, 9089–9096. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 1–19. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Chetty, M.; Armah, E.K.; Asante-Sackey, D. Treatment of Water and Wastewater for Reuse and Energy Generation-Emerging Technologies; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Gunnerson, C.; Stuckey, D. Integrated Resource Recovery-Anaerobic Digestion; UNDP Proje: Washington, DC, USA, 1986. [Google Scholar]
- Hossain, M.A. Removal of Copper from Water by Adsorption onto Banana Peel as Bioadsorbent. Int. J. Geomate 2012, 2, 227–234. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, Z.; Zhang, Y.; Xu, R.; Zheng, Y.; Hu, J.; Li, X.; Jia, M.; Xiong, W.; Cao, J. Influence of nanoscale zero-valent iron and magnetite nanoparticles on anaerobic digestion performance and macrolide, aminoglycoside, β-lactam resistance genes reduction. Bioresour. Technol. 2019, 294, 122139. [Google Scholar] [CrossRef]
- Adeleke, O.; Akinlabi, S.; Jen, T.-C.; Dunmade, I. Towards sustainability in municipal solid waste management in South Africa: A survey of challenges and prospects. Trans. R. Soc. S. Afr. 2021, 76, 53–66. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Syaichurrozi, B.I.; Sumardiono, S. Kinetic Model of Biogas Yield Production from Vinasse at Various Initial pH: Comparison between Modified Gompertz Model and First Order Kinetic Model. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 2798–2805. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, G.; Yu, H.-Q. Kinetic modeling of batch hydrogen production process by mixed anaerobic cultures. Bioresour. Technol. 2006, 97, 1302–1307. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Rathilal, S.; Tetteh, E.K. Coagulation Treatment of Wastewater: Kinetics and Natural Coagulant Evaluation. Molecules 2021, 26, 698. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Verma, P. Advanced microalgae-based renewable biohydrogen production systems: A review. Bioresour. Technol. 2020, 320, 124301. [Google Scholar] [CrossRef]
- Pessoa, M.; Sobrinho, M.M.; Kraume, M. The use of biomagnetism for biogas production from sugar beet pulp. Biochem. Eng. J. 2020, 164, 107770. [Google Scholar] [CrossRef]
- Abdelradi, F. Food waste behaviour at the household level: A conceptual framework. Waste Manag. 2018, 71, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Cho, K.; Bae, H.; Lee, C. The biostimulation of anaerobic digestion with (semi)conductive ferric oxides: Their potential for enhanced biomethanation. Appl. Microbiol. Biotechnol. 2015, 99, 10355–10366. [Google Scholar] [CrossRef] [PubMed]


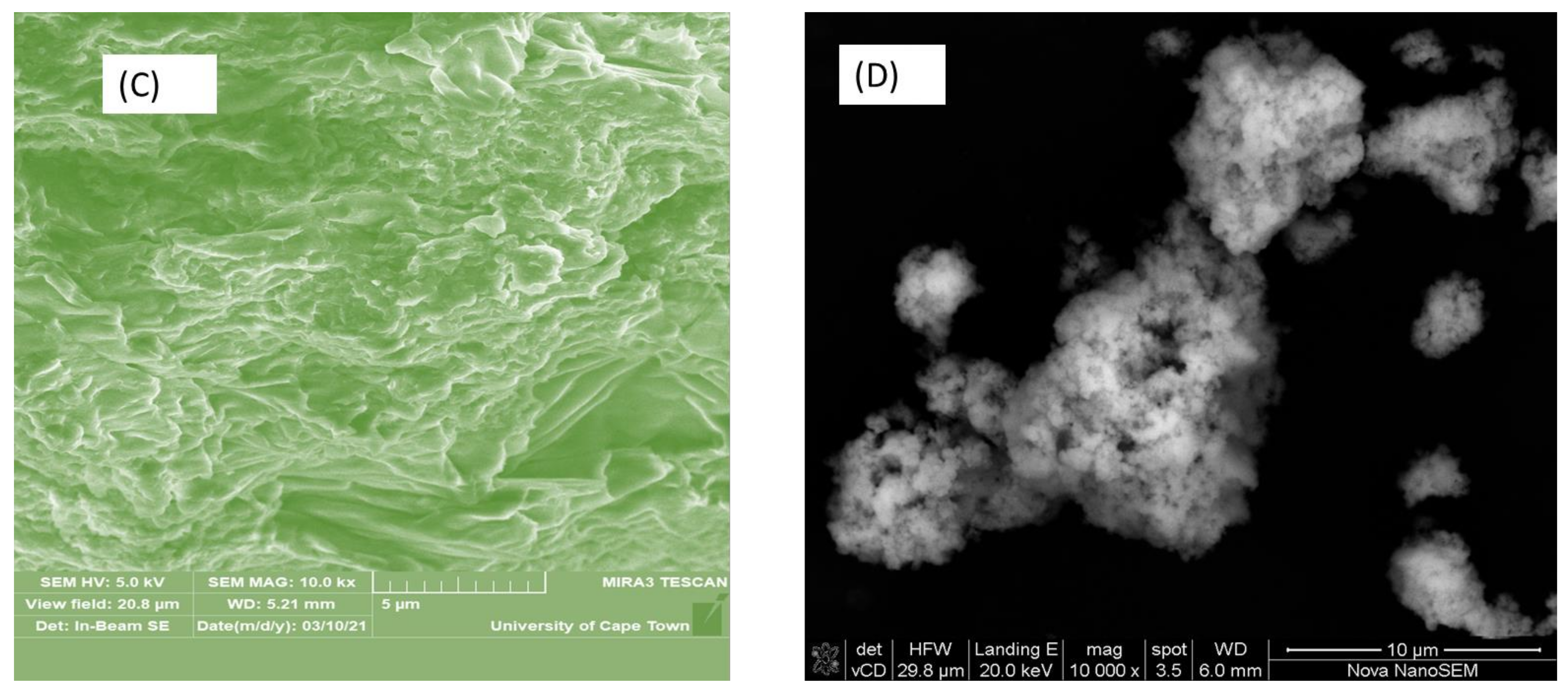

| Biosorbent | Waste or Raw Material Used | Treatment Process | Results | Reference |
|---|---|---|---|---|
| Calcined banana Peels | Synthetic water prepared by diluting concentrated Mn(VII) and Fe(II) with deionised water | Adsorption | The biochar from banana peels was treated with pristine and phosphoric acid; the phosphoric acid pre-treatment had a better absorption efficiency than the pristine pre-treatment. | [40] |
| Raw Banana Peels | Automotive industrial wastewater Dirty water (river and rainwater) | Primary water treatment Water purification | The process had the highest removal of copper (93.52%) and lead (87.44%). The physical test met the quality conditions except for temperatures that exceeded the quality conditions of the maximum standard value.- Bacteriologically there were a lot of total coliforms exceeding the maximum standard conditions. | [24] |
| Egg shell | Real electroplating wastewaters containing Cr, Pb and Cd and synthetic wastewater containing heavy metals (Cr, Pb and Cd) | Jar-test coagulation process | The reuse of waste eggshell in the removal of toxic heavy metals, i.e., Cd and Cr in synthetic wastewater was much enhanced when calcined eggshell was added; however, removal of Pb was rather favourable with natural eggshell. | [23] |
| Fe3O4 | Anaerobic sludge acquired from an Anaerobic-Anoxic-Oxic (AAO) reactor | Batch anaerobic digestion process | There was a 28% increase in biogas yield and COD removal of 14,760 mg/L in the reactor with Fe3O4 | [41] |
| * Calcined banana Peels | Domestic and municipal wastewater | Biochemical methane potential (BMP) test | 32.258 mL/day biogas yield, 73.53%, 71.05% and 88.93% COD, color and turbidity removal, respectively. | This study |
| * Raw Banana Peels | Domestic and municipal wastewater | BMP | 33.226 mL/day biogas yield, 72.69%, 70.35% and 94.13% COD, color and turbidity removal, respectively | This study |
| * Egg shell | Domestic and municipal wastewater | BMP | 32.581 mL/day biogas yield, 73.11%, 69.65% and 94.26% COD, color and turbidity removal, respectively. | This study |
| * Fe3O4 | Domestic and municipal wastewater | BMP | 37.807 mL/day biogas yield, 92.59%, 74.86% and 94.13% COD, color and turbidity removal, respectively. | This study |
| Parameters | Results |
|---|---|
| Chemical oxygen demand (COD) (mg/L) | 2380 ± 57.6 |
| Color (Pt.Co) | 57 ± 12.5 |
| Turbidity (NTU) | 17.32 ± 2.2 |
| Total solids (TS) (mg TS/L) | 204.5 ± 24.6 |
| Volatile solids (VS) (mg VS/L) | 106 ± 32.6 |
| pH | 6.59 ± 1.3 |
| Set-Up | Biosorbent Type | Biosorbent Loading (g) | Wastewater (mL) | Inoculum (mL) |
|---|---|---|---|---|
| A | Calcined banana peels (BI) | 1.5 | 500 | 300 |
| B | Crushed eggshell (CE) | 1.5 | 500 | 300 |
| C | Banana peels (PB) | 1.5 | 500 | 300 |
| D | Magnetite (Fe3O4) | 1.5 | 500 | 300 |
| E | Control (no loading) | n/a | 500 | 300 |
| Bioreactor | Biosorbent Added (g) | Average Biogas Yield (mL/day) | Cumulative Biogas Yield (mL/day) |
|---|---|---|---|
| A | 1.5 | 32 | 1000 |
| B | 1.5 | 34 | 1030 |
| C | 1.5 | 33 | 1010 |
| D | 1.5 | 40 | 1117 |
| E | No additives | 25 | 775 |
| Set-Up | A | B | C | D | E |
|---|---|---|---|---|---|
| Yt (mL/g COD) | 1 × 103 | 1.03 × 103 | 1.01 × 103 | 1.17 × 103 | 775 |
| Ym (mL/g COD) | 3.68 × 105 | 2.06 × 105 | 3.71 × 105 | 2.96 × 105 | 3.08 × 105 |
| k (1/day) | 8 × 10−4 | 1.4 × 10−4 | 8 × 10−4 | 1.3 × 10−4 | 7 × 10−4 |
| SSR | 5.92 × 105 | 7.44 × 105 | 7.64 × 105 | 4.57 × 105 | 4.19 × 105 |
| R2 | 0.927 | 0.919 | 0.919 | 0.968 | 0.923 |
| Predicted value (mL/g COD) | 874 | 878 | 864 | 1.20 × 103 | 655 |
| Difference between measured (Yt) and predicted values | 126 | 152 | 146 | 31 | 120 |
| Set-Up | A | B | C | D | E |
|---|---|---|---|---|---|
| Y(t) (mL/g COD) | 1 × 103 | 1.03 × 103 | 1.01 × 103 | 1.17 × 103 | 775 |
| Ym (mL/g COD) | 1.89 × 103 | 1.75 × 103 | 1.57 × 103 | 1.46 × 103 | 1.40 × 103 |
| ʎ (days) | 23.58 | 22.49 | 21.45 | 15.79 | 23.33 |
| k (1/day) | 0.081 | 0.094 | 0.106 | 0.123 | 0.884 |
| SSR | 3.79 × 105 | 3.03 × 105 | 2.71 × 105 | 4.16 × 105 | 1.64 × 105 |
| R2 | 0.991 | 0.993 | 0.994 | 0.993 | 0.993 |
| Predicted value (mL/g COD) | 1.09 × 103 | 1.12 × 103 | 1.09 × 103 | 1.25 × 103 | 841 |
| Difference between measured (Yt) and predicted values (mL) | 92 | 86 | 81 | 78 | 66 |
| Set-Up | A | B | C | D | E |
|---|---|---|---|---|---|
| Yt (mL) | 1 × 103 | 1.03 × 103 | 1.01 × 103 | 1.17 × 103 | 7.75 × 102 |
| Ym (mL) | 5.33 × 105 | 2.08 × 105 | 4.12 × 105 | 1.81 × 105 | 1.82 × 105 |
| Rmax (mL/day) | 3.6 × 10−5 | 2.2 × 10−5 | 1.9 × 10−5 | 3.7 × 10−5 | 0.16 |
| KCH (1/day) | 6.73 | 1.59 | 0.28 | 1.71 | 2 × 10−5 |
| SSR | 5.92 × 105 | 7.42 × 105 | 7.65 × 105 | 4.56 × 105 | 4.21 × 105 |
| R2 | 9.28 × 10−1 | 9.19 × 10−1 | 9.2 × 10−1 | 9.69 × 10−1 | 9.24 × 10−1 |
| Predicted value (mL/g COD) | 876 | 880 | 864 | 1.20 × 103 | 654 |
| Difference between measured (Yt) and predicted values (mL) | 124 | 150 | 164 | 31 | 121 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S.; Armah, E.K.; Adedeji, J.; Chollom, M.N.; Chetty, M. Effect of Engineered Biomaterials and Magnetite on Wastewater Treatment: Biogas and Kinetic Evaluation. Polymers 2021, 13, 4323. https://doi.org/10.3390/polym13244323
Amo-Duodu G, Tetteh EK, Rathilal S, Armah EK, Adedeji J, Chollom MN, Chetty M. Effect of Engineered Biomaterials and Magnetite on Wastewater Treatment: Biogas and Kinetic Evaluation. Polymers. 2021; 13(24):4323. https://doi.org/10.3390/polym13244323
Chicago/Turabian StyleAmo-Duodu, Gloria, Emmanuel Kweinor Tetteh, Sudesh Rathilal, Edward Kwaku Armah, Jeremiah Adedeji, Martha Noro Chollom, and Maggie Chetty. 2021. "Effect of Engineered Biomaterials and Magnetite on Wastewater Treatment: Biogas and Kinetic Evaluation" Polymers 13, no. 24: 4323. https://doi.org/10.3390/polym13244323









