Effect of Locust Bean Gum-Sodium Alginate Coatings Combined with High CO2 Modified Atmosphere Packaging on the Quality of Turbot (Scophthalmus maximus) during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Additives
2.2. Preparation of Turbot Samples
2.3. Microbiological Analysis
2.4. Water Migration
2.5. Analysis of Thiobarbituric Acid (TBA)
2.6. Determination of Total Volatile Basic Nitrogen (TVB-N)
2.7. Determination of Trimethylamine-Nitrogen (TMA)
2.8. K Value
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
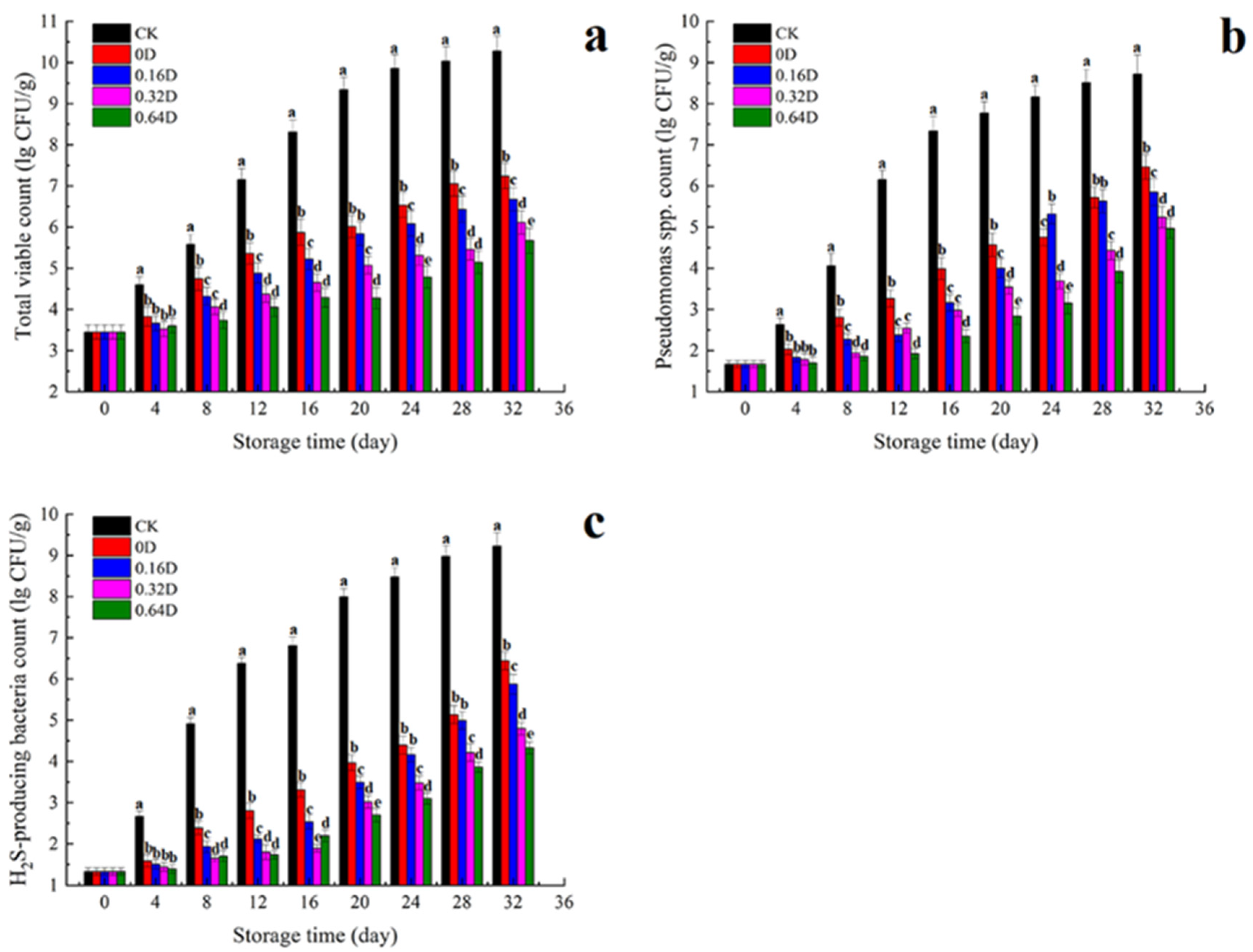
3.1. Microbiological Results
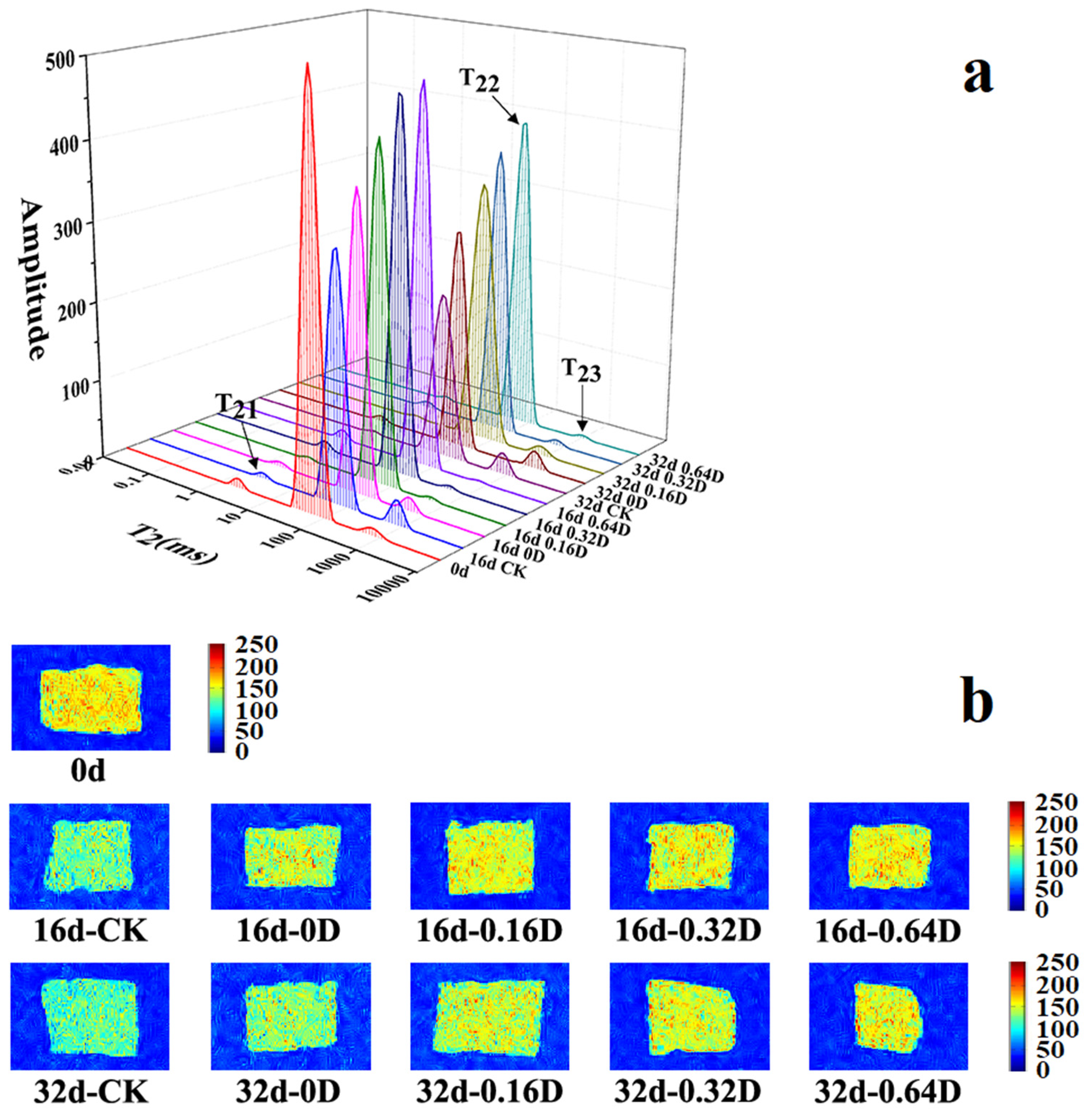
3.2. Water Distribution
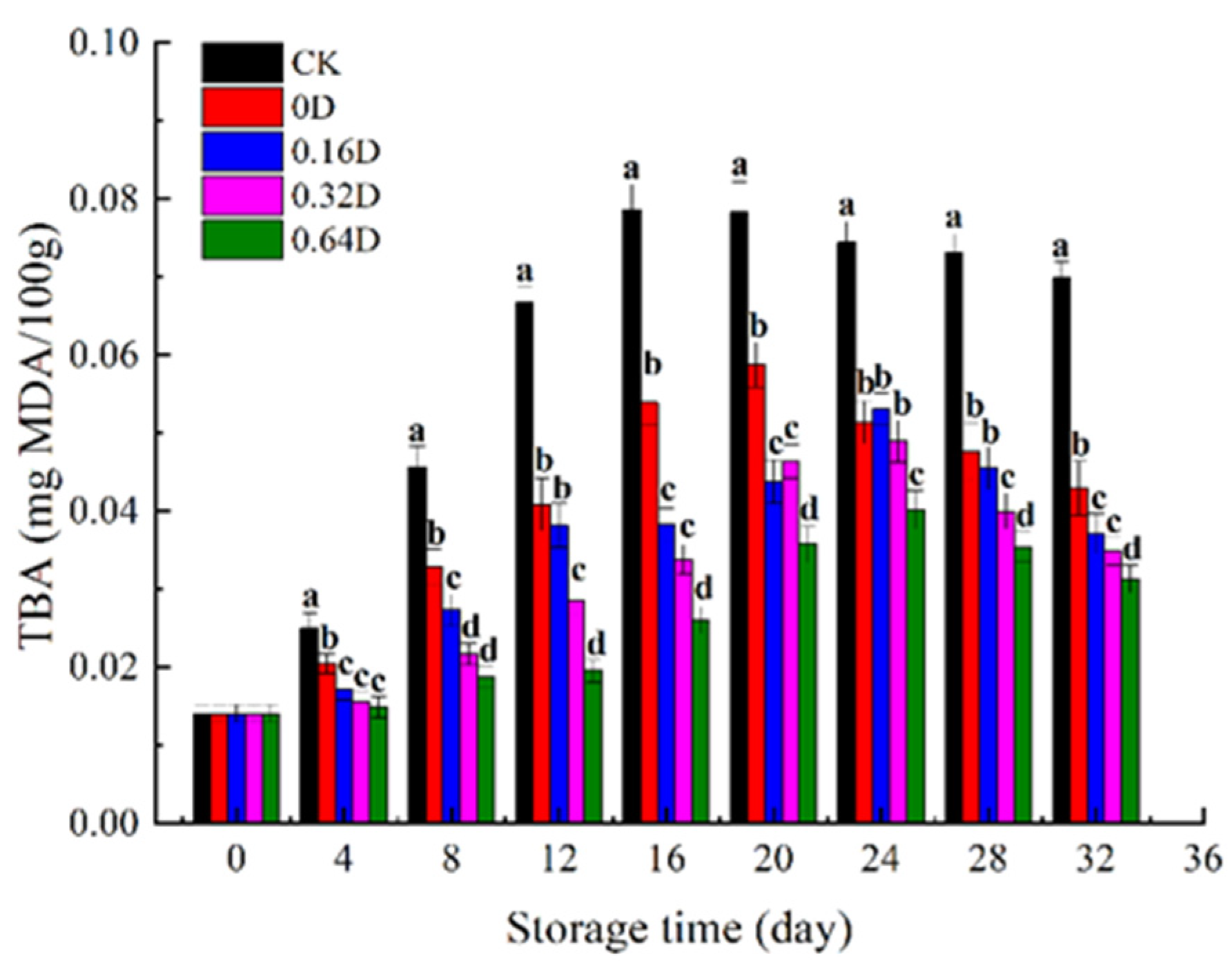
3.3. TBA Analysis
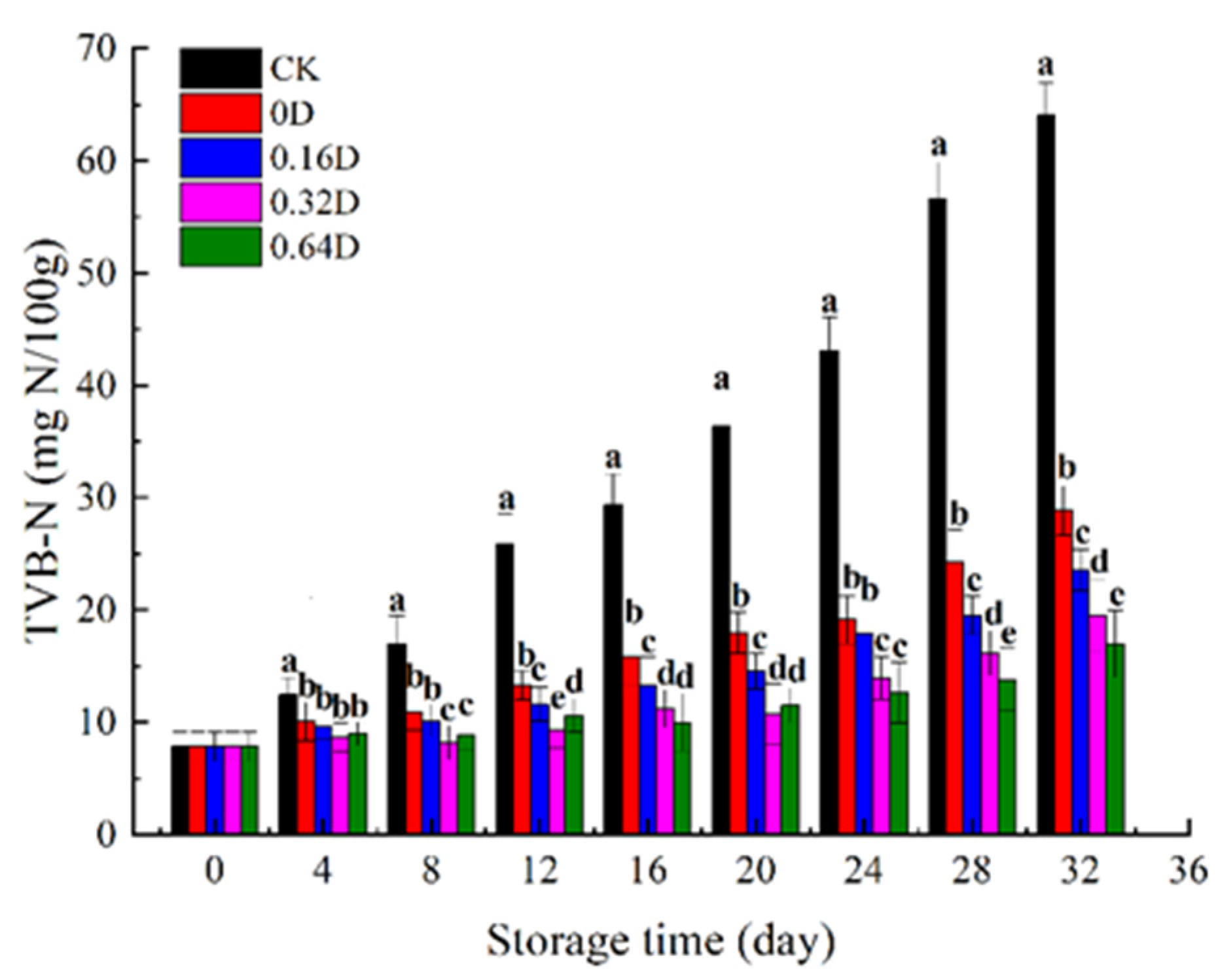
3.4. Changes in TVB-N
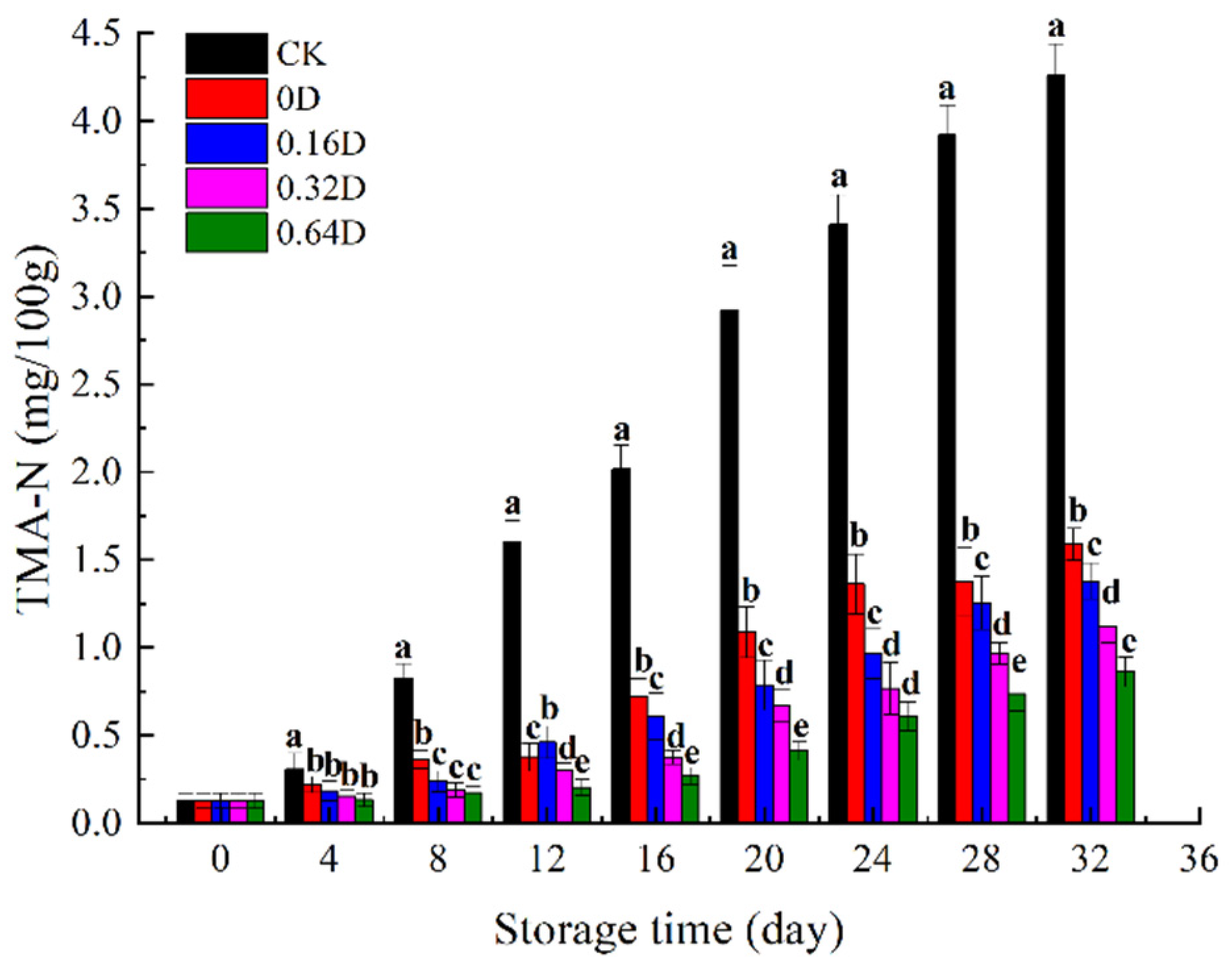
3.5. Changes in TMA-N
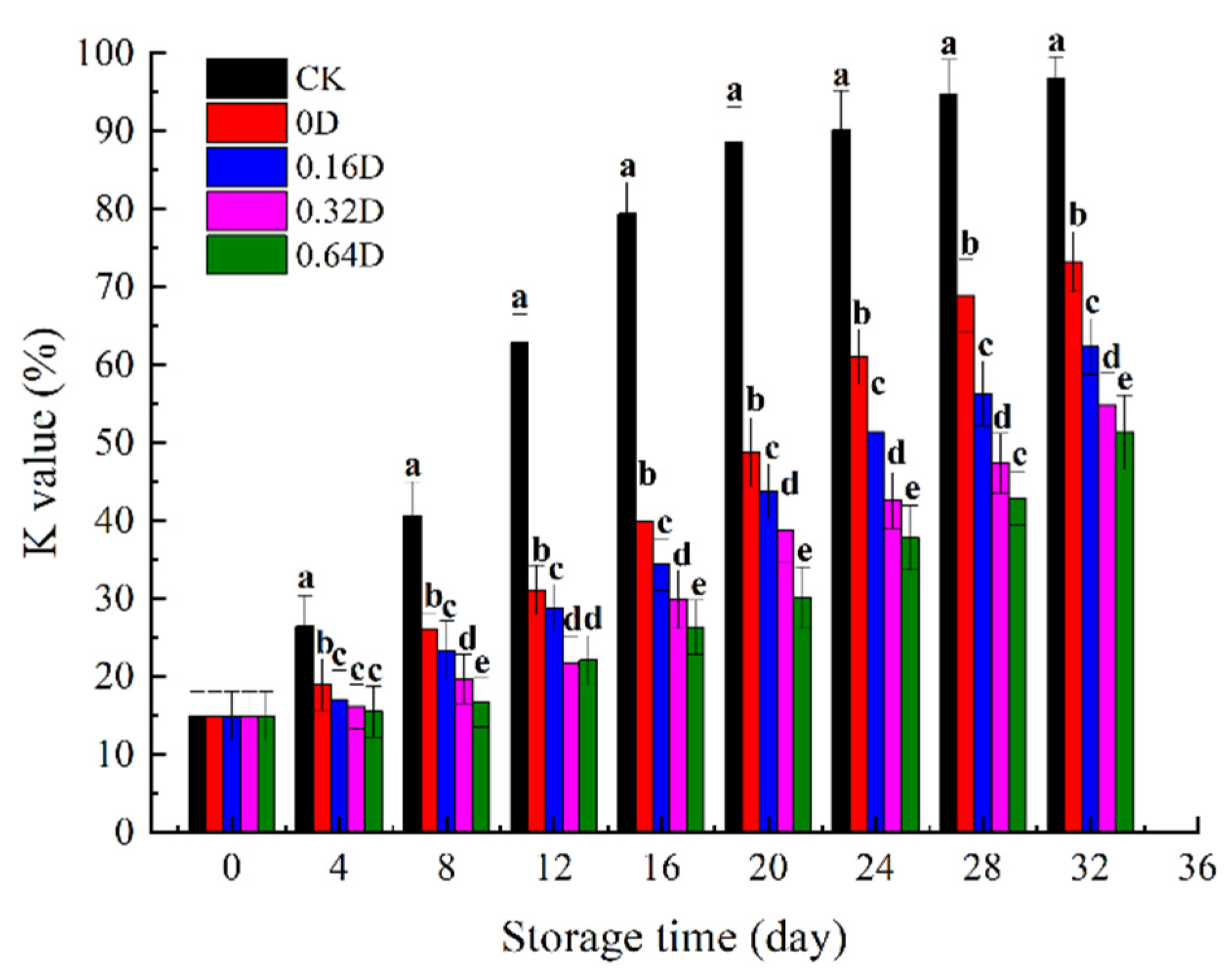
3.6. K Value
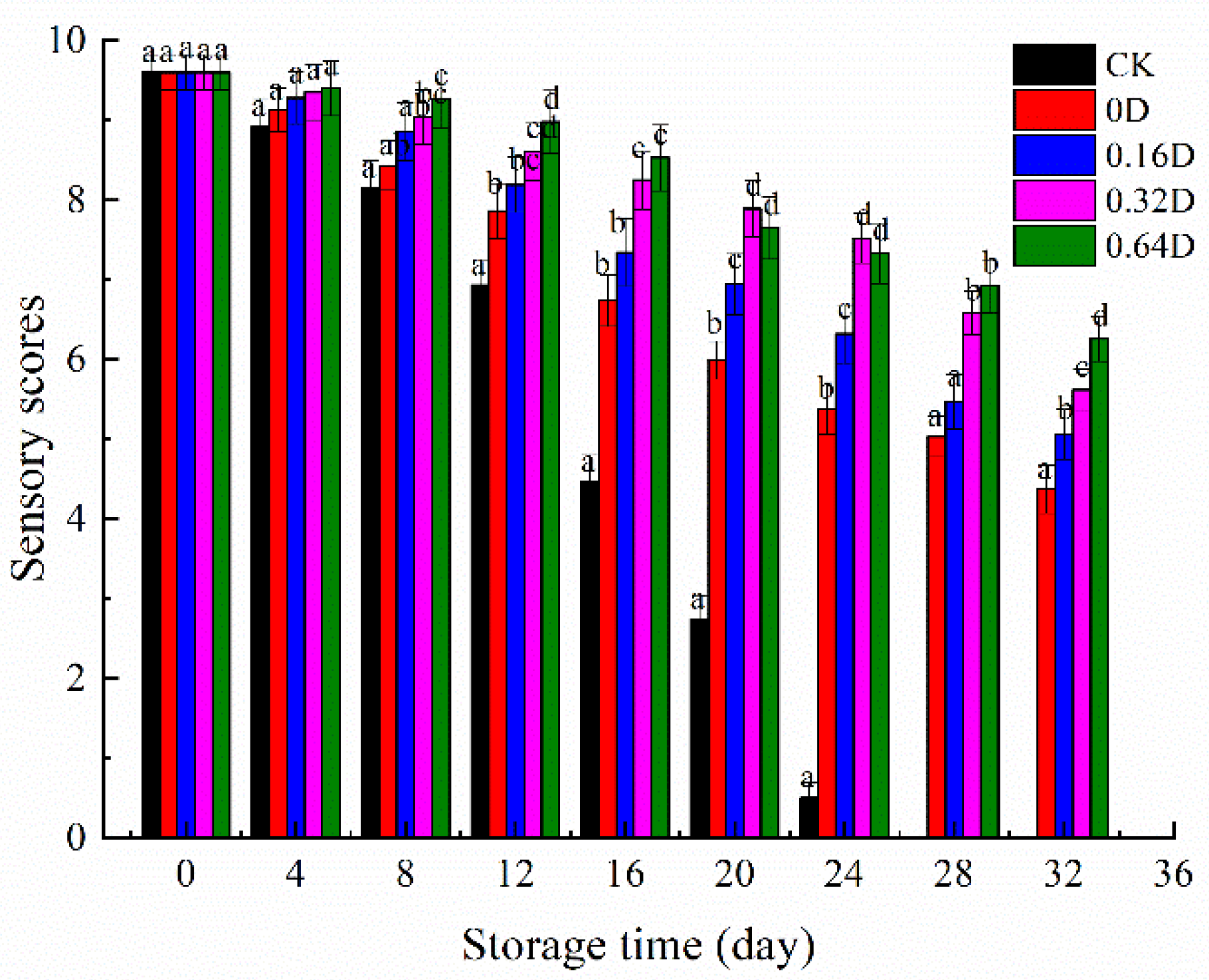
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, J.; Tang, L.; Li, S.; Li, G.; Mo, Z. The Efficacy and Side-Effects of Oil-Based Adjuvants Emulsified Vibrio Anguillarum Bivalent Inactivated Vaccine in Turbot (Scophthalmus maximus) under Production Mode. Aquaculture 2020, 524, 735259. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on Natural Preservatives for Extending Fish Shelf Life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Sun, X.; Chen, H.; He, B.; Mei, Y.; Wang, D.; Li, J. Effect of the Combination of Vanillin and Chitosan Coating on the Microbial Diversity and Shelf-Life of Refrigerated Turbot (Scophthalmus maximus) Filets. Front. Microbiol. 2020, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Peng, Y.; Mei, J.; Xie, J. Effects of Microencapsulated Eugenol Emulsions on Microbiological, Chemical and Organoleptic Qualities of Farmed Japanese Sea Bass (Lateolabrax japonicus) during Cold Storage. LWT 2019, 118, 108831. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Q.; Chu, Y.; Lan, W.; Mei, J.; Xie, J. Effects of Chitosan and Sodium Alginate Active Coatings Containing ε-Polysine on Qualities of Cultured Pufferfish (Takifugu obscurus) during Cold Storage. Int. J. Biol. Macromol. 2020, 160, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, L.; Yu, S.; Liu, J.; Shi, J. High Performance of Alginate/Polyvinyl Alcohol Composite Film Based on Natural Original Melanin Nanoparticles Used as Food Thermal Insulating and UV–Vis Block. Carbohydr. Polym. 2020, 233, 115884. [Google Scholar] [CrossRef]
- Lu, J.; Yang, H.; Hao, J.; Wu, C.; Liu, L.; Xu, N.; Linhardt, R.J.; Zhang, Z. Impact of Hydrolysis Conditions on the Detection of Mannuronic to Guluronic Acid Ratio in Alginate and Its Derivatives. Carbohydr. Polym. 2015, 122, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Hashemi, M.; Aminzare, M.; Bidkorpeh, F.G.; Ebrahimi, M.; Jannat, B.; Tepe, B.; Noori, S.M.A. Effects of Sodium Alginate and Chitosan Coating Combined with Three Different Essential Oils on Microbial and Chemical Attributes of Rainbow Trout Fillets. J. Aquat. Food Prod. Technol. 2020, 29, 253–263. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Xia, Y. Ammonium Persulphate Induced Synthesis of Polymethyl Methacrylate Grafted Sodium Alginate Composite Films with High Strength for Food Packaging. Int. J. Biol. Macromol. 2018, 124, 1238–1245. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.-H. Physical and Bioactivities of Biopolymeric Films Incorporated with Cellulose, Sodium Alginate and Copper Oxide Nanoparticles for Food Packaging Application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver Loaded Hydroxyethylacryl Chitosan/Sodium Alginate Hydrogel Films for Controlled Drug Release Wound Dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef]
- Upadhyay, M.; Adena, S.K.R.; Vardhan, H.; Yadav, S.K.; Mishra, B. Locust Bean Gum and Sodium Alginate Based Interpenetrating Polymeric Network Microbeads Encapsulating Capecitabine: Improved Pharmacokinetics, Cytotoxicity & in Vivo Antitumor Activity. Mater. Sci. Eng. C 2019, 104, 109958. [Google Scholar] [CrossRef]
- Laha, B.; Goswami, R.; Maiti, S.; Sen, K.K. Smart Karaya-Locust Bean Gum Hydrogel Particles for the Treatment of Hypertension: Optimization by Factorial Design and Pre-Clinical Evaluation. Carbohydr. Polym. 2019, 210, 274–288. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Maheriya, P.; Nagar, B.J. Locust Bean Gum in the Development of Sustained Release Mucoadhesive Macromolecules of Aceclofenac. Carbohydr. Polym. 2014, 113, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ramírez, C.; Mazo, P.; Zuluaga, R.; Gañán, P.; Álvarez-Caballero, J. Characterization of Chitosan Extracted from Fish Scales of the Colombian Endemic Species Prochilodus magdalenae as a Novel Source for Antibacterial Starch-Based Films. Polymers 2021, 13, 2079. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Physical and Antimicrobial Properties of Sodium Alginate/Carboxymethyl Cellulose Films Incorporated with Cinnamon Essential Oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuo, S.; He, L.; Cheng, C.; Zhu, B.; Lu, Y.; Wu, Q.; Shang, W.; Ge, W.; Shi, L. Daphnetin Prevents Methicillin-Resistant Staphylococcus aureus Infection by Inducing Autophagic Response. Int. Immunopharmacol. 2019, 72, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Y.; Xiao, Q.; Li, D. Daphnetin Activates the Nrf2-Dependent Antioxidant Response to Prevent Arsenic-Induced Oxidative Insult in Human Lung Epithelial Cells. Chem. Interact. 2019, 302, 93–100. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, L.; Yang, H.; Xiao, Y.; Xiong, Y.L. Super-Chilling (−0.7 °C) with High-CO2 Packaging Inhibits Biochemical Changes of Microbial Origin in Catfish (Clarias gariepinus) Muscle during Storage. Food Chem. 2016, 206, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, Y.; Yu, J.; Ling, J.; Shang, H.; Liu, Z. The Combined Efficacy of Superchilling and High CO2 Modified Atmosphere Packaging on Shelf Life and Quality of Swimming Crab (Portunus trituberculatus). J. Aquat. Food Prod. Technol. 2017, 34, 59–664. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.; Devlieghere, F. High Pressure Carbon Dioxide Inactivation of Microorganisms in Foods: The Past, the Present and the Future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Q.; Mei, J.; Xie, J. Shelf-Life Extension of Refrigerated Turbot (Scophthalmus maximus) by Using Weakly Acidic Electrolyzed Water and Active Coatings Containing Daphnetin Emulsions. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Mei, J.; Xie, J. Effect of Locust Bean Gum-Sodium Alginate Coatings Incorporated with Daphnetin Emulsions on the Quality of Scophthalmus maximus at Refrigerated Condition. Int. J. Biol. Macromol. 2020, 170, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, Z.; Tan, M.; Mei, J.; Xie, J. Evaluation of Weakly Acidic Electrolyzed Water and Modified Atmosphere Packaging on the Shelf Life and Quality of Farmed Puffer Fish (Takifugu obscurus) during Cold Storage. J. Food Saf. 2020, 40. [Google Scholar] [CrossRef]
- Li, N.; Mei, J.; Shen, Y.; Xie, J. Quality Improvement of Half-Smooth Tongue Sole (Cynoglossus Semilaevis) Fillets by Chitosan Coatings Containing Rosmarinic Acid during Storage. CyTA J. Food 2018, 16, 1018–1029. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Y.; Xie, J. Evaluation of Water Dynamics and Protein Changes in Bigeye Tuna (Thunnus obesus) during Cold Storage. LWT 2019, 108, 289–296. [Google Scholar] [CrossRef]
- Morachis-Valdez, A.G.; Santillán-Álvarez, Á.; Gómez-Oliván, L.M.; García-Argueta, I.; Islas-Flores, H.; Dublán-García, O. Effects of Peppermint Extract and Chitosan-Based Edible Coating on Storage Quality of Common Carp (Cyprinus carpio) Fillets. Polymers 2021, 13, 3243. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The Effects of Edible Chitosan-Based Coatings on Flavor Quality of Raw Grass Carp (Ctenopharyngodon idellus) Fillets during Refrigerated Storage. Food Chem. 2017, 242, 412–420. [Google Scholar] [CrossRef]
- Meral, R.; Alav, A.; Karakas, C.; Dertli, E.; Yilmaz, M.T.; Ceylan, Z. Effect of Electrospun nisin and Curcumin Loaded Nanomats on the Microbial Quality, Hardness and Sensory Characteristics of Rainbow Trout Fillet. LWT 2019, 113, 108292. [Google Scholar] [CrossRef]
- Jia, Z.; Shi, C.; Zhang, J.; Ji, Z. Comparison of Freshness Prediction Method for Salmon Fillet during Different Storage Temperatures. J. Sci. Food Agric. 2021, 101, 4987–4994. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.; Yang, C.; Wan, X.; Ding, H.H.; Shi, Y.; Zhao, C. Bacterial Spoilage Profiles in the Gills of Pacific Oysters (Crassostrea gigas) and Eastern Oysters (C. virginica) during Refrigerated Storage. Food Microbiol. 2019, 82, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.L.; da Silveira Alvares, T.; Sampaio, G.S.L.; Cabral, C.C.; Araujo, J.V.A.; Franco, R.M.; Mano, S.B.; Conte-Junior, C.A. Influence of Vacuum and Modified Atmosphere Packaging in Combination with UV-C Radiation on the Shelf Life of Rainbow Trout (Oncorhynchus mykiss) Fillets. Food Control 2016, 60, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Kimbuathong, N.; Leelaphiwat, P.; Harnkarnsujarit, N. Inhibition of Melanosis and Microbial Growth in Pacific White Shrimp (Litopenaeus vannamei) Using High CO2 Modified Atmosphere Packaging. Food Chem. 2019, 312, 126114. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiang, W.; Fan, H.; Xie, J.; Qian, Y.-F. Study on the Mobility of Water and Its Correlation with the Spoilage Process of Salmon (Salmo solar) Stored at 0 and 4 °C by Low-Field Nuclear Magnetic Resonance (LF NMR 1H). J. Food Sci. Technol. 2017, 55, 173–182. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, Y.; Jia, S.; Hong, H.; Liu, Y.; Luo, Y. Effects of Pomegranate Peel Extract on Quality and Microbiota Composition of Bighead Carp (Aristichthys nobilis) Fillets during Chilled Storage. Food Microbiol. 2019, 82, 445–454. [Google Scholar] [CrossRef]
- Qin, N.; Zhang, L.; Zhang, J.; Song, S.; Wang, Z.; Regenstein, J.M.; Luo, Y. Influence of Lightly Salting and Sugaring on the Quality and Water Distribution of Grass Carp (Ctenopharyngodon idellus) during Super-Chilled Storage. J. Food Eng. 2017, 215, 104–112. [Google Scholar] [CrossRef]
- Reza, S.; Bapary, M.A.J.; Ahasan, C.T.; Islam, N. Kamal Shelf Life of Several Marine Fish Species of Bangladesh during Ice Storage. Int. J. Food Sci. Technol. 2009, 44, 1485–1494. [Google Scholar] [CrossRef]
- Lan, W.; Hu, X.; Sun, X.; Zhang, X.; Xie, J. Effect of the Number of Freeze-Thaw Cycles Number on the Quality of Pacific White Shrimp (Litopenaeus vannamei): An Emphasis on Moisture Migration and Microstructure by LF-NMR and SEM. Aquac. Fish. 2019, 5, 193–200. [Google Scholar] [CrossRef]
- Albertos, I.; Martin-Diana, A.B.; Cullen, P.; Tiwari, B.K.; Ojha, K.S.; Bourke, P.; Rico, D. Shelf-Life Extension of Herring (Clupea harengus) Using in-Package Atmospheric Plasma Technology. Innov. Food Sci. Emerg. Technol. 2019, 53, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lin, B.; Hong, Y.; Liu, T.; Huang, Z.; Wang, R.; Wang, Z. Assessing the Moisture Migration during Microwave Drying of Coal Using Low-Field Nuclear Magnetic Resonance. Dry. Technol. 2017, 36, 567–577. [Google Scholar] [CrossRef]
- Neta, Z.M.; de Almeida, N.M.; Grisi, C.V.B.; de Sousa, S.; Cordeiro, A.M.T.D.M. Elaboration and Quality Control of the Piracui from Trahira (Hoplias malabaricus) during Storage. Int. J. Gastron. Food Sci. 2020, 23, 100287. [Google Scholar] [CrossRef]
- Halldorsdottir, S.M.; Kristinsson, H.G.; Sveinsdottir, H.; Thorkelsson, G.; Hamaguchi, P.Y. The Effect of Natural Antioxidants on Haemoglobin-Mediated Lipid Oxidation during Enzymatic Hydrolysis of Cod Protein. Food Chem. 2013, 141, 914–919. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid Oxidation and Improving the Oxidative Stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Piccini, J.; Evans, D.R.; Quaranta, H.O. Comparison of TBA Number of Irradiated Fish with Sensory Quality. Food Chem. 1986, 19, 163–171. [Google Scholar] [CrossRef]
- Volpe, M.; Siano, F.; Paolucci, M.; Sacco, A.; Sorrentino, A.; Malinconico, M.; Varricchio, E. Active Edible Coating Effectiveness in Shelf-Life Enhancement of Trout (Oncorhynchusmykiss) Fillets. LWT 2015, 60, 615–622. [Google Scholar] [CrossRef]
- Shokri, S.; Parastouei, K.; Taghdir, M.; Abbaszadeh, S. Application an Edible Active Coating Based on Chitosan-Ferulago Angulata Essential Oil Nanoemulsion to Shelf Life Extension of Rainbow Trout Fillets Stored at 4 °C. Int. J. Biol. Macromol. 2020, 153, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Yaghoubi, M.; Nemati, Z.; Farmani, B.; Khaneghah, A.M. Efficacy of Stinging Nettle Extract in Combination with ε-Polylysine on the Quality, Safety, and Shelf Life of Rainbow Trout Fillets. Food Sci. Nutr. 2021, 9, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Chaijan, S.; Panpipat, W.; Panya, A.; Cheong, L.-Z.; Chaijan, M. Preservation of Chilled Asian Sea Bass (Lates calcarifer) Steak by Whey Protein Isolate Coating Containing Polyphenol Extract from Ginger, Lemongrass, or Green Tea. Food Control 2020, 118, 107400. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.; Chen, L.; Yang, H. Effect of Vacuum Impregnated Fish Gelatin and Grape Seed Extract on Metabolite Profiles of Tilapia (Oreochromis niloticus) Fillets during Storage. Food Chem. 2019, 293, 418–428. [Google Scholar] [CrossRef]
- Peng, J.; Zheng, F.; Wei, L.; Lin, H.; Jiang, J.; Hui, G. Jumbo Squid (Dosidicus gigas) Quality Enhancement Using Complex Bio-preservative during Cold Storage. J. Food Meas. Charact. 2017, 12, 78–86. [Google Scholar] [CrossRef]
| Sample | Daphnetin Content (g/L) | Modes of Packaging |
|---|---|---|
| CK | - | Air |
| 0D | - | Modified atmosphere |
| 0.16D | 0.16 | Modified atmosphere |
| 0.32D | 0.32 | Modified atmosphere |
| 0.64D | 0.64 | Modified atmosphere |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Liu, W.; Mei, J.; Xie, J. Effect of Locust Bean Gum-Sodium Alginate Coatings Combined with High CO2 Modified Atmosphere Packaging on the Quality of Turbot (Scophthalmus maximus) during Refrigerated Storage. Polymers 2021, 13, 4376. https://doi.org/10.3390/polym13244376
Cao J, Liu W, Mei J, Xie J. Effect of Locust Bean Gum-Sodium Alginate Coatings Combined with High CO2 Modified Atmosphere Packaging on the Quality of Turbot (Scophthalmus maximus) during Refrigerated Storage. Polymers. 2021; 13(24):4376. https://doi.org/10.3390/polym13244376
Chicago/Turabian StyleCao, Jie, Wenru Liu, Jun Mei, and Jing Xie. 2021. "Effect of Locust Bean Gum-Sodium Alginate Coatings Combined with High CO2 Modified Atmosphere Packaging on the Quality of Turbot (Scophthalmus maximus) during Refrigerated Storage" Polymers 13, no. 24: 4376. https://doi.org/10.3390/polym13244376
APA StyleCao, J., Liu, W., Mei, J., & Xie, J. (2021). Effect of Locust Bean Gum-Sodium Alginate Coatings Combined with High CO2 Modified Atmosphere Packaging on the Quality of Turbot (Scophthalmus maximus) during Refrigerated Storage. Polymers, 13(24), 4376. https://doi.org/10.3390/polym13244376








