Charge Storage and Solar Rechargeable Battery Devices Based on Electrodes Electrochemically Modified with Conducting Polymer Nanowires
Abstract
:1. Introduction
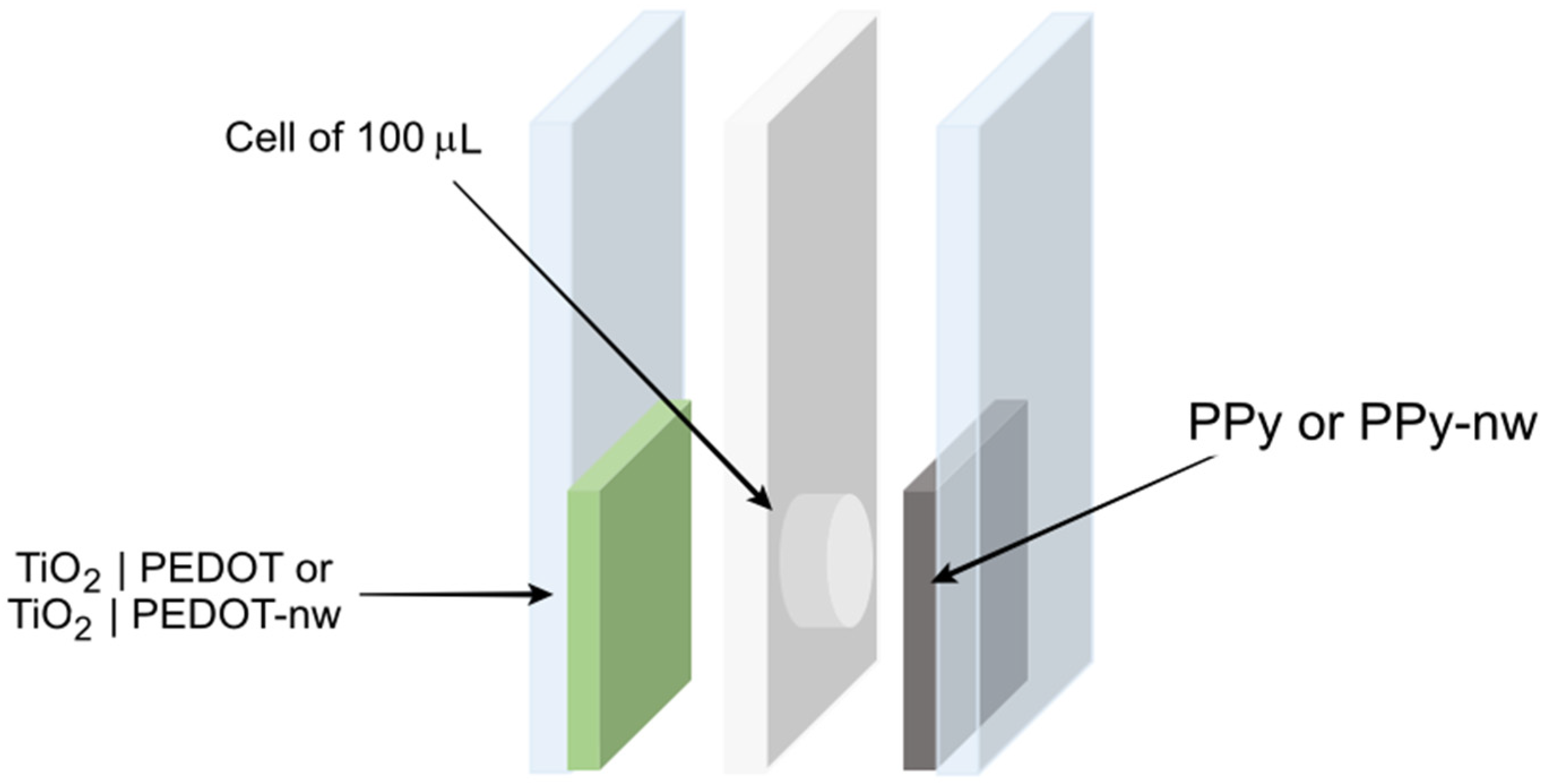
2. Materials and Methods
3. Results and Discussion
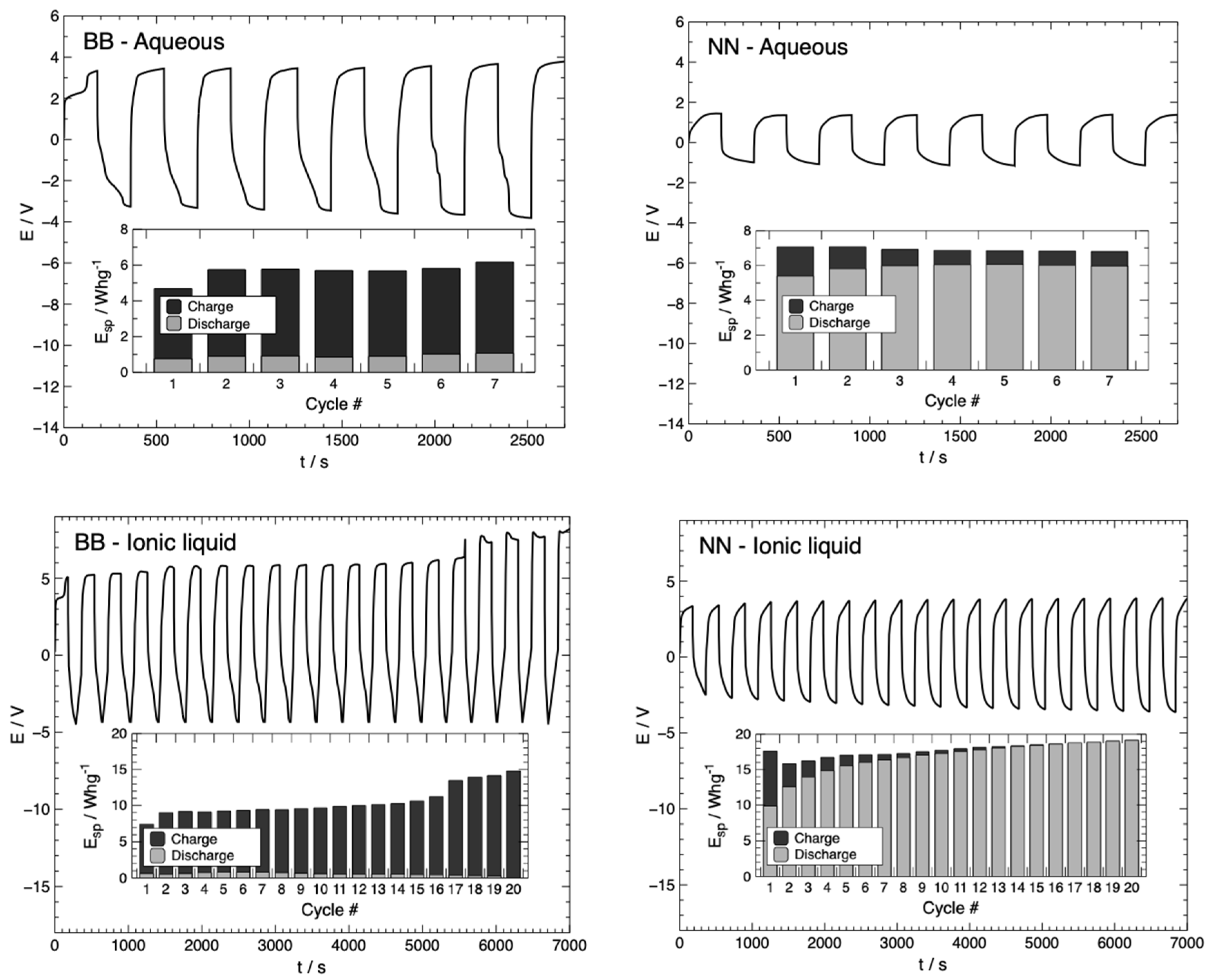
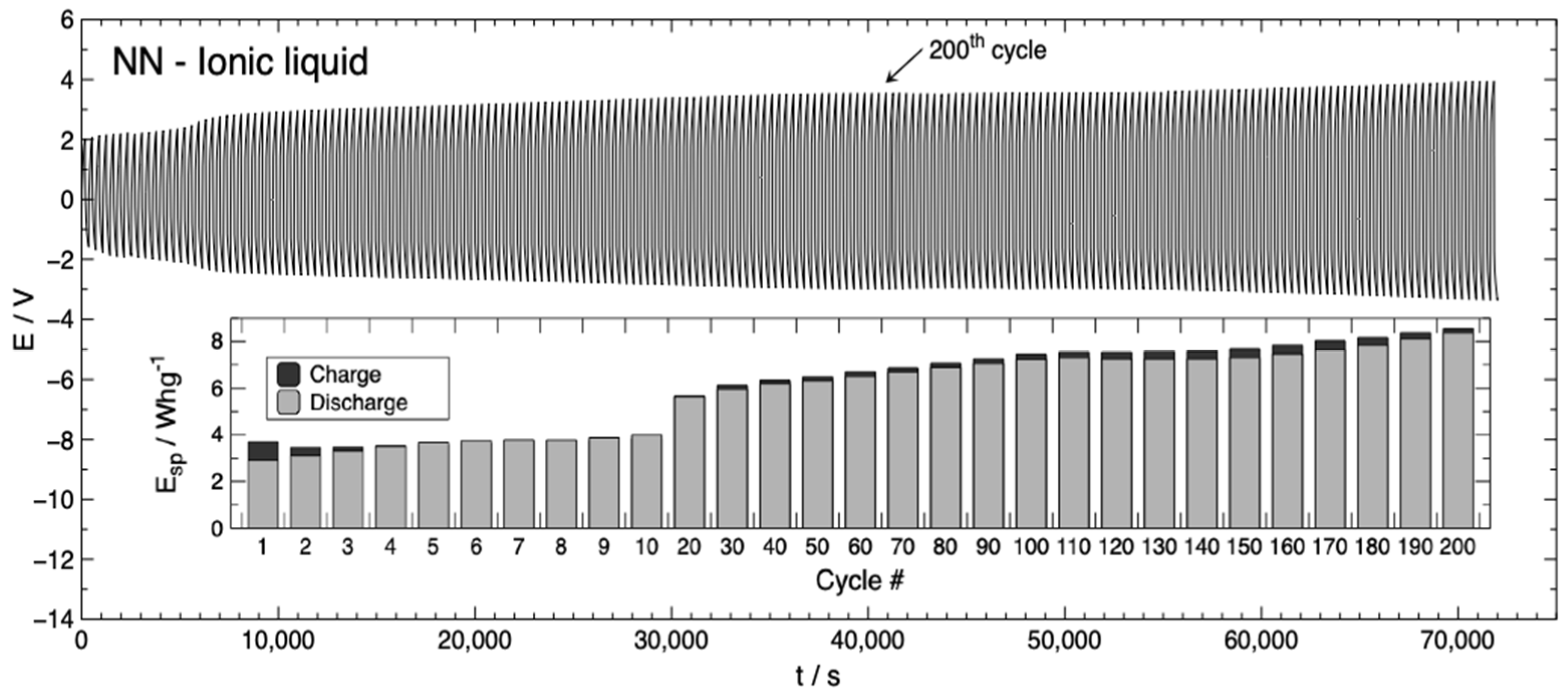
3.1. Battery Characterization
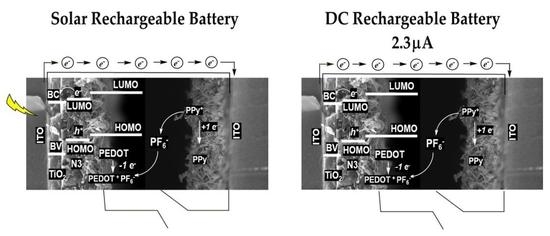
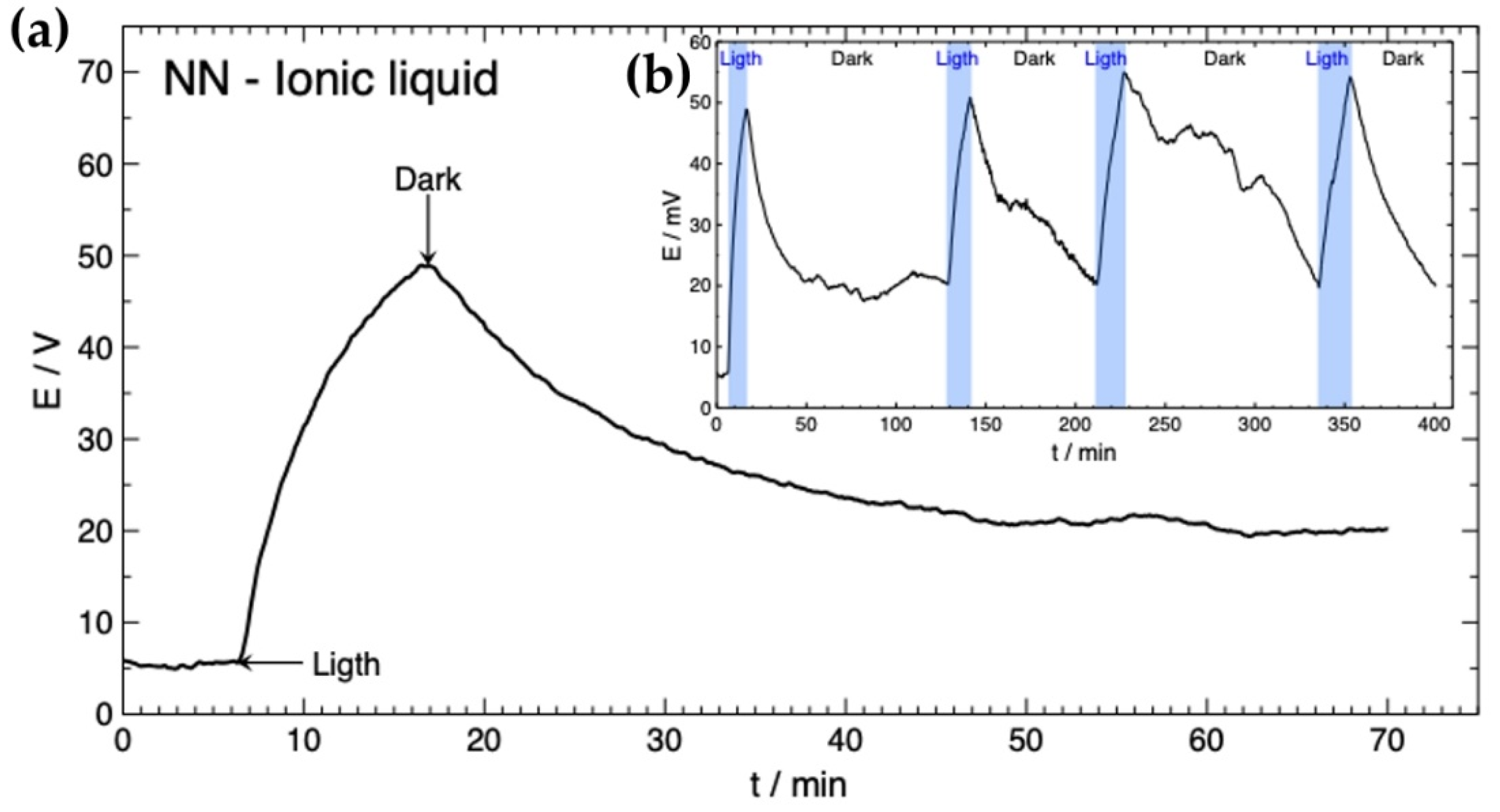
3.2. Solar Rechargeable Battery Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muench, S.; Wild, A.; Friebe, C.; Haupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef] [PubMed]
- Hillman, A.R.; Daisley, S.J.; Bruckenstein, S. Kinetics and mechanism of the electrochemical p-doping of PEDOT. Electrochem. Commun. 2007, 9, 1316–1322. [Google Scholar] [CrossRef]
- Hillman, A.R.; Daisley, S.J.; Bruckenstein, S. Solvent effects on the electrochemical p-doping of PEDOT. Phys. Chem. Chem. Phys. 2007, 9, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Hillman, A.R.; Daisley, S.J.; Bruckenstein, S. Ion and solvent transfers and trapping phenomena during n-doping of PEDOT films. Electrochem. Commun. 2008, 53, 3763–3771. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Park, C.; Kim, H.S.; Park, J.H.; Chung, K.Y.; Ahn, H. Controlling Vanadate Nanofiber Interlayer via Intercalation with Conducting Polymers: Cathode Material Design for Rechargeable Aqueous Zinc Ion Batteries. Adv. Funct. Mater. 2021, 31, 2100005. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Xia, X.H.; Wang, X.L.; Xiang, J.Y.; Zhang, L. Porous NiO/Poly(3,4-ethylenedioxythiophene) Films as Anode Materials for Lithium Ion Batteries. J. Power Sources 2010, 195, 1207–1210. [Google Scholar] [CrossRef]
- Ramirez, A.M.; del Valle, M.A.; Armijo, F.; Diaz, F.R.; Pardo, M.A.; Ortega, E. Enhancement of Electrodes Modified by Electrodeposited PEDOT-nanowires with Dispersed Pt Nanoparticles for Formic Acid Electro-oxidation. J. Appl. Polym. Sci. 2017, 134, 44723. [Google Scholar] [CrossRef]
- Martinez-Rojas, F.; Castaneda, E.; Armijo, F. Conducting polymer applied in a label-free electrochemical immunosensor for the detection prostate-specific antigen using its redox response as an analytical signal. J. Electroanal. Chem. 2021, 880, 114877. [Google Scholar] [CrossRef]
- Martinez-Rojas, F.; Diculescu, V.C.; Armijo, F. Electrochemical Immunosensing Platform for the Determination of the 20S Proteasome Using an Aminophenylboronic/Poly-indole-6-carboxylic Acid-Modified Electrode. ACS Appl. Bio Mater. 2020, 3, 4941–4948. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Y.H.; Morrin, A.; Ding, C.F.; Luo, X.L. Preparation and electrochemical sensing application of porous conducting polymers. Trac-Trend Anal. Chem. 2021, 135, 116155. [Google Scholar] [CrossRef]
- Liu, W.N.; Li, X.X.; Li, W.J.; Ye, Y.M.; Wang, H.; Su, P.P.; Yang, W.; Yang, Y. High-performance supercapacitors based on free-standing SiC@PEDOT nanowires with robust cycling stability. J. Energy Chem. 2022, 66, 30. [Google Scholar] [CrossRef]
- Kondratiev, V.V.; Babkova, T.A.; Tolstopjatova, E.G. PEDOT-supported Pd nanoparticles as a catalyst for hydrazine oxidation. J. Solid State Electrochem. 2013, 17, 1621–1630. [Google Scholar] [CrossRef]
- Kshirasagar, K.J.; Markad, U.S.; Saha, A.; Sharma, K.K.K.; Sharma, G.K. Facile synthesis of palladium nanoparticle doped polyaniline nanowires in soft templates for catalytic applications. Mater. Res. Express 2017, 4, 025015. [Google Scholar] [CrossRef]
- Schoetz, T.; Craig, B.; de Leon, C.P.; Bund, A.; Ueda, M.; Low, C.T.J. Aluminium Poly(3,4-ethylenedioxythiophene) Rechargeable Battery with Ionic Liquid Electrolyte. J. Energy Storage 2020, 28, 101176. [Google Scholar] [CrossRef]
- Jung, Y.J.; Singh, N.; Choi, K.S. Cathodic Deposition of Polypyrrole Enabling the One-Step Assembly of Metal-Polymer Hybrid Electrodes. Angew. Chem. Int. Ed. 2009, 48, 8481–8484. [Google Scholar] [CrossRef]
- Walcarius, A. Electrochemistry with Micro- and Mesoporous Silicates. In Ordered Porous Solids—Recent Advances and Prospects; Elsevier: Amsterdam, The Netherlands, 2009; pp. 523–557. [Google Scholar]
- Walcarius, A. Electroinduced Surfactant Self-Assembly Driven to Vertical Growth of Oriented Mesoporous Films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef]
- Ullah, W.; Herzog, G.; Vila, N.; Walcarius, A. Electrografting and electropolymerization of nanoarrays of PANI filaments through silica mesochannels. Electrochem. Commun. 2021, 122, 106896. [Google Scholar] [CrossRef]
- Laskowski, L.; Laskowska, M.; Vila, N.; Schabikowski, M.; Walcarius, A. Mesoporous Silica-Based Materials for Electronics-Oriented Applications. Molecules 2019, 24, 2395. [Google Scholar] [CrossRef] [Green Version]
- Del Valle, M.A.; Ramirez, A.M.R.; Diaz, F.R.; Pardo, M.A.; Ortega, E.; Armijo, F. Influence of the Electrolyte Salt on the Electrochemical Polymerization of Pyrrole. Effects on p-Doping/Undoping, Conductivity and Morphology. Int. J. Electrochem. Sc. 2018, 13, 12404. [Google Scholar] [CrossRef]
- Ramirez, A.M.R.; Gacitua, M.A.; Ortega, E.; Diaz, F.R.; del Valle, M.A. Electrochemical in Situ Synthesis of Polypyrrole Nanowires. Electrochem. Commun. 2019, 102, 94–98. [Google Scholar] [CrossRef]
- Del Valle, M.A.; Ramirez, A.M.; Hernandez, L.A.; Armijo, F.; Diaz, F.R.; Arteaga, G.C. Influence of the Supporting Electrolyte on the Electrochemical Polymerization of 3,4-Ethylenedioxythiophene. Effect on p- and n-Doping/Undoping, Conductivity and Morphology. Int. J. Electrochem. Sci. 2016, 11, 7048–7065. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.D.; Hu, W.D.; Qiao, J.L.; Zhang, L.; Zhang, J.J. A Review of Electrolyte Materials and Compositions for Electrochemical Supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, K.; Hata, F.; Uto, S. Structure and size of ions electrochemically doped in conducting polymer. J. Micromech. Microeng. 2018, 28, 054003. [Google Scholar] [CrossRef]
- Ehsani, A.; Kowsari, E.; Najafi, M.D.; Safari, R.; Shiri, H.M. Influence of Ionic Liquid on Pseudocapacitance Performance of Electrochemically Synthesized Conductive Polymer: Electrochemical and Theoretical Investigation. J. Colloid Interface Sci. 2017, 500, 315–320. [Google Scholar] [CrossRef]
- Liu, P.; Yang, H.X.; Ai, X.P.; Li, G.R.; Gao, X.P. A Solar Rechargeable Battery Based on Polymeric Charge Storage Electrodes. Electrochem. Commun. 2012, 16, 69–72. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Cattin, L.; Bernede, J.C.; Diaz, F.R.; Gacitua, M.A.; del Valle, M.A. Nanostructured TiO2 and PEDOT Electrodes with Photovoltaic Application. Nanomaterials 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- East, G.A.; del Valle, M.A. Easy-to-make Ag/AgCl Reference Electrode. J. Chem. Educ. 2000, 77, 97. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J.M. Electrochemically Assisted Self-assembly of Meso porous Silica Thin Films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]
- Evans, R.G.; Klymenko, O.V.; Price, P.D.; Davies, S.G.; Hardacre, C.; Compton, R.G. A Comparative Electrochemical Study of Diffusion in Room Temperature Ionic Liquid Solvents Versus Acetonitrile. ChemPhysChem 2005, 6, 526–533. [Google Scholar] [CrossRef]
- Chockla, A.M.; Klavetter, K.C.; Mullins, C.B.; Korgel, B.A. Solution-Grown Germanium Nanowire Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 4658–4664. [Google Scholar] [CrossRef]
- Takshi, A.; Yaghoubi, H.; Tevi, T.; Bakhshi, S. Photoactive Supercapacitors for Solar Energy Harvesting and Storage. J. Power Sources 2015, 275, 621–626. [Google Scholar] [CrossRef]
- Lei, B.; Li, G.R.; Chen, P.; Gao, X.P. A Solar Rechargeable Battery Based on Hydrogen Storage Mechanism in Dual-phase Electrolyte. Nano Energy 2017, 38, 257–262. [Google Scholar] [CrossRef]

| Battery | |||||
|---|---|---|---|---|---|
| Ah g−1 | Wh kg−1 | W kg−1 | |||
| Charge | Discharge | Charge | Discharge | ||
| BB-Aqueous | 148 | 423 | 153 | 8469 | 3067 |
| NN-Aqueous | 2471 | 3794 | 860 | 75,884 | 17,196 |
| BB-Ionic liquid | 184 | 655 | 153,333 | 13,109 | 3,066,667 |
| NN-Ionic liquid | 2868 | 7901 | 1182 | 158,027 | 23,645 |
| Battery Configuration | I | Cycle n°/Stability | Ref | |||
|---|---|---|---|---|---|---|
| A g−1 | Ah g−1 | Wh kg−1 | W kg−1 | |||
| Al|EMImCl-AlCl3|PEDOT | 0.03 | 179 | 233 | 146 | 100/94% | [14] |
| SS|E-AVNF|Zn | 0.50 | 326 | 276 | 16,000 | 1000/94% | [5] |
| NiO/PEDOT|EC/DEC + LiPF6|Li | 0.72 | 650 | n/a | n/a | 50/80% | [6] |
| Sn|PPy|Cu | n/a | 942 | n/a | n/a | 50/47% | [15] |
| ITO|TiO2|PEDOTnw|BMIMPF6|PPymw|ITO | 57.4 | 2868 | 1075 | 21,495 | 200/98% | This work |
| Active Layer Material | Medium | OCV/mV | Ref | |
|---|---|---|---|---|
| Anode | Cathode | |||
| TiO2|PEDOT a,b | PPy c | LiClO4 + TBP in PC | 510 | [26] |
| ZnTpp|PEDOT:PSS | Carbon paper | MV in TB | 430 | [32] |
| N749|PEDOT:PSS | Carbon paper | MV in TB | 198 | |
| TiO2|N719 a | Hydrogen storage alloy d | LiClO4 + PEDOT-modified Nafion® | 700 | [33] |
| TiO2|N3|PEDOTnw | PPynw | Ionic liquid | 35 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, A.M.; Gacitúa, M.A.; Díaz, F.R.; del Valle, M.A. Charge Storage and Solar Rechargeable Battery Devices Based on Electrodes Electrochemically Modified with Conducting Polymer Nanowires. Polymers 2021, 13, 4375. https://doi.org/10.3390/polym13244375
Ramírez AM, Gacitúa MA, Díaz FR, del Valle MA. Charge Storage and Solar Rechargeable Battery Devices Based on Electrodes Electrochemically Modified with Conducting Polymer Nanowires. Polymers. 2021; 13(24):4375. https://doi.org/10.3390/polym13244375
Chicago/Turabian StyleRamírez, Andrés Mauricio, Manuel Alejandro Gacitúa, Fernando Raúl Díaz, and María Angélica del Valle. 2021. "Charge Storage and Solar Rechargeable Battery Devices Based on Electrodes Electrochemically Modified with Conducting Polymer Nanowires" Polymers 13, no. 24: 4375. https://doi.org/10.3390/polym13244375
APA StyleRamírez, A. M., Gacitúa, M. A., Díaz, F. R., & del Valle, M. A. (2021). Charge Storage and Solar Rechargeable Battery Devices Based on Electrodes Electrochemically Modified with Conducting Polymer Nanowires. Polymers, 13(24), 4375. https://doi.org/10.3390/polym13244375