Facile Preparation of Tunicate-Inspired Chitosan Hydrogel Adhesive with Self-Healing and Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Hydrogels
2.3. Gelation Time
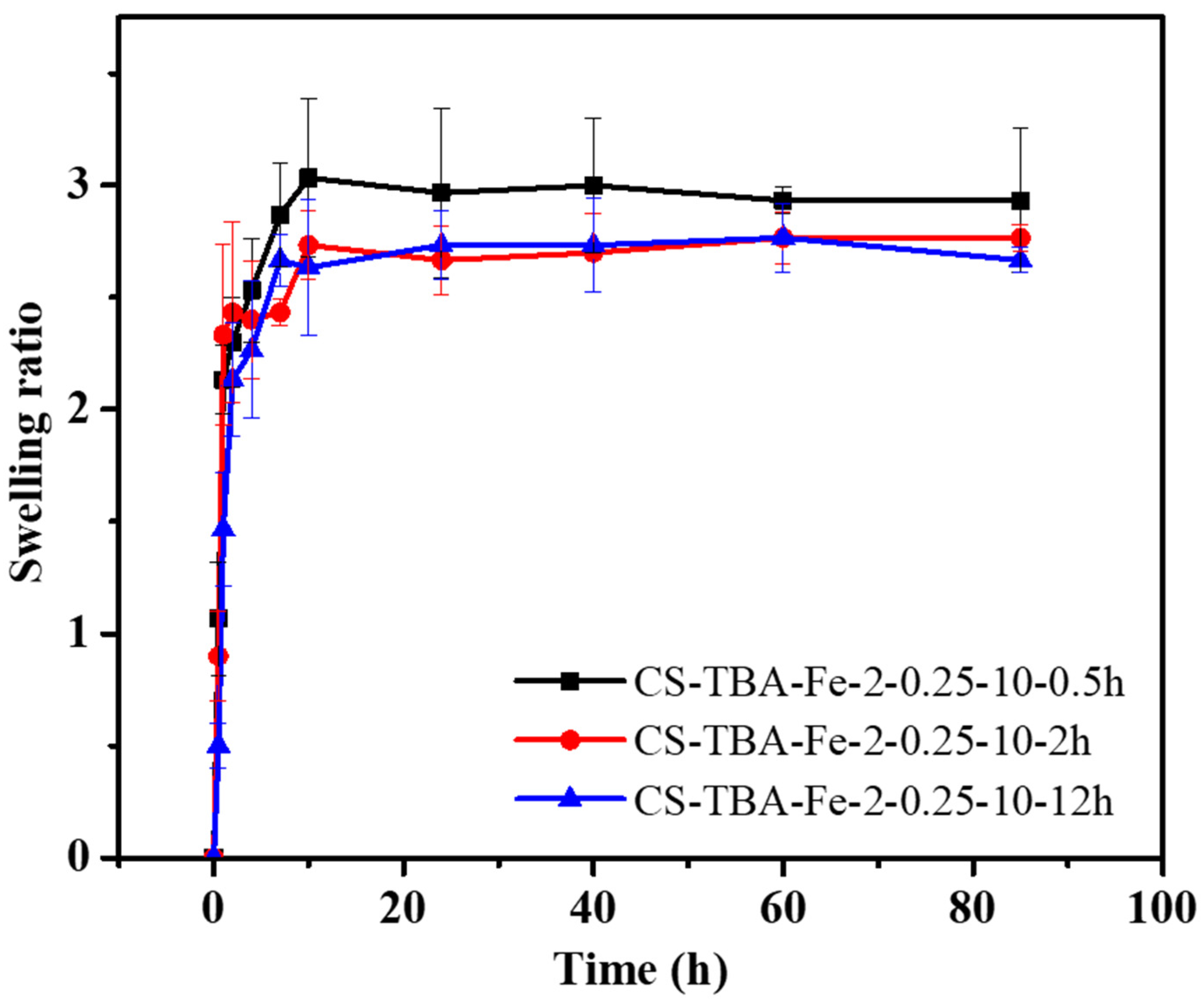
2.4. Swelling Ratio
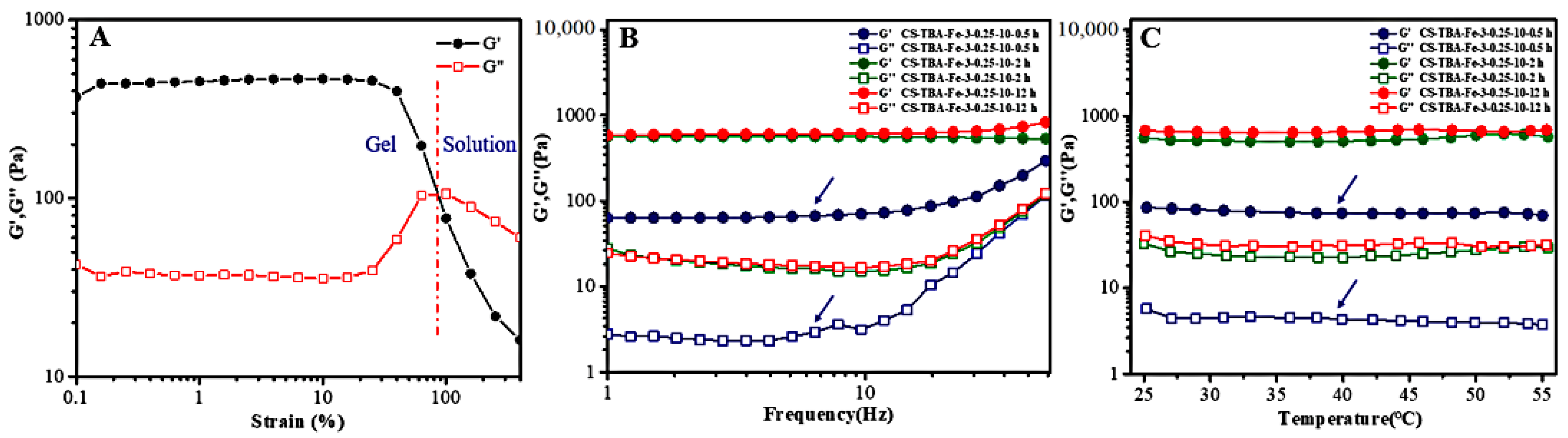
2.5. Rheological Properties
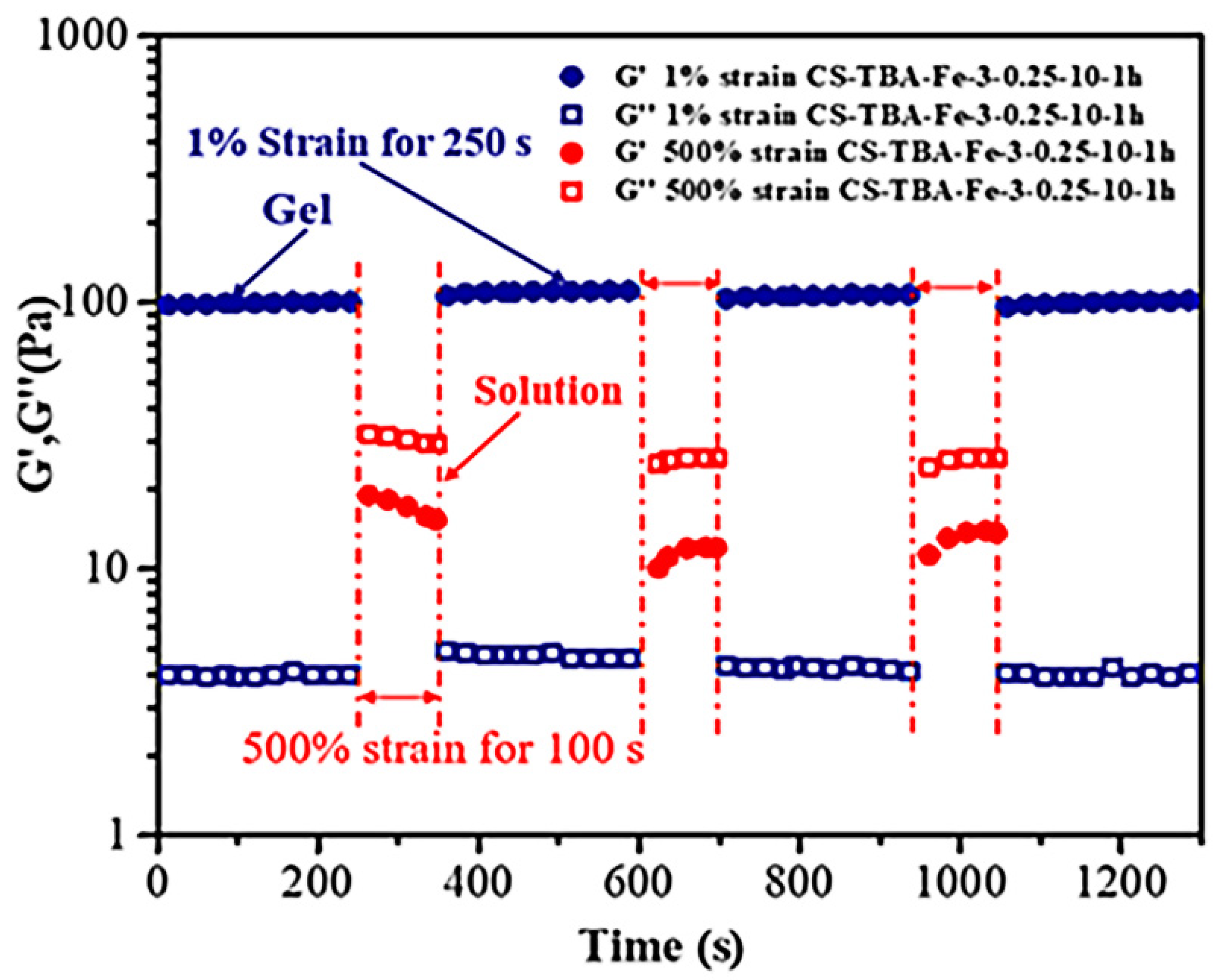
2.6. Self-Healing Test
2.7. Tissue Adhesive Strength
2.8. In Vitro Antibacterial Test
3. Results
3.1. Synthesis and Characterization of CS-TBA-Fe Hydrogel
3.2. The Gelation Time of CS-TBA-Fe Hydrogel
3.3. Swelling Capacities of CS-TBA-Fe Hydrogels
3.4. Rheological Behavior of CS-TBA-Fe Hydrogels
3.5. Self-Healing Ability of CS-TBA-Fe Hydrogels
3.6. Adhesion Strength of CS-TBA-Fe Hydrogels
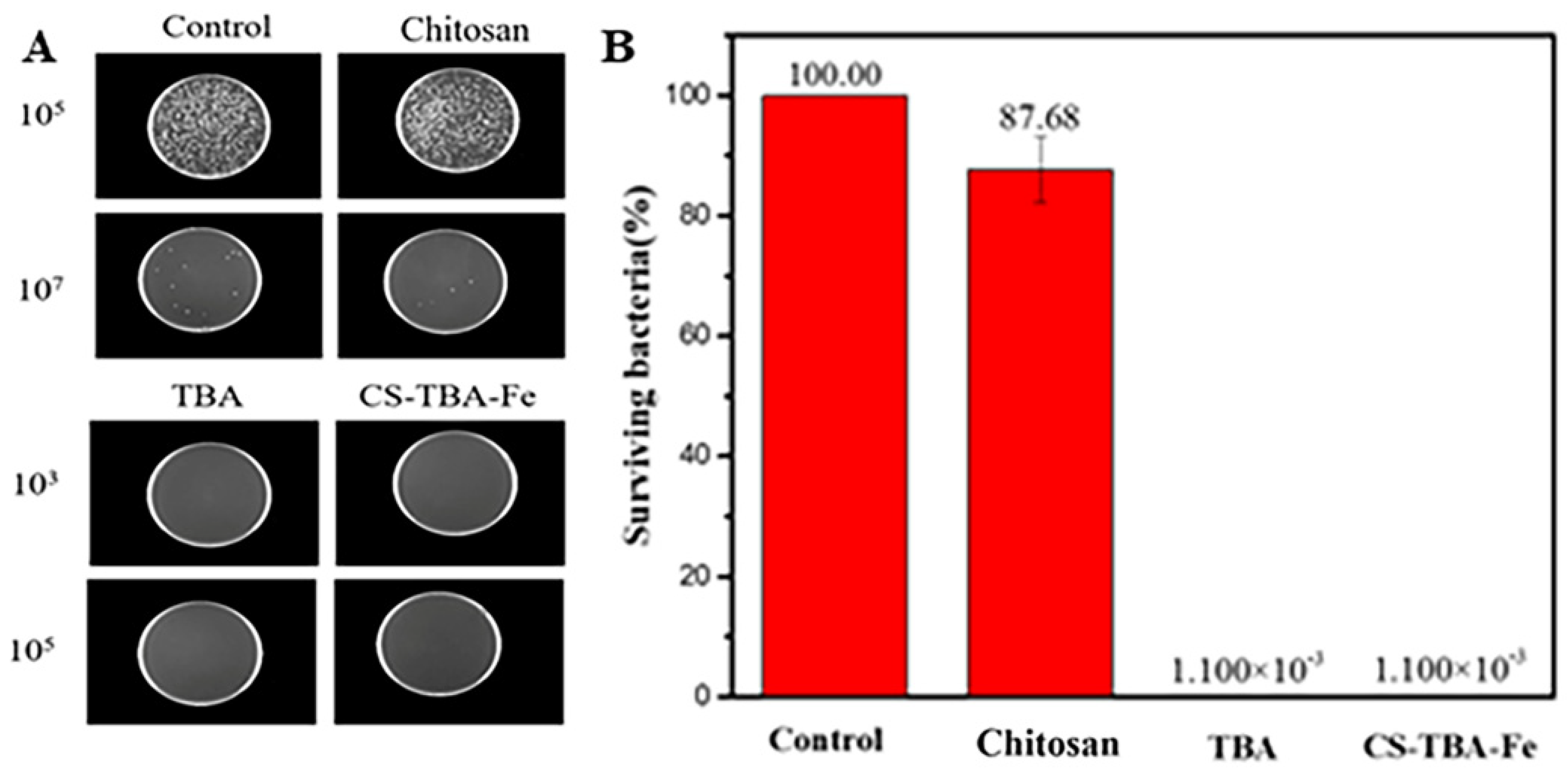
3.7. Antibacterial Activity of CS-TBA-Fe Hydrogels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, R.; Li, J.; Chen, W.; Xu, T.; Yun, S.; Xu, Z.; Xu, Z.; Sato, T.; Chi, B.; Xu, H. A Biomimetic musselinspired Ε-Poly-l-lysine Hydrogel with robust tissue-anchor and anti-infection capacity. Adv. Funct. Mater. 2017, 27, 1604894. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Wang, Q.; Wang, R.; Xie, Y.; Zhao, W.; Zhao, C. Design of antibacterial poly(ether sulfone) membranes via covalently attaching hydrogel thin layers loaded with Ag nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 15962–15974. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Yang, J. Design strategies and applications of tissue bioadhesives: Design strategies and applications of tissue. Macromol. Biosci. 2013, 13, 271–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-C. Recent advances in crosslinking chemistry of biomimetic poly(ethylene glycol) hydrogels. RSC Adv. 2015, 5, 39844–39853. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, H.; Lam, K.Y. A multiphysics model of photo-sensitive hydrogels in response to light-thermo-PH-salt coupled stimuli for biomedical applications. Bioelectrochemistry 2020, 135, 107584. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Guo, J.; Zhang, Z.; Zhang, X.; Zhao, Y. Bio-Inspired Stretchable, Adhesive, and Conductive Structural Color Film for Visually Flexible Electronics. Adv. Funct. Mater. 2020, 30, 2000151. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, H.; Wu, H.; Wang, Y.; Liu, Q.; Wang, Y.; Lincoln, S.F.; Guo, X.; Wang, J. Cyclodextrin Hydrogels: Rapid removal of aromatic micropollutants and adsorption mechanisms. J. Chem. Eng. Data 2020, 65, 678–689. [Google Scholar] [CrossRef]
- Kotturi, H.; Abuabed, A.; Zafar, H.; Sawyer, E.; Pallipparambil, B.; Jamadagni, H.; Khandaker, M. Evaluation of Polyethylene Glycol Diacrylate-Polycaprolactone scaffolds for tissue engineering applications. J. Funct. Biomater. 2017, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Bao, Z.; Gao, M.; Sun, Y.; Nian, R.; Xian, M. The recent progress of tissue adhesives in design strategies, adhesive mechanism and applications. Mater. Sci. Eng. C 2020, 111, 110796. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, H.; Mizuno, Y.; Chen, X.; Nishiguchi, A.; Taguchi, T. Prevention of pulmonary air leaks using a biodegradable tissue-adhesive fiber sheet based on alaska pollock gelatin modified with decanyl groups. Biomater. Sci. 2021, 9, 861–873. [Google Scholar] [CrossRef]
- Lye, I.; Corley, A.; Bartnikowski, N.; Fraser, J. In vitro testing of cyanoacrylate tissue adhesives and sutures for extracorporeal membrane oxygenation cannula securement. Aust. Crit. Care 2019, 32, S11. [Google Scholar] [CrossRef]
- Mandell, S.P.; Gibran, N.S. Fibrin sealants: Surgical hemostat, sealant and adhesive. Expert Opin. Biol. Ther. 2014, 14, 821–830. [Google Scholar] [CrossRef]
- Ramirez-Barron, S.N.; Sanchez-Valdes, S.; Betancourt, R.; Gallardo, C.A.; Puente-Urbina, B.; Rodriguez-Fernández, O.S.; Carneiro-da Cunha, M.G.; dos Santos-Correia, M.T.; Sanchez-Martinez, Z.V. Preparation and characterization of gelatin-gallic Acid/ZnO nanocomposite with antibacterial properties as a promising multi-functional bioadhesive for wound dressing applications. Int. J. Adhes. Adhes. 2021, 104, 102749. [Google Scholar] [CrossRef]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly curable chitosan–PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef]
- Fürst, W.; Banerjee, A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann. Thorac. Surg. 2005, 79, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Wang, L.; Zhang, X.; Heng, B.C.; Wang, D.A.; Ge, Z. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioact. Mater. 2021, 6, 1689–1698. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Lee, M.Y.; Cha, J.M.; Min, K.H.; Lee, S.C.; Shin, S.Y.; Bae, H. Injectable hydrogel derived from chitosan with tunable mechanical properties via hybrid-crosslinking system. Carbohydr. Polym. 2021, 251, 117036. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, F.; Hou, Z.; Chen, Y.; Luo, X. Injectable self-healing CuS nanoparticle complex hydrogels with antibacterial, anti-cancer, and wound healing properties. Chem. Eng. J. 2021, 409, 128224. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Sha, D.; Liu, Y.; Xu, J.; Shi, K.; Yu, C.; Ji, X. Injectable and antibacterial ε-poly(l-lysine)-modified poly(vinyl alcohol)/chitosan/AgNPs hydrogels as wound healing dressings. Polymer 2021, 212, 123155. [Google Scholar] [CrossRef]
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2018, 179, 273–281. [Google Scholar] [CrossRef]
- Wang, T.; Mu, X.; Li, H.; Wu, W.; Nie, J.; Yang, D. The photocrosslinkable tissue adhesive based on copolymeric dextran/HEMA. Carbohydr. Polym. 2013, 92, 1423–1431. [Google Scholar] [CrossRef]
- Mirshafian, R.; Wei, W.; Israelachvili, J.N.; Waite, J.H. α,β-dehydro-dopa: A hidden participant in Mussel adhesion. Biochemistry 2016, 55, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Lee, J.; Lee, J.N.; Lee, H.; Park, W.H. Mussel-inspired poly(γ-glutamic acid)/nanosilicate composite hydrogels with enhanced mechanical properties, tissue adhesive properties, and skin tissue regeneration. Acta Biomater. 2021, 126, 537. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Hou, Z.; Zhou, L.; Zhang, P.; Ouyang, Y.; Wang, P.; Chen, Y.; Luo, X. Injectable self-healing hydrogels containing CuS nanoparticles with abilities of hemostasis, antibacterial activity, and promoting wound healing. ACS Biomater. Sci. Eng. 2021, 7, 335–349. [Google Scholar] [CrossRef]
- Dong, C.; Fan, H.; Tang, F.; Gao, X.; Feng, K.; Wang, J.; Jin, Z. Mussel byssus cuticle-inspired ultrastiff and stretchable triple-crosslinked hydrogels. J. Mater. Chem. B 2021, 9, 373–380. [Google Scholar] [CrossRef]
- Filippidi, E.; Cristiani, T.R.; Eisenbach, C.D.; Waite, J.H.; Israelachvili, J.N.; Ahn, B.K.; Valentine, M.T. Toughening elastomers using mussel-inspired iron-catechol complexes. Science 2017, 358, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-modified hyaluronic acid hydrogel adhesives with fast-forming and high tissue adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, X.; Lv, X.; Zhang, M.; Huang, X.; Shi, Y.; Lu, C.; Song, J.; Yang, H. Bioinspired mineral–organic bone adhesives for stable fracture fixation and accelerated bone regeneration. Adv. Funct. Mater. 2020, 30, 1908381. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, S.; Wang, X.; Zhang, Y.; Chen, L. Noncompressible hemostasis and bone regeneration induced by an absorbable bioadhesive self-healing hydrogel. Adv. Funct. Mater. 2021, 31, 2009189. [Google Scholar] [CrossRef]
- Chen, W.; Wang, R.; Xu, T.; Ma, X.; Yao, Z.; Chi, B.; Xu, H. A mussel-inspired poly(γ-glutamic acid) tissue adhesive with high wet strength for wound closure. J. Mater. Chem. B 2017, 5, 5668–5678. [Google Scholar] [CrossRef]
- Tang, X.; Wang, X.; Sun, Y.; Zhao, L.; Li, D.; Zhang, J.; Sun, H.; Yang, B. Magnesium Oxide-assisted dual-cross-linking bio-multifunctional hydrogels for wound repair during full-thickness skin injuries. Adv. Funct. Mater. 2021, 31, 2105718. [Google Scholar] [CrossRef]
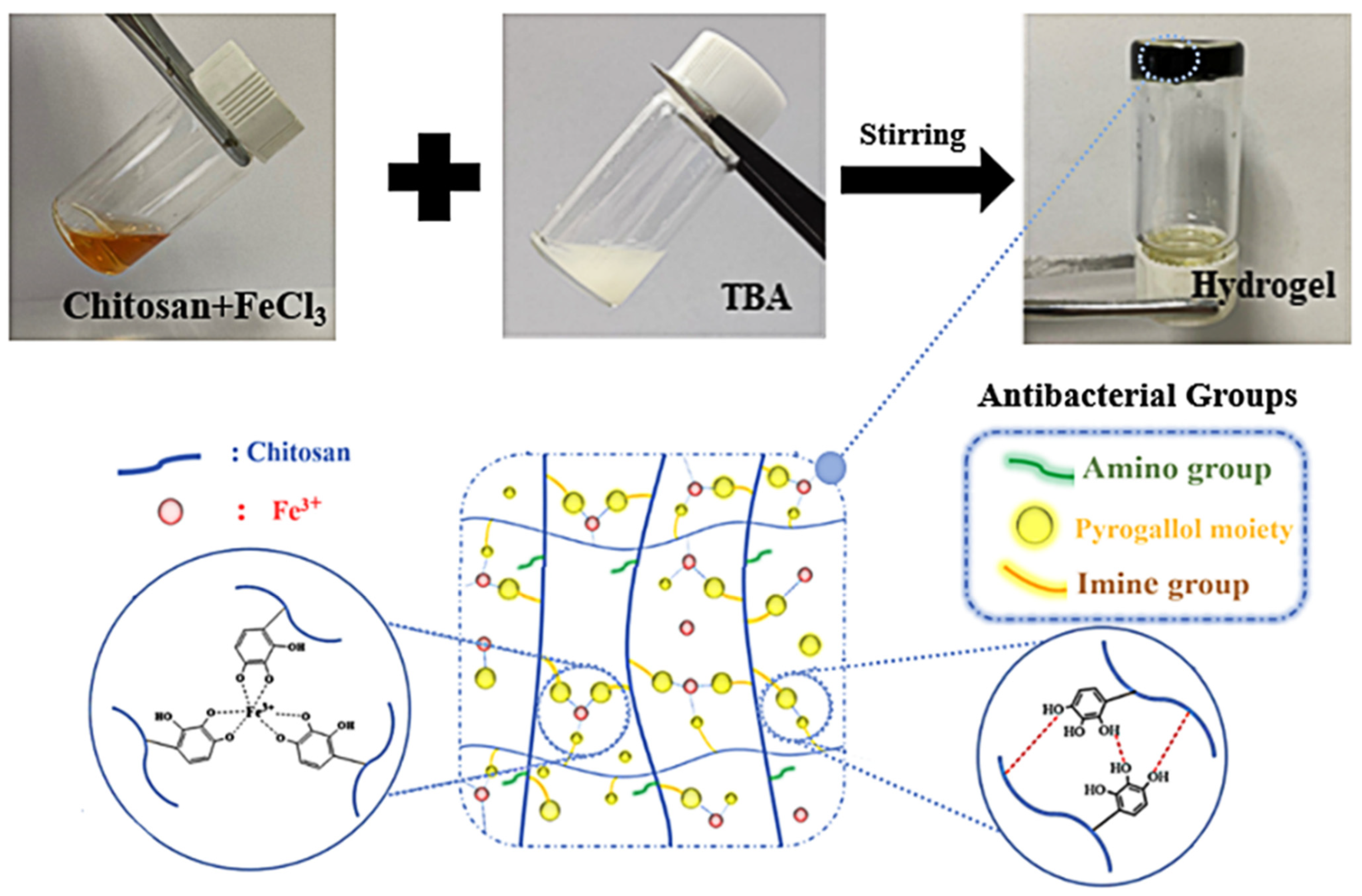



| CS-TBA Formula | Gelation Time |
|---|---|
| CS-TBA-Fe-1-25-0 | not gel |
| CS-TBA-Fe-2-15-0 | 10 h |
| CS-TBA-Fe-2-25-0 | 2 h 11 m |
| CS-TBA-Fe-3-25-0 | 21 m 31 s |
| CS-TBA-Fe-3-25-0 (0.2M HCl) | not gel |
| Sample | OD600 | Average | Bacterial Activity (%) | ||
|---|---|---|---|---|---|
| Control | 0.0903 | 0.0789 | 0.0812 | 0.0835 | 10.6 |
| CS-TBA-Fe | 0.778 | 0.783 | 0.788 | 0.783 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Liu, R.; Liu, H.; Wang, R.; Xi, Z.; Lin, Y.; Wang, J. Facile Preparation of Tunicate-Inspired Chitosan Hydrogel Adhesive with Self-Healing and Antibacterial Properties. Polymers 2021, 13, 4322. https://doi.org/10.3390/polym13244322
He X, Liu R, Liu H, Wang R, Xi Z, Lin Y, Wang J. Facile Preparation of Tunicate-Inspired Chitosan Hydrogel Adhesive with Self-Healing and Antibacterial Properties. Polymers. 2021; 13(24):4322. https://doi.org/10.3390/polym13244322
Chicago/Turabian StyleHe, Xiang, Ruyue Liu, Huiqing Liu, Ruixiao Wang, Zhenhao Xi, Yixiang Lin, and Jie Wang. 2021. "Facile Preparation of Tunicate-Inspired Chitosan Hydrogel Adhesive with Self-Healing and Antibacterial Properties" Polymers 13, no. 24: 4322. https://doi.org/10.3390/polym13244322







